Abstract
Skeletal metastasis is a common cause of severe morbidity, reduction in quality of life (QOL) and often early mortality. Its prevalence is rising due to a higher rate of diagnosis, better systemic treatment, longer lives with the disease and higher disease burden rate. As people with cancer live longer and with rising sensitivity of body imaging and surveillance, the incidence of pathological fracture, metastatic epidural cord compression is rising and constitutes a challenge for the orthopedic surgeon to maintain their QOL. Metastatic disease is no longer a death sentence condemning patients to “terminal care.” In the era of multidisciplinary care and effective systemic targeted and nontargeted therapy, patient expectations of QOL, even during palliative end of care period is high. We lay emphasis on proving the diagnosis of metastasis by biopsy and histopathology and discuss imaging modalities to help estimate fracture risk and map disease extent. This article discusses at length the evidence and decision-making process of various modalities to treat skeletal metastasis. The modalities range from radiation including image-guided, stereotactic and whole body radiation, systemic targeted or hormonal therapy, spinal decompression with or without stabilization, extended curettage with stabilization, resection in select cases with megaprosthetic or biological reconstruction, percutaneous procedures using radio frequency ablation, cementoplasties and discusses the role of emerging modalities like high frequency ultrasound-guided ablation, cryotherapy and whole body radionuclide therapy. The focus lies on the role of multidisciplinary care, which considers complex decisions on patient centric prognosis, comorbidities, cost, feasibility and expectations in order to maximize outcomes on QOL issues.
Keywords: Bone metastases, pathological fracture, skeletal metastases
MeSH terms: Metastases, fracture, pathological, bone cancer
INTRODUCTION
Bone is among the most frequent sites of metastatic disease next only to lung and liver. Approximately, 70% of patients who die of breast or prostate cancer have also had bone metastases.1 Thyroid, lung, and kidney cancers also have a propensity to involve the skeleton. Approximately, 30-65% of patients with metastatic lung cancers will develop bone metastases,2 as will approximately 47% of patients with advanced thyroid cancer and 30% of patients with advanced renal carcinoma.3 It is reported that 9-29% of patients with metastases will have a pathological fracture4 and 90% of fractures require surgery.5 With advances in treatment, patients with cancer, especially prostate and breast cancer are living longer and thus there is greater responsibility on the orthopedic surgeon to preserve the quality of life (QOL) by preventing and managing skeletal-related events (SRE). SRE include pain, vertebral fractures, cord compression and pathological fractures of long bones. All these have an impact on mobility and functional independence and therefore greatly impact the QOL.
A lot has changed in the last decade in terms of imaging, detection, medical and surgical management. Management of skeletal metastases is no longer a simple task of fixing a fracture, but involves a multidisciplinary coordination between medical oncologists, radiation oncologists and the surgeon. This article updates the orthopedic surgeon on the current concepts in workup and managing skeletal metastases.
IMAGING MODALITIES IN METASTATIC DISEASE
The radiographic appearance of metastatic bone tumors have traditionally been described as osteolytic, osteoblastic, or mixed lytic and sclerotic [Figure 1]. Recently, an osteoporotic pattern has been described where the lesions appear as “faded” bone without discrete areas of cortical destruction or increased density in theX-ray.6 It is estimated that there is at least 25-75% loss of mineral before a lesion is visible on X-ray7,8 implying that the bone is already significantly weak by the time the lesion is radiologically visible. The extent of bony destruction helps assess the risk of fracture. Lytic lesionsdestroying 50% or more of the diaphyseal cortex can result in a 60-90% reduction in bone strength, significantly increasing the risk of fracture.9
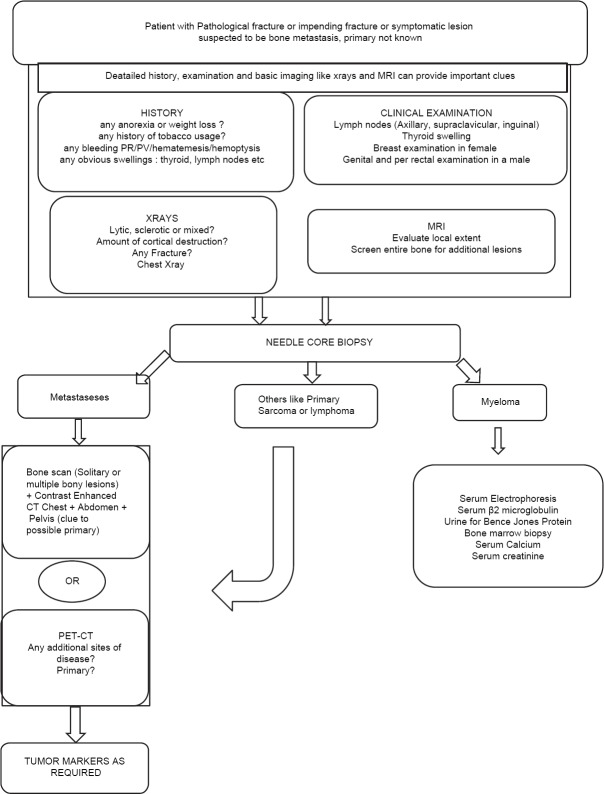
Figure 1.
Varied radiological appearance of metastasis-lytic, mixed and blastic. (a) The radiograph of forearm with elbow joint anteroposterior view showing lytic metastasis from lung carcinoma occurring distal to the elbow (Note that metastases below the knee and elbow are rare) (b) The radiograph of hip joint with proximal thigh anteroposterior view showing an impending fracture from a lytic metastasis arising from a colonic primary. Pure lytic lesions are common with renal, lung, thyroid, uterine, adrenal, GI malignancies and melanoma (c) X-ray pelvis with both hip joints anteroposterior view showing extensive lytic and sclerotic metastases to pelvis and both femori from breast cancer. Mixed lesions are known with breast, lung, ovary, cervix, testicular malignancies and lymphoma (d) X-ray pelvis with both hip joints anteroposterior view showing pure sclerotic or blastic metastasis arising from a prostate carcinoma in a 70-year-old male. Pure blastic lesions are common with prostate, bladder cancers, medullary carcinoma thyroid and bronchial carcinoids
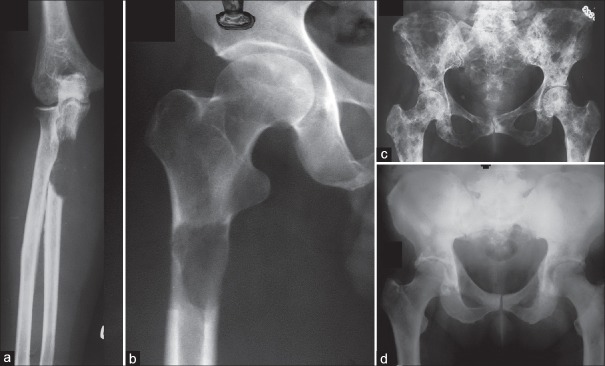
Some rough guidelines for prophylactic fixation have been proposed based on the amount of cortical destruction on X-rays. Fidler10 in 1973 suggested prophylactic fixation of the long bone for more than 50% cortical destruction. In 1982, Harrington,11 after a review of the literature, added the criteria of lesion length more than 2.5 cm, fracture of adjacent lesser trochanter and persistent pain after radiation. It was far too simplistic not factoring several variables and therefore not accurate. In 1989, Mirels12 proposed a more refined system [Table 1]. Prophylactic fixation was recommended for a score greater than 9 which predicted a 33% risk a score <7 had a low risk of fracture. Though not categorical or absolute, subsequent studies have validated this system as having high sensitivity but low specificity.12,13,14
Table 1.
Mirel's score
More accuracy would involve algorithm based evaluation of computerized tomography (CT) images compared withthe opposite side to assess the bony rigidity. This has been used and reported for children with benign tumors.15 CT-based finite element modeling has also been used to predict fracture risk in long bones.16 Magnetic resonance imaging (MRI), Dual energy X-ray absorptiometry (DEXA) and quantitative CT have been tried to evaluate fracture risk in vertebral bodies.17 Once developed, these would provide a far higher accuracy in estimating fracture risk. Currently, however, none of these can still be used practically in the clinic, and Mirels score remains the standard of care. Despite all the enumerated scoring systems to predict fracture risk, pain at rest and activity remains the single most sensitive symptom to predict fracture risk and increasingly more clinicians rely on this to make judgment calls despite equivocal scores.
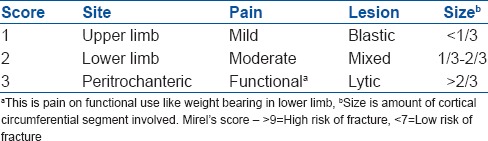
Computerized tomography and MRI can evaluate suspicious findings on Technetium-99 (99mTc) bone scans and can provide better spatial resolution and three-dimensional anatomic information about the skeleton as well as softtissue involvement [Figure 2]. CT is excellent at assessing the cortical lesions whereas trabecular lesions may be best seen on MRI.8 MRI is highly sensitive to detect small skeletal metastases not yet detectable on bone scans by revealing abnormal bone marrow; Though MRI is very sensitive, it cannot help differentiate between infection, fracture or inflammation, all of which cause similar marrow signal changes.
Figure 2.
The plain radiograph (a) Underestimates the disease extent identified on magnetic resonance imaging (MRI) images (b) MRI can also identify and delineate soft tissue involvement, joint effusion, skip lesions and other crucial information like relation to neurovascular structures which can critically affect treatment plan
Once a lesion is identified as metastatic disease, the clinician needs to know if the lesion is solitary or if there are other sites of disease. 99mTc bone scintigraphy screens the entire skeleton, is very sensitive and has a relative low cost making it the initial imaging modality for detection of bone metastases. It accumulates in areas of increased osteoblastic activity and increased blood flow. Osteoblastic metastases are, therefore, more reliably picked up. Technetium bone scan is false negative for highly aggressive and rapidly growing lytic tumors like myeloma because of minimal reactive bone formation.18,19 Lytic lesions can appear as “cold” defects but are frequently overlooked.7 In contrast to plain films, as little as 5-10% change in the ratio of lesion to normal bone is required to detect an abnormality on bone scan. It is estimated that bone scan can detect malignant bone lesions 2-18 months earlier than the radiograph can.7 This explains why tumors known to have a propensity for skeletal metastases undergo regular screening with bone scan. Bone scan has published sensitivity rates between 62% and 100%, with a specificity of 78-100%.7 False positives can occur in any area of high bone turnover related to trauma, infection, or arthropathy.7,20,21 To overcome lack of specificity, correlative imaging with radiographs, CT, or MRI is recommended, especially in the context of equivocal findings (single “hot spot” or few lesions). Lesions like enchondromas, bone islands or healed nonossifying fibromas can appear as hot spots and need differentiation from a metastatic lesion. These are generally obvious on an X-ray. Sometime CT accurately characterizes the lesion. Single-photon emission computed tomography (SPECT) improves the sensitivity and specificity of 99mTc bone scans for detection of small bone metastases,22,23 by superimposing the hot spot on a CT cut. A focal hot spot with a lytic area is significant for suspected metastases, but MRI still provides better tomographic images. Tuberculosis, particularly in the spine can be a confounding factor in the Asian subcontinent and can coexist with metastatic disease. We recommend a biopsy prior to treatment in any skeletal lesion. The presence of multiple lesions in patients with a known malignancy is more suggestive of metastatic disease, although other nonneoplastic conditions like infection and arthropathy can also have multifocal areas of uptake. Any suspected lesion is further worked up with local X-rays, a focal MRI or CT. MRI is the most accurate when bony destruction is minimal.
Lytic lesions negative on bone scan are better detected by metabolic scans such as fluorodeoxyglucose-positron emission tomography (FDG-PET) because they have a higher than normal glucose metabolism. The poor specificity of the FDG-PET, as well as its lack of anatomic detailing, makes CT and MRI necessary for further characterization. As FDG-PET relies on glucose uptake by the tumor, it can detect early metastases without any bony destruction. It can also help in assessing the response to treatment by showing a corresponding reduction in activity of the tumor. As more knowledge accumulates, its use is increasing for several malignancies such as lung, breast, and head and neck cancers. Although FDG-PET is superior in detecting osteolytic metastases, it is less sensitive than 99mTc bone scans for detection of osteoblastic metastases.24,25 It is thus more sensitive than bone scan for myeloma, equivalently sensitive for breast and lung cancers, and less sensitive for prostate cancer. The aggressiveness of the tumor may also influence the sensitivity of detecting bone metastases using FDG-PET.26 Skeletal PET using F-18 sodium fluoride (NaF-18), a positron-emitting bone-seeking tracer, may improve the utility of PET. The available literature shows that NaF-18 PET is substantially more sensitive and specific than 99mTc bone scan and SPECT27,28 for detection of metastases, especially for osteolytic lesions, but has a higher cost and involves greater radiation dose.29 Addition of CT greatly improves the utility of PET by providing better resolution.28
Whole body MRI as a tool for systemic surveillance with its zero radiation and excellent anatomic detailing is useful in myeloma for screening the skeleton but unlike PET cannot be used to assess response. PET MRI is a new modality, which also helps decrease radiation and its potential side-effects, while retaining excellent biologic imaging capabilities. Studies are underway to determine its utility and effectiveness in systemic surveillance.
BIOPSY AND TUMOR MARKERS
A biopsy is a must to prove that the lesion is metastatic. A needle biopsy generally is adequate for most lesions as it provides a diagnosis in over 90% of cases.30,31,32 It is important to accurately target the lesion using image guidance (USG/CT/C Arm). A Jamshidi or other trocar cannula based needle is used for entering the bone. For lesions with a soft tissue mass, a tru-cut spring loaded core biopsy needle is adequate and simple to use. Apart from morphology, immunohistochemistry can help give a clue to the possible primary in metastatic disease. Where the primary diagnosis of malignancy has already been made, an FNAC may be used to simply confirm metastatic disease. If the orthopedic surgeon is unfamiliar with the method of doing a needle biopsy or the pathologist is uncomfortable in reporting on the needle biopsy (this often happens in inexperienced centers), it is best to refer the patient to an oncology setup.
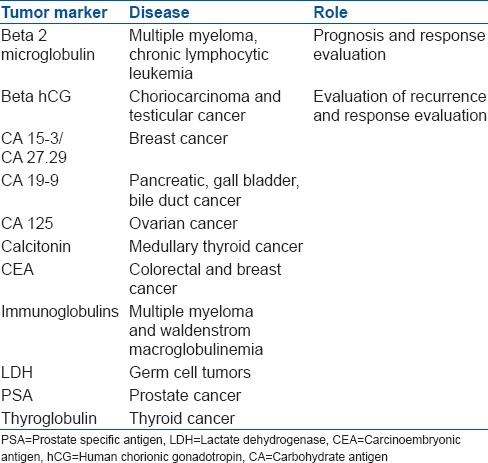
Tumor markers are substances that are detected in body fluids (blood/serum/urine) or in tissue (tumor/marrow/bone) that help diagnose, screen, monitor response to therapy and detect a recurrence by minimally or noninvasive methods. Most markers have been proteins that represent a unique genetic signature associated with a particular malignancy. A few commonly used ones are enumerated in the Table 2.
Table 2.
Tumor markers
In spite of full workup like tumor markers, PET-CT and modern immunohistochemistry, the primary may be unknown in about 3-5% of these cases.33
CLINICAL PRESENTATION AND WORKUP
Pathological fracture or impending fracture or painful bony lesion without any known history or treatment for cancer
This is the commonest scenario in which a patient presents to an orthopedic surgeon. Proximal femur and proximal humerus are the most common sites, but any long bone could be affected. Alternatively, the spine may be involved, with or without a vertebral fracture or neurological deficit. The workup is outlined in Figure 3. It is very important not to assume diagnosis of a metastatic lesion just because the patient is elderly as infections and primary sarcomas can have a similar appearance. It is also equally important not to assume that the fracture has resulted from osteoporosis. Either way, one should not be in a hurry to operate as unplanned surgery can lead to inappropriate treatment with permanent, irreversible harm caused to the patient. Assuming metastatic disease and performing intralesional surgery can be a disaster for a primary bone sarcoma [Figure 4]. This is more likely to happen in the proximal femur where chondrosarcomas also present in the elderly and are not always mineralized. Pathological fracture is not an emergency, and a clinician will find it worthwhile to do a complete workup prior to any surgery.
Figure 3.
Workup of a patient with a symptomatic bony lesion or impending fracture or pathological fracture from suspected metastases
Figure 4.
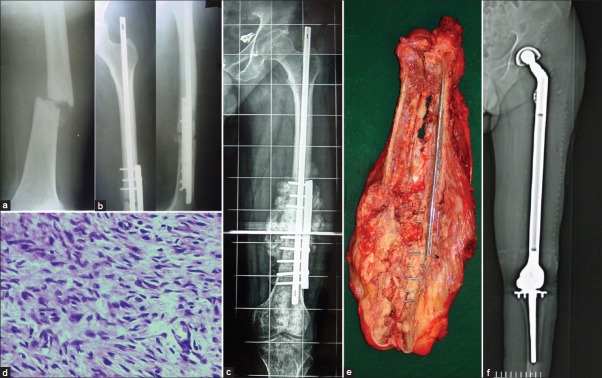
(a) A 50-year-old male with a pathological fracture shaft femur was assumed to have metastasis (b) He underwent immediate fixation with an intramedullary Nail and a derotation plate. No prior biopsy was performed. Figure shows the postoperative X-ray (c) Rapid growth of a bony hard mass at the fracture site a few weeks later prompted a referral. Note the exuberant bone formation (d) Histology proved to be an osteosarcoma (e) Resection involved removing the entire femur. Specimen is bivalved to show the tumor (f) Postoperative X-ray showing the total femur replacement. This would not be necessary were a proper workup done prior to surgery
It is important to pay attention to small details like revealing an unexpected unpleasant diagnosis to the patient and their family. Diagnosis of metastasis is often not a “death sentence” and must not prompt resignation and advice for “terminal palliative care.” In modern times, patients with metastatic disease can live for several years and early referral to a multidisciplinary oncology team is advised. Once the decision for surgery is made at a multidisciplinary meeting, the surgeon can choose the surgical strategy outlined in the sections below. It is important to estimate a patient's general condition, disease stage and expected survival prior to embarking on any intervention.
Primary is known: The skeletal lesion is picked up on workup or during periodic followup
This presentation is now becoming more frequent as routine surveillance screening with a technetium bone scan or PET-CT often picks up an asymptomatic bony lesion or on followup. Alternatively a bony lesion is detected because of symptoms in a patient known to have a primary cancer. Asymptomatic lesions tend to be smaller, and an opinion is sought by the treating oncologist as to the best line of treatment. A surgeon must not assume that a lesion is metastases even if the primary is known [Figure 5] as infection, benign lesions or primary bone sarcomas (second malignancy) can confound the diagnosis. A needle biopsy can establish the diagnosis. A discussion with the multidisciplinary team which includes at least a medical oncologist and radiation oncologist and preferably also an interventional radiologist is required to make the final treatment decision.
Figure 5.
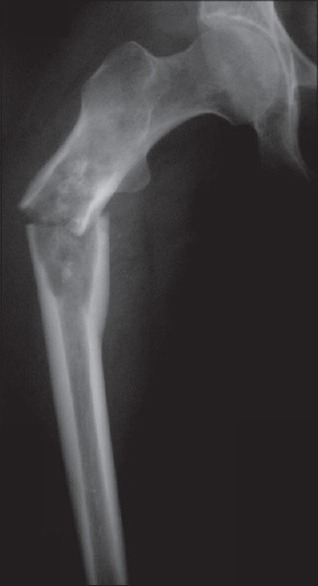
This 54-year-old lady was treated for breast cancer 4 years prior to having this pathological fracture through the proximal femur. It was assumed to be metastasis. A radiologically obvious chondrosarcoma was missed. The treatment and prognosis were radically different, reinforcing the need for histopathological confirmation with a biopsy
TREATMENT OF SKELETAL METASTASES
The treatment of skeletal metastases is aimed first at maintaining or improving QOL and then at disease control and possible cure. Asymptomatic Metastases detected on workup may require systemic therapy and radiation, primarily aimed at disease control and preventing SRE that include pain, cord compression and fracture. For symptomatic lesions, the treatment is aimed at pain elimination and maintaining ambulation and neurological function. For every lesion, a decision is made by a multidisciplinary team regarding need for analgesia, radiation, systemic medical treatment (chemotherapy, hormonal therapy, targeted therapy or agents to improve bone strength) and need for intervention either minimally invasive (radiofrequency ablation [RFA], cementoplasty, vertebroplasty etc.) or open orthopedic surgery (decompression of spinal cord or vertebral or long bone stabilization). Decision making is best-customized to the individual patient but still based done on evidence-based protocols. With the improvement in systemic treatment, radiation as well as percutaneous interventions, the need for open surgery is much reduced. Logically, the least invasive methods are used upfront reserving the surgical option for the nonresponsive cases in pathological fracture or paraplegia from spinal cord compression.
Bone strengthening (systemic treatment) treatment for prevention of skeletal-related events
The advent of bisphosphonates revolutionized prevention of SRE in both lytic and blastic metastases. By their action on bone turnover they exerted numerous beneficial effects like decreased lysis and associated hypercalcemia, reduced microfractures and insufficiency fractures and associated pain and vertebral fracture induced cord compressions resulting in reduced hospitalizations and improved QOL parameters. These drugs have been useful in pure lytic, blastic and mixed lesions. Zoledronate and pamidronate are time tested pyrophosphate analogues that the work by inhibiting farnesyl pyrophosphataseand causing osteoclast lysis and apoptosis.34 There is also some evidence suggesting direct antineoplastic activity. Bisphosphonates have been shown to reduce bone-related skeletal events and are widely used for bone metastases in breast cancer, multiple myeloma, lung cancer, and prostate cancer (which also has a level of osteolytic activity).35,36 The primary side-effects associated with bisphosphonate treatment include anemia, gastrointestinal symptoms (e.g. nausea, vomiting, diarrhea, or constipation), fatigue, fever, weakness, arthralgia, myalgia and less commonly, hypocalcaemia. It is essential to measure serum creatinine before zoledronic acid administration and adjust dosage for renal insufficiency. Since bisphosphonates are known to decrease Vitamin D levels, it is recommended to supplement Vitamin D and calcium before and throughout the duration of bisphosphonate treatment. Osteonecrosis of the jaw (ONJ) is rare, but serious complication of bisphosphonate use most commonly associated with the amino bisphosphonates. A dental evaluation and preemptive dental treatment is recommended to reduce ONJ.37,38,39
Denosumab, a fully human monoclonal antibody to RANKL, is a more potent inhibitor of osteoclastogenesis andbone resorption than bisphosphonates. It has been approved for the treatment of postmenopausal osteoporosis as well as bone metastases from solid tumors and multiple myeloma. It has convenient subcutaneous dosing and is virtually free of immediate adverse events. The incidence of hypocalcemia is a little higher40 making Vitamin D and calcium supplementation mandatory. The incidence of ONJ is similar but acute reaction like bodyache and flu-like syndrome is much less common than with bisphosphonates.40 Denosumab was reported superior to zoledronic acid in preventing SRE in a recently concluded randomized trial.40 It was also easier to administer, it had virtually no short term side-effects and did not need dose adjustments for renal toxicity. The main drawback currently is its high cost. The dosing schedule in all trials has been 120 mg subcutaneously as a monthly dose.
RADIATION THERAPY
External radiation has a strong role in palliation of pain and local tumor control. Various forms of radiation include external-beam radiation therapy (EBRT) given as multiple fractions or as a single fraction in high dose, stereotactic body radiotherapy and cyber knife radiosurgery. Sophisticated methods of two-dimensionaland three-dimensional conformal radiation therapy (RT), image-guided intensity modulated RT and tomotherapy, have improved precise delivery of radiation and minimized side effects.
The decision for dosage and fraction is determined by location, symptoms, tumor volume and indication for treatment viz. palliation of pain versus medium term tumor control. In most cases for palliative pain relief, equal pain relief is noted by conventional 30 Gy given in 10 fractions versus single dose of 8-10 Gy.41 Although rate of re-radiation is higher in a single fraction group,42 it is attractive for its convenience and rapid relief. Evidence shows no difference for response in spinal versus non spinal site and histology of the disease.41,42,43 The decision is thus made on life expectancy and patient expectations viz. a patient who has a life expectancy of less than 3 months shall most likely benefit by a single fraction high dose strategy.
The role of hemi-body or whole body radiation is going down with increasing availability of radionuclide therapy that is especially useful in multiple sclerotic bone metastases. Radioiodine is a part of standard treatment for metastases from thyroid cancer. The isotopes for palliative painrelief in use include strontium 89, samarium153 and phosphorous 32. Samarium is the most commonly used isotope in the North American continent. These radionuclides attach to hydroxyapatite and are effective in areas of increased bone turnover. Side effects include debilitating marrow depression which may worsen with repetitive and heavily pre-treated patients. In most cases, adequate repetitive treatment is possible with a tolerable myelosupression. Access and availability in India is yet an unresolved issue. There are no randomized trials evaluating the efficacy of combining bisphosphonates or denosumab with RT. A recent Cochrane review showed equal efficacy of various radionuclides in symptom relief and marrow suppression.44
A radiation oncologist's opinion on radio responsiveness of the disease to be treated is a must. E.g., radio resistant painful skeletal metastases from renal cell cancer are unlikely to respond and perhaps are best treated with a surgical modality.
MINIMALLY INVASIVE MODALITIES
Upto 30% of patients do not have adequate relief after EBRT. In the modern world, percutaneous modalities like RFA, cryoablation and high intensity focused ultrasound and microwave can relieve the pain and improve bone strength without risk of morbidity from open surgery. RFA is the most common modality used, and multiple studies have shown successful pain relief, decrease in opioid use, and improvement in QOL with minimum complications.45,46,47 Most studies have combined cementoplasty (cement injection) after RF ablation particularly for periacetabular lesions. Upto 80% patients have improvement on pain and mobility scores and are able to ambulate independently with low interventional morbidity and complications.48,49 Cryoablation, with its ability to visualize the ice ball beyond which there is no thermal injury has an advantage over RFA where burn margins are not visible on CT. The postoperative inflammation and pain reported by RF ablated patients seems to be less in cryoablated patients. Although not compared head to head with RF ablation, cryoablation seems to be an attractive modality.50,51
High intensity focused ultrasound (HIFU) uses focused ultrasound energy to generate heat. Magnetic resonance guidance is used with HIFU to allow accurate visualization. Published data shows promising results with almost complete pain relief in a high percentage of individuals.52,53,54,55,56 Lesions less than a cm from the skin surface are unsuitable due to the risk of skin burn. Microwave is another form of thermoablative method under investigation with the advantage of higher ablation volume and faster ablation time.57,58,59,60
Decision making factors and perioperative considerations
Once a lesion has been completely characterized by full workup including a biopsy, a plan of management is made. Very few conditions like a fracture or impending fracture or neurological deficit warrant early or emergent surgery. Even in emergency, an MRI followed by a biopsy and frozen section can provide a diagnosis in a very short time. If metastasis is strongly suspected as when the primary is known and imaging documents multiple lesions, a frozen section at time of surgery can be used before proceeding to intralesional palliative surgery. This approach is contraindicated in solitary lesions where resection may be considered for metastatic disease and where there is a higher likelihood of the lesion being infection, primary sarcoma or another nonmetastatic lesion. A surgeon must assess whether surgery will provide a QOL. Fixing a painful fracture even in a nonambulatory patient with a limited lifespan is justified if pain relief was not obtained through pharmacological methods or other nonsurgical methods. The most common indications for surgery would be a pathological fracture or an impending fracture. Failure of nonsurgical treatment and pain is also an indication for surgery.
After full staging, a decision is made whether the intention of treatment is curative or palliative. For instance, a patient with operable renal cell cancer and a solitary bone metastasis in femur is selected for R0 resection at both the sites of disease, whereas, same disease with multiple skeletal and spinal metastases is selected for intralesional treatment with palliative intent. The expected lifespan also is an important decision making factor. A longer lifespan favors resection rather than the curettage, for example in hormone-sensitive breast cancer with multiple skeletal metastases and impending fracture of the femoral neck. Longer survival increases the risk of local recurrence with an intralesional surgery and therefore resection may be preferred. It is better to overestimate the survival than underestimate as resurgery in metastatic disease may not be possible or may be risky with advancing disease and deteriorating general condition. The site of disease also matters as disease close to the joint is best resected whencompared to diaphyseal disease. Tumors non-responsive to radiation like renal cell cancer are more likely to require surgery. An unfit patient with poor general condition, coagulopathy or hypercalcemia will also not be a candidate for surgery. Conditions like parkinsonism that make a patient unreliable for following instructions of limited weight bearing make the surgical option more likely. Finally, the experience and expertise of the surgeon as well as available infrastructure facilities will decide the option of treatment. For example, nonavailability of tumor embolization may go against doing the intralesional surgery in the proximal femur for a large thyroid metastasis.
Once surgery is considered, other factors must be considered that will affect the patient outcome. The patient should have a general condition which allows them to withstand the stress of an operation. Many cancer patients are elderly and have limited physiological reserve due to age or the treatment particularly chemotherapy. It is estimated that 60% of patients may have medical comorbidities.61 It is important to have a physician and anesthetist evaluate this prior to surgery. Highest mortality related to surgery and anesthesia occurs from cardiac-related events. Patients with renal failure are at particular risk for nonfatal perioperative complications (60%), particularly hyperkalemia, pneumonia, and hypotension.62 The serum albumin is a quick method to screen the patient's nutritional status. A serum albumin less than 2 g/dL is an indicator of severe nutritional impairment and a risk for wound healing problems.63 Cancer patients often suffer from an array of electrolyte disturbances related to calcium, magnesium, potassium, sodium and phosphorus which may require correction prior to surgery. The importance and risks with these are commonly underestimated. The risk of hypercalcemia in particular is high and has been estimated to be 5-20%.64
Ewer and Ali65 offer a risk categorization that is geared specifically to oncology patients, which is based on the physical examination, laboratory data, cardiac function, arterial blood gases, and ventilation mechanics. Nonsurgical methods are recommendedfor patients in the high-risk category.
Blood transfusions are frequently needed perioperatively. Oncology patients are often immunocompromised and susceptible to blood-borne pathogens, in particular, cytomegalovirus. Irradiated blood products reduce the risk of this transmissible viral agent. Leukocyte depletion filters reduce the febrile reaction. Intraoperative autologous blood transfusion can reduce the need for banked blood particularly in planned elective surgery. Platelet transfusions and Fresh frozen plasma may be required to correct coagulation disorder. Preoperative tumor embolization upto 24–36 h prior to surgery particularly for very vascular lesions like metastases from kidney and thyroid cancer can reduce the bleeding and need for transfusion.
Deep venous thrombosis is also a perioperative risk both from the malignancy and from immobility in these patients. Adequate prophylaxis or sometimes placement of filters in the inferior vena cava may be required.
Cardiopulmonary dysfunction and hypotension arising from marrow embolism during or following intramedullary instrumentation either for nailing or joint replacement is an underestimated and potentially fatal but avoidable complication.66 This results from release of showers of vasoactive, inflammatory and thrombogenic substances from the intramedullary canal.67 The risk of systemic hypotension after manipulation and pressurization of the femoral canal is highly variable and has been reported from 5% to 50%.68 Choong reported a 20% incidence of hypotension and mortality of 10% or more has been reported after hypotensive episodes.67,69 Strategies suggested reducing this incidence include pulse lavage to remove tissue and vasoactive products from the intramedullary canal.70,71,72 Flexible, small diameter drive shafts to allow the intramedullary contents to escape up the femoral canal,73 and proximal and distal venting of the canal to reduce build-up of pressure.74 Additional steps like maintaining normovolemia increased inspired oxygen and decreased volatile agent concentration,68 and maintaining aortic perfusion pressure with vasopressors like norepinephrine is recommended.75,76 It is best to perform this surgery electively in the normal daylight working hours when best anesthesia and surgical expertise is available.67
If nonsurgical option is chosen, it avoids dangers of open surgery but involves protected weight bearing with crutches or walker and possible use of external orthosis along with radiation. Fracture risk is still present and can happen without warning. Weight bearing can be resumed once lesion has healed radiologically.
Surgery for spinal metastases
Vertebrae are common sites for skeletal involvement, thoracic followed by lumbar and less commonly cervical and sacral segments. Autopsy studies have shown that as many as 70% of patients with cancer have spinal metastases.77 Spinal metastases with spinal cord compression occur in 5-14% of cancer patients.77,78 Most metastatic disease is extradural, but rarely intradural or even intramedullary deposits can occur. Surgical techniques today allow more extensive disease to be tackled with lesser morbidity and sometimes even disease control. The main indications for surgery being restoration of neurological function and spinal stability and rarely disease control.
The MRI is able to assess the extent of epidural disease and cord compression. MRI also reveals if the compression is from the soft epidural tumor or from a bony retropulsed fragment. Round cell tumors like Ewings sarcoma and lymphoma as well as prostate metastases are extremely sensitive to systemic treatment (former to steroids and chemotherapy drugs and latter to androgen deprivation by castration) and can be given a chance at recovery without open surgery [Figure 6]. Tumors located in the junctional regions such as the occipitocervical, cervicothoracic, thoracolumbar, and lumbosacral junctions and in the mobile segments of the spine (C3 through C6 and L2 through L4) are more likely to be associated with instability. Movement-related pain which is absent on recumbency, more than 50% vertebral height collapse, bilateral facet and pedicle involvement or any subluxation or translation points to instability. Severe instability also requires surgery as systemic treatment is unlikely to provide the required stability.79
Figure 6.
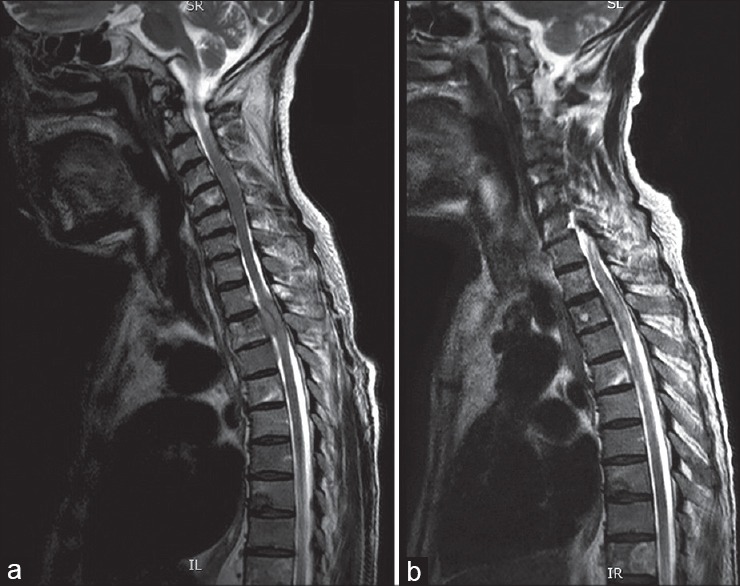
MRI T2W sagittal image showing (a) metastasis with cord compression (prostate) (b) Response to orchidectomy can often be dramatic as shown in the image on the right and may obviate the need for immediate surgery
Historically, surgery without fixation was equivalent to EBRT.80 Patchell et al.81 showed that surgery with EBRT was clearly superior to EBRT alone in maintaining ambulation. 62% of the patients who were nonambulatory at the time of recruitment regained the ability to walk. Literature reveals ambulatory rates ranging from 74% to 100% after surgery, with 57-82% of nonambulatory patients regaining ambulation.82 Cochrane database review83 of 2008 reported that surgery prior to radiotherapy gave better recovery of function in selected cases. The authors suggest that radiotherapy alone may suffice for ambulant patients with stable spines but there is evidence of benefit from decompressive surgery in ambulant patients with poor prognostic factors for radiotherapy and in nonambulant patients with a single area of compression, paraplegia <48 h, nonradiosensitive tumours and a predicted survival of more than 3 months. Improvement in pain has been observed in more than 90% of patients who underwent instrumented stabilization.84 In contrast, Rades et al. in a matched pair analysis found no benefit of surgery with RT compared to RT alone, particularly in elderly patients.85,86 we would recommend a customized approach taking into account several patient and disease-related factors as discussed above.
The amount of surgery ranges from simple decompression to an enbloc resection and fixation. Several rating scales have been proposed87,88 especially to aid selection of patients for more extensive resection. Patients with solitary metastases with favorable histology like breast cancer are more often selected for the enbloc resections in view of longer life and therefore need to maintain QOL. Solitary Metastases from lung or Renal cancer which are often radioresistant are also candidates for resection rather than simple decompression and fixation.89 The approach and the fixation are dictated by the lesion and in this modern era of superspecialization, we recommend that the spine specialists be involved in the surgical care of these patients. As a basic rule, one must do the minimum required to achieve the goal of pain-free ambulation for as long as possible. The anterior or posterior approach depends on the exact site, experience of the surgeon and the procedure to be carried out. The posterior approach is most favored due to the ease, familiarity and ability to decompress as well as stabilize well. Preoperative embolization helps reduce bleeding and transfusion-related morbidity particularly in hypervascular tumors like thyroid and renal cell carcinoma (RCC) metastases. Preoperative embolization also allows better vision and therefore better decompression.
Surgery for metastases of appendicular skeleton
The surgical procedure chosen will have to be customized to the region involved, skill and experience of the surgeon, infrastructure available, costs that patient can bear and expected survival.
Solitary lesions require special mention as they are biologically different and associated with better survival. In selected cases, resection of solitary lesions may improve disease-free survival and even cure.90,91,92,93,94 If the primary is unknown after the extensive workup, a solitary metastatic lesion should be resected rather than treated with intralesional surgery if morbidity is acceptable.95
The usual surgical options vary from internal fixation with a nail or plate with or without cement and endoprosthetic arthroplasty. The exact choice depends on the location, amount of bone loss and responsiveness of lesion to systemic therapy. Non responsive lesions are best resected to minimize the risk and morbidity of a local recurrence [Figure 7]. It may be wise to resect those lesions where patient is expected to have long survival [Figure 8]. Periarticular lesions are often resected and reconstructed with arthroplasty. Diaphyseal lesions can also be resected and reconstructed with a diaphyseal prosthesis [Figure 9] but they are mostly treated with intralesional surgery with or without cement depending on the bone loss. Nails or rods being inside the medullary canal are considered load sharing compared to plates which are external to the bone and considered load bearing. As a general rule, nails are preferred in the lower limbs and plates in the upper limb. Wherever possible, the maximum length of the bone is stabilized inorder to minimize a future fracture at another location. Even when a closed nailing is done for an impending fracture, the lesion should be debulked maximum possible, especially in lesions less responsive to radiation as this reduces the risk and morbidity of local recurrence.96 Bone cement or polymethyl methacrylate (PMMA) is excellent in filling up areas of bone loss and providing compressive strength to the construct. Bone grafting is, usually, avoided in metastatic lesions as radiation used postoperatively would prevent incorporation. Doses below 3000 rad have been shown not to prevent callus formation.97 Besides, the goal is to provide immediate mobility for which strength provided by PMMA is ideal. For the same reasons, cemented arthroplasty is preferred over uncemented arthroplasty. With extensive bone loss, resection is preferred. The rate of pathological fracture healing has been reported as 67% for myeloma, 44% for RCC, 37% for breast carcinoma and 0% for lung carcinom.97 The surgeon is advised to assume that the fracture will not heal, and patient will survive, so that a durable construct is provided. Better to overestimate survival than underestimate. One must keep in mind the fact that a patient operated for metastatic disease may never be fit to undergo another surgery for a complication like implant failure or for local recurrence.
Figure 7.
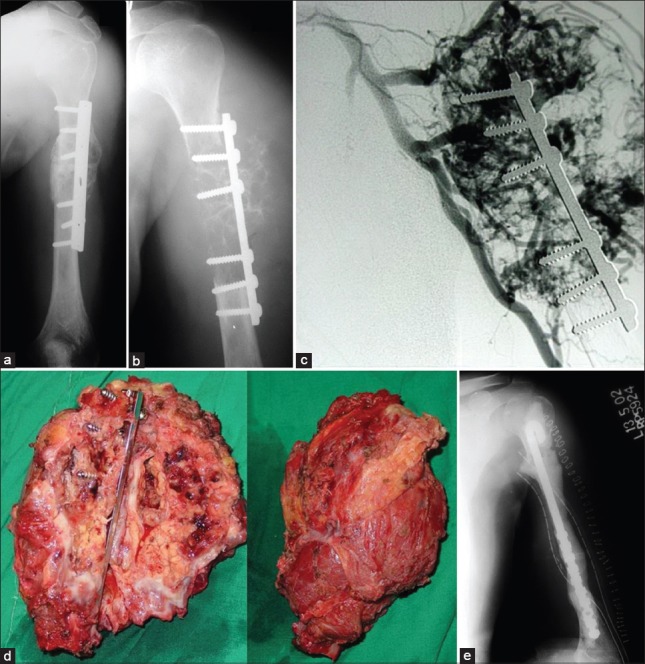
X-ray of the arms with shoulder joint anteroposterior view (a and b) showing local recurrence after previous cementing + fixation in renal cell carcinoma metastasis (c) Angiogram showing the hypervascularity. Preoperative embolisation is a must especially if intralesional surgery is contemplated (d) Resection specimen (e) Postoperative X-ray showing a nail-cement spacer. This is a cost effective construct in the upperlimb and sometimes in the lowerlimb
Figure 8.
(a) Postoperative X-ray after intralesional curetting and cementing of a metastatic lesion from breast cancer (b) Local recurrence is seen within a year despite postoperative radiotherapy (c) Progressive disease which required resection. In patients with long expected survival, primary resection may obviate need for more surgery in future
Figure 9.
A solitary diaphyseal metastasis from uterine leiomyosarcoma treated with resection and diaphyseal prosthesis. It allowed immediate weight bearing ambulation and preservation of native hip and knee
Pelvic and periacetabular defects
Surgery is only required for those lesions that compromise the load transfer from the lower limb to spine. These are lesions that affect the superior and medial acetabular walls, as well as the medial column of the pelvis and the posterior ilium in the region of the sacroiliac joint. Posterior Ilium lesions not involving the acetabulum can be treated by intralesional debulking and cement augmentation. Acetabular lesions that are contained (with an intact medial wall) can be reconstructed by a cemented arthroplasty. Uncemented cups run the risk of loosening and migration from failure to osteointegrate from radiation used postoperatively or from osteolysis due to disease progression. Protrusio acetabular cups compensate for deficiencies ofthe medial wall, while cement and pin fixation5,97,98,99,100,101 (modified Harrington method) can be used effectively to reconstruct large defects in the acetabular column and dome.102 Massive bone loss may require resection or reconstruction with an acetabular prosthesis. Stemmed acetabular implants now available (an inverted ice cream cone prosthesis or pedestal cup) allow anchorage of the acetabular shell into the posterior ilium with the stem [Figure 10]. Immediate weight bearing is possible with these implants. Alternatively, a customs acetabular or pelvic prosthesis may be used. As these resections and reconstructions are massive and involve significant blood loss and risk of complications, it is important to have determined preoperatively that the benefits outweigh the risks. Pain relief and immediate weight bearing are the obvious advantages of this reconstruction.102
Figure 10.
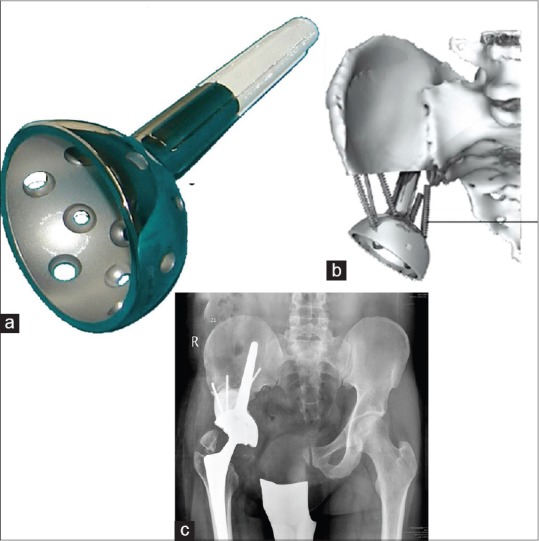
(a) Inverted ice cream cone prosthesis from Stanmore implants worldwide (Stanmore, UK) is useful in reconstructing large periacetabular defects and allows immediate weight bearing (b) The stem is hydroxyapatite coated and anchors in the strong posterior ilium while cement screw struts fashioned allow additional mechanical integrity. It can often be combined with a constrained liner to prevent dislocations (c) Postoperative x-ray showing the implant in place
Proximal femur
Lesions of the femoral neck and head are best treated with hip arthroplasty as internal fixation has high failure rate.100 Hemiarthroplasty is adequate when acetabulum is not involved,103,104 else an acetabular surfacing is done as well. A routine acetabular replacement would increase the risk of dislocation and is, therefore, reserved for lesions that involve the acetabulum as well. For extensive lesions, proximal femur megaprosthesis is used. A constrained liner can be used in cases judged to be at high risk for dislocation. Cemented stem is preferred as uncemented stems may loosen and fail over a period of time due to nonintegration after radiation or tumor-induced osteolysis. The benefit of a long stem has been that it prevents fracture from another lesion that may appear in the future as entire length of bone can be spanned. However, long stemmed implants increase the chance of embolism from longer canal instrumentation increase.105,106 The best indication for a longer stem is when a diaphyseal disease is present in addition to the disease in the neck or head. With modern surveillance, should a distal disease appear, it can be tackled on its merit subsequently.
The management of intertrochanteric lesions is more controversial, and opinion is divided between fixation and prosthetic replacement. Most orthopedic surgeons are familiar with the DHS and also the now available gamma nail. If fixation is used it is imperative to confirm that the head and neck offer good purchase as the construct depends on this. The challenge lies in reconstructing the void in the calcar area with cement [Figure 11]. The great advantage is lesser surgery and hip joint preservation. If replacement arthroplasty is chosen, a calcar replacement type or megaprosthesis is chosen.107 The diseased bone is completely or almost completely excised reducing the risk of local recurrence. Additionally, there is no reliance on that diseased bone for weight bearing. We have generally preferred this option owing to its reliability in restoring ambulation though there is a higher risk of infection, trochanteric fracture or hip dislocation as compared to internal fixation.
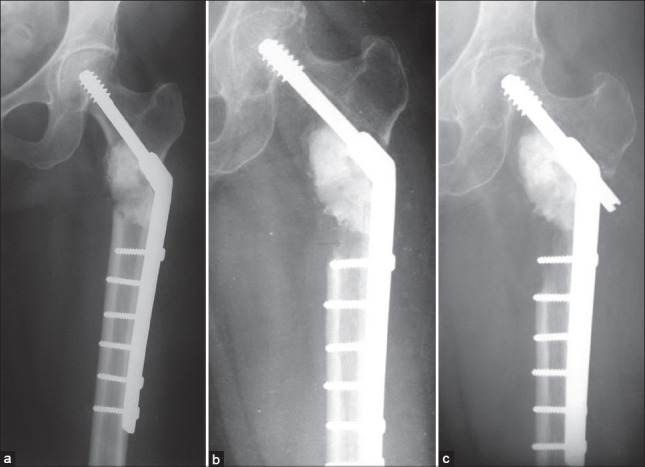
Figure 11.
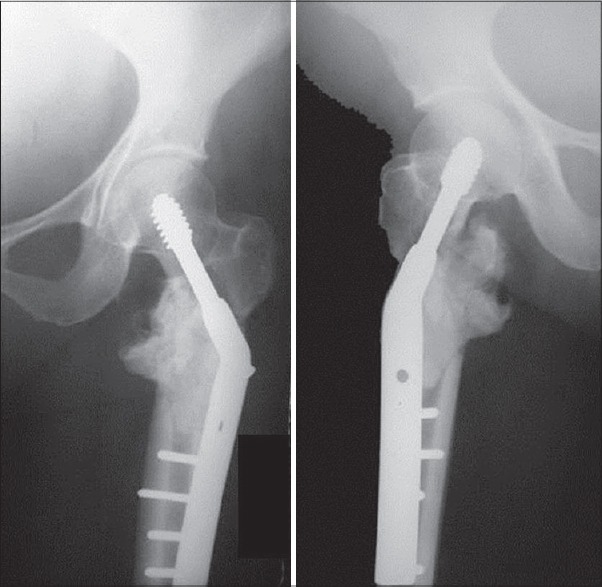
X-ray of hip joint anteroposterior view showing challenge of cementing in intertrochanteric lesions. Calcar has to be built up to allow least chances of failure in a high stress zone
The subtrochanteric zone is a high-stress zone with loads reaching 6 times body weight.108 This poses a great challenge to most internal fixation devices, particularly the nail-plate devices. The implants of choice are, therefore, the intramedullary nail or prosthetic replacement. The nail is preferred for smaller lesions where adequate fixation is possible, and replacement preferred for more bone loss, and also if secure purchase in the head and neck is in doubt.
Femoral diaphysis
Lesions of the femoral diaphysis are best treated with an intramedullary nail that not only spans the entire femur but also includes fixation into the femoral neck and head. It is important to ensure that there is no disease in head and neck and that the bone is strong enough for the purchase of interlocking screw failing which failure rates are higher. Calcar replacing or megaprosthetic arthroplasty are preferred in these situations.107 Titanium nails would be preferred to stainless steel as they can be thinner and stronger allowing lesser instrumentation of the canal and lesser risk of failure. For solitary lesions, resection is done, and reconstruction options available include a diaphyseal replacement prosthesis, a nail-cement spacer or reconstruction with bone (allograft or autograft depending on the length of the defect).
For distal femoral defects, curettage and cementing with or without fixation can be used for smaller defects. Larger defects can be treated with resection and megaprosthetic replacement.
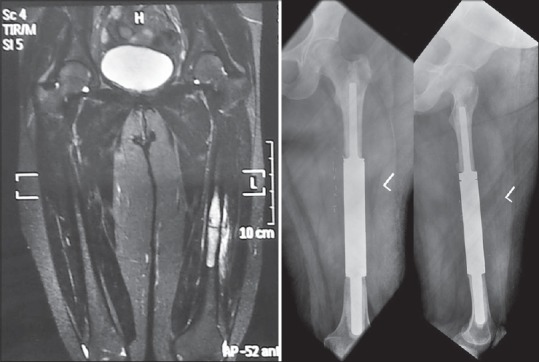
Lesions in Tibia are much less common than in femur and are managed much like in femur. Curettage or resection of proximal tibial lesions depends on the size of the lesion. Sometimes a resection of both distal femur and proximal tibia is required to restore ambulation [Figure 12]. Tibial shaft lesions are treated with the same principles as femoral shaft lesions [Figure 13]. Shows a distal femoral fracture with a tibial lesion in a patient with myeloma treated with a distal femur megaprosthesis and a custom long stem tibial component to bypass the tibial lesion which was curetted and cemented.
Figure 12.
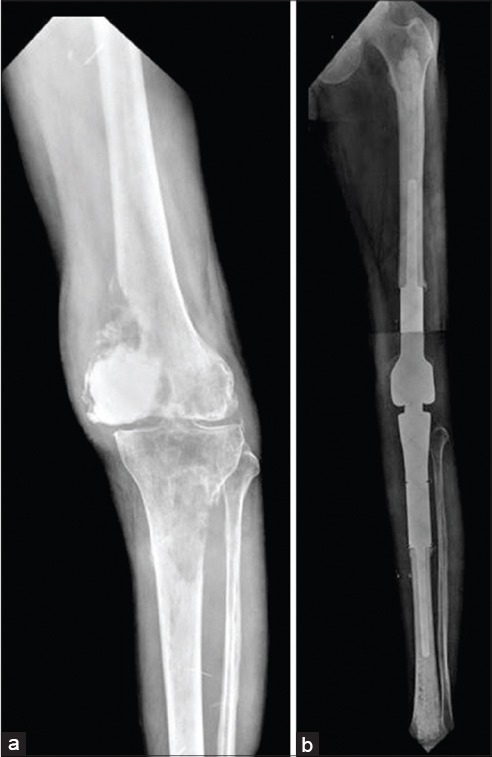
(a) Extensive involvement of distal femur and tibia in a case of renal cell carcinoma resistant to therapy. Intralesional surgery with cement had been done earlier (b) Composite resection of distal femur with proximal tibia over a fixed hinge prosthesis allowing immediate weight bearing
Figure 13.
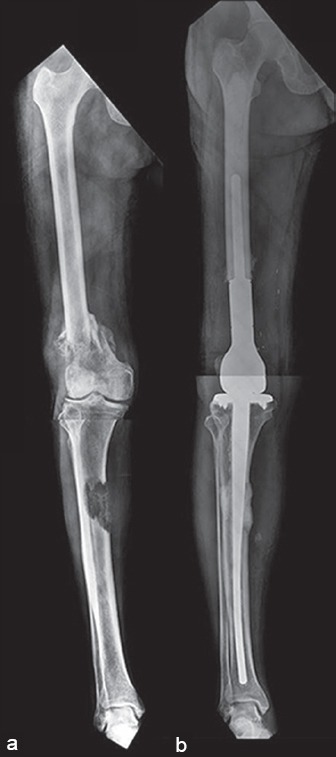
(a) Preoperative X-rays of a 70 year old with recalcitrant but indolent myeloma affecting the distal femur and tibial shaft (b) Reconstruction was done in a single step with a distal femur megaprosthesis with a custom tibial tray with a long stem. The patient, a practicing lawyer was back to work in 4 weeks after surgery
METASTASES IN THE UPPER EXTREMITY
In contrast to the lower extremity, the upper extremity being non weight bearing has much less need for endoprosthetic reconstructions. Plate or rod fixation with cement is generally adequate for most lesions with prosthesis being reserved for proximal humerus fractures with massive bone loss. Whether a standard Neers type prosthesis is used or a megaprosthesis is used depends on remaining bone stock. Resections generally require a megaprosthetic type of replacement [Figure 14]. This type of replacement is associated with poor shoulder function, as a normal attachment sites for the rotator cuff musculature are lost. A reverse type of shoulder prosthesis can be used where glenoid is preserved and where the deltoid muscle and axillary nerve are spared [Figure 15]. A reverse shoulder can provide stability as well as reasonable active flexion and abduction. Both nails and plates can be used depending on the situation as well as surgeon preference. As in the femur, we prefer to fix long to avoid future disease related fractures in the same bone. For scapular lesions, a partial or total scapulectomy is performed generally when resection is chosen. Distal humerus and forearm bone are rarely involved, and resection is required only for large lesions or solitary metastases. Custom implants can sometimes provide good solutions in the forearm [Figure 16].
Figure 14.
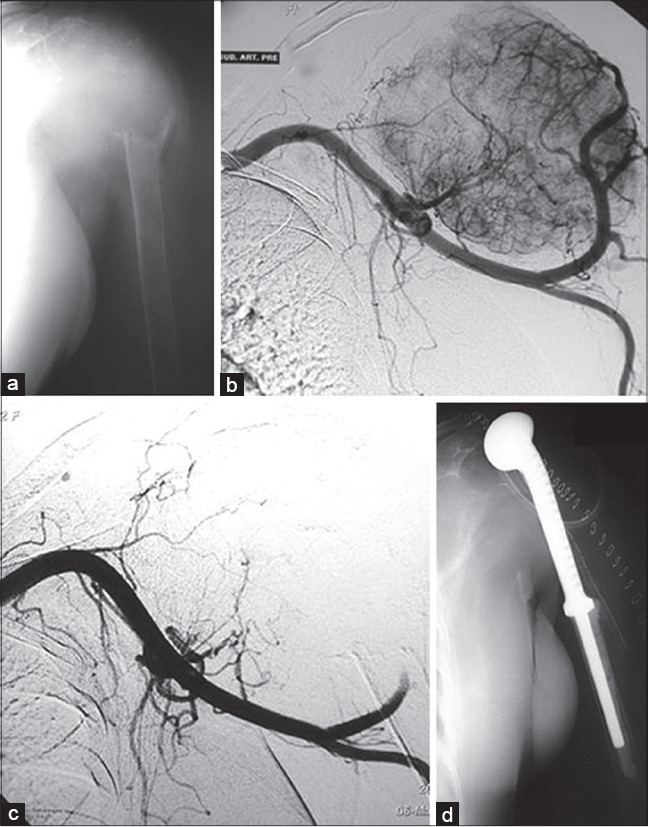
(a) Proximal humeral metastasis from thyroid cancer in a 55-year-old lady (b) Hypervascularity seen on the angiogram (c) preoperative embolization has obliterated hypervascularity (d) Resection was done as thyroid malignancies are associated with relatively long survivals even with extensive bony metastases. Resection greatly minimizes the risk of local recurrence. The embolization makes the surgery easier
Figure 15.
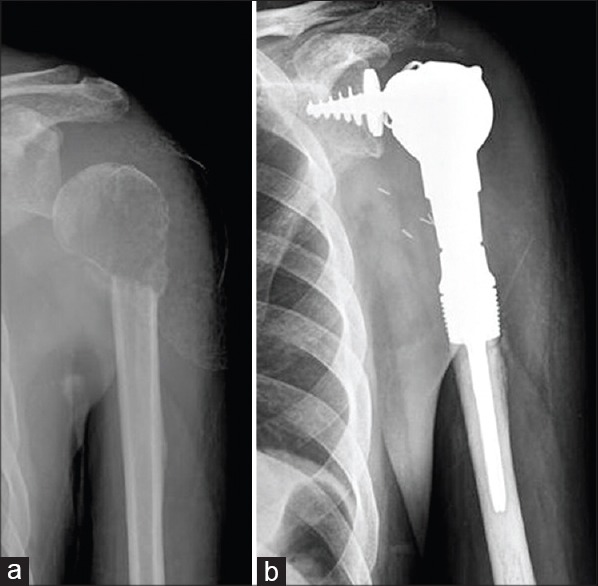
(a) The radiograph depicts a 56-year-old male who presented with a pathological fracture while serving during a game of tennis. Biopsy revealed a metastatic cancer. Positron emission tomography-computerised tomography (PET-CT) showed this to be solitary metastases from renal cell carcinoma (b) Resection and reconstruction with a reverse shoulder type of implant (Stanmore Implants Worldwide, UK) preserving the deltoid muscle and axillary nerve allows better abduction and flexion
Figure 16.
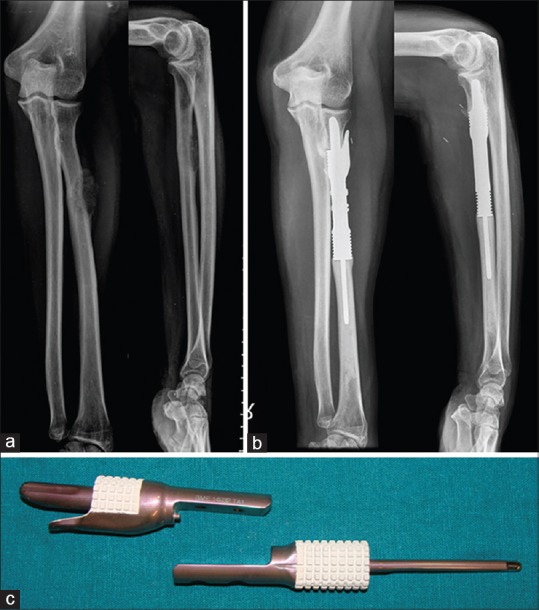
(a) Proximal radius lesion which on biopsy was a metastatic carcinoma. Positron emission tomography-computerised tomography revealed a lung primary with this lesion being a solitary metastasis (b) After chemotherapy, both lesions underwent resection. The radius was reconstructed with a custom intercalary prosthesis (c) Custom prosthesis, proximal and distal segments
CONCLUSIONS
With the increasing survivals of patients with metastatic disease, a proper workup prior to surgery including a biopsy and early involvement of a multidisciplinary oncology team is recommended. Percutaneous modalities can relieve pain and provide rigidity to bone without the morbidity of open surgery. Surgery should be well planned and properly executed. We emphasize that pathological fractures are not an emergency and proper workup is mandatory before any intervention. The patient's longevity should be over estimated than underestimated, and one must assume that fracture will not heal and plan a construct accordingly. Perioperative steps and intraoperative care can reduce bleeding and embolic events. Resections are done in selected cases particularly for solitary metastases. Wherever possible, an opinion from an orthopedic oncologist be sought to ensure that best possible care is being provided. Even if cure is not possible, proper management of metastatic disease can provide QOL for a reasonable amount of time.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Rubens RD, Mundy GR. London (UK): Martin Dunitz; 2000. Cancer and the Skeleton. [Google Scholar]
- 2.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: A phase iii, double-blind, randomized trial-The zoledronic acid lung cancer and other solid tumors study group. J Clin Oncol. 2003;21:3150–7. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 3.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: A randomized, double-blind, multicenter, comparative trial. Cancer. 2003;98:1735–44. doi: 10.1002/cncr.11701. [DOI] [PubMed] [Google Scholar]
- 4.Aaron AD. Current concepts review-treatment of metastatic adenocarcinoma of the pelvis and the extremities*. J Bone Joint Surg. 1997;79:917–32. doi: 10.2106/00004623-199706000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Harrington KD. Orthopaedic management of extremity and pelvic lesions. Clin Orthop Relat Res. 1995:136–47. [PubMed] [Google Scholar]
- 6.Theriault RL. Biology of bone metastases. Cancer Control J Moffitt Cancer Cent. 2012;19:92–101. doi: 10.1177/107327481201900203. [DOI] [PubMed] [Google Scholar]
- 7.Even-Sapir E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J Nucl Med. 2005;46:1356–67. [PubMed] [Google Scholar]
- 8.Rosenthal DI. Radiologic diagnosis of bone metastases. Cancer. 1997;80(8 Suppl):1595–607. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1595::aid-cncr10>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Costelloe CM, Rohren EM, Madewell JE, Hamaoka T, Theriault RL, Yu TK, et al. Imaging bone metastases in breast cancer: Techniques and recommendations for diagnosis. Lancet Oncol. 2009;10:606–14. doi: 10.1016/S1470-2045(09)70088-9. [DOI] [PubMed] [Google Scholar]
- 10.Fidler M. Prophylactic internal fixation of secondary neoplastic deposits in long bones. Br Med J. 1973;1:341–3. doi: 10.1136/bmj.1.5849.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington KD. New trends in the management of lower extremity metastases. Clin Orthop Relat Res. 1982:53–61. [PubMed] [Google Scholar]
- 12.Damron TA, Morgan H, Prakash D, Grant W, Aronowitz J, Heiner J. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin Orthop Relat Res. 2003:S201–7. doi: 10.1097/01.blo.0000093842.72468.73. [DOI] [PubMed] [Google Scholar]
- 13.Evans AR, Bottros J, Grant W, Chen BY, Damron TA. Mirels’ rating for humerus lesions is both reproducible and valid. Clin Orthop Relat Res. 2008;466:1279–84. doi: 10.1007/s11999-008-0200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van der Linden YM, Dijkstra PD, Kroon HM, Lok JJ, Noordijk EM, Leer JW, et al. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br. 2004;86:566–73. [PubMed] [Google Scholar]
- 15.Leong NL, Anderson ME, Gebhardt MC, Snyder BD. Computed tomography-based structural analysis for predicting fracture risk in children with benign skeletal neoplasms: Comparison of specificity with that of plain radiographs. J Bone Joint Surg Am. 2010;92:1827–33. doi: 10.2106/JBJS.I.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong J, Cabe GD, Tedrow JR, Hipp JA, Snyder BD. Failure of trabecular bone with simulated lytic defects can be predicted non-invasively by structural analysis. J Orthop Res. 2004;22:479–86. doi: 10.1016/j.orthres.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Keyak JH, Kaneko TS, Rossi SA, Pejcic MR, Tehranzadeh J, Skinner HB. Predicting the strength of femoral shafts with and without metastatic lesions. Clin Orthop Relat Res. 2005;439:161–70. doi: 10.1097/01.blo.0000174736.50964.3b. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig H, Kumpan W, Sinzinger H. Radiography and bone scintigraphy in multiple myeloma: A comparative analysis. Radiography. 1982;55:173–81. doi: 10.1259/0007-1285-55-651-173. [DOI] [PubMed] [Google Scholar]
- 19.Woolfenden JM, Pitt MJ, Durie BG, Moon TE. Comparison of bone scintigraphy and radiography in multiple myeloma. Radiology. 1980;134:723–8. doi: 10.1148/radiology.134.3.7355226. [DOI] [PubMed] [Google Scholar]
- 20.Resnick DL. Philadelphia: WB Saunders; 2002. Diagnosis of Bone and Joint Disorders. [Google Scholar]
- 21.Rybak LD, Rosenthal DI. Radiological imaging for the diagnosis of bone metastases. Q J Nucl Med. 2001;45:53–64. [PubMed] [Google Scholar]
- 22.Ryan PJ, Fogelman I. The bone scan: Where are we now? Semin Nucl Med. 1995;25:76–91. doi: 10.1016/s0001-2998(95)80020-4. [DOI] [PubMed] [Google Scholar]
- 23.Savelli G, Maffioli L, Maccauro M, De Deckere E, Bombardieri E. Bone scintigraphy and the added value of SPECT (single photon emission tomography) in detecting skeletal lesions. Q J Nucl Med. 2001;45:27–37. [PubMed] [Google Scholar]
- 24.Kao CH, Hsieh JF, Tsai SC, Ho YJ, Yen RF. Comparison and discrepancy of 18f-2-deoxyglucose positron emission tomography and Tc-99m MDP bone scan to detect bone metastases. Anticancer Res. 1999;20:2189–92. [PubMed] [Google Scholar]
- 25.Gallowitsch HJ, Kresnik E, Gasser J, Kumnig G, Igerc I, Mikosch P, et al. F-18 fluorodeoxyglucose positron-emission tomography in the diagnosis of tumor recurrence and metastases in the followup of patients with breast carcinoma: A comparison to conventional imaging. Invest Radiol. 2003;38:250–6. doi: 10.1097/01.RLI.0000063983.86229.f2. [DOI] [PubMed] [Google Scholar]
- 26.Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35:135–42. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-fluoride: Applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 28.Sapir EE, Mishani E, Flusser G, Ur M. 18f-fluoride positron emission tomography and positron emission tomography/computed tomography. Semin Nucl Med. 2007;37:462–9. doi: 10.1053/j.semnuclmed.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Hetzel M, Arslandemir C, König HH, Buck AK, Nüssle K, Glatting G, et al. F-18 NaF PET for detection of bone metastases in lung cancer: Accuracy, cost-effectiveness, and impact on patient management. J Bone Miner Res. 2003;18:2206–14. doi: 10.1359/jbmr.2003.18.12.2206. [DOI] [PubMed] [Google Scholar]
- 30.Ball AB, Fisher C, Pittam M, Watkins RM, Westbury G. Diagnosis of soft tissue tumours by tru-Cut biopsy. Br J Surg. 1990;77:756–8. doi: 10.1002/bjs.1800770713. [DOI] [PubMed] [Google Scholar]
- 31.Kissin MW, Fisher C, Carter RL, Horton LW, Westbury G. Value of tru-cut biopsy in the diagnosis of soft tissue tumours. Br J Surg. 1986;73:742–4. doi: 10.1002/bjs.1800730921. [DOI] [PubMed] [Google Scholar]
- 32.Pramesh CS, Deshpande MS, Pardiwala DN, Agarwal MG, Puri A. Core needle biopsy for bone tumours. Eur J Surg Oncol. 2001;27:668–71. doi: 10.1053/ejso.2001.1198. [DOI] [PubMed] [Google Scholar]
- 33.Briasoulis E, Pavlidis N, Felip E. ESMO Guidelines Working Group. Cancers of unknown primary site: ESMO clinical recommendations for diagnosis, treatment and followup. Ann Oncol. 2009;20(Suppl 4):154–5. doi: 10.1093/annonc/mdp159. [DOI] [PubMed] [Google Scholar]
- 34.Lipton A. Implications of bone metastases and the benefits of bone-targeted therapy. Semin Oncol. 2010;37:S15–29. doi: 10.1053/j.seminoncol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Berenson JR, Lichtenstein A, L, Dimopoulos MA, Bordoni R, George S, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. Myeloma Aredia Study Group. N Engl J Med. 1996;334:488–93. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 36.Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. N Engl J Med. 1996;335:1785–92. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 37.Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: A review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136:1117–24. doi: 10.1007/s00432-010-0907-7. [DOI] [PubMed] [Google Scholar]
- 38.Palaska PK, Cartsos V, Zavras AI. Bisphosphonates and time to osteonecrosis development. Oncologist. 2009;14:1154–66. doi: 10.1634/theoncologist.2009-0115. [DOI] [PubMed] [Google Scholar]
- 39.Fehm T, Felsenberg D, Krimmel M, Solomayer E, Wallwiener D, Hadjii P. Bisphosphonate-associated osteonecrosis of the jaw in breast cancer patients: Recommendations for prevention and treatment. Breast. 2009;18:213–7. doi: 10.1016/j.breast.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 41.Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–76. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 43.Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: Single fraction versus multifraction radiotherapy – A systematic review of randomised trials. Clin Oncol (R Coll Radiol) 2003;15:345–52. doi: 10.1016/s0936-6555(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 44.Roqué I, Figuls M, Martinez-Zapata MJ, Coello PA, Català E, Garcia JL, Ferrandiz M. Radioisotopes for metastatic bone pain. Cochrane Libr. 2003 doi: 10.1002/14651858.CD003347. [DOI] [PubMed] [Google Scholar]
- 45.Dupuy DE, Hong R, Oliver B, Goldberg SN. Radiofrequency ablation of spinal tumors: Temperature distribution in the spinal canal. AJR Am J Roentgenol. 2000;175:1263–6. doi: 10.2214/ajr.175.5.1751263. [DOI] [PubMed] [Google Scholar]
- 46.Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: A multicenter study. J Clin Oncol. 2004;22:300–6. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 47.Dupuy DE, Liu D, Hartfeil D, Hanna L, Blume JD, Ahrar K, et al. Percutaneous radiofrequency ablation of painful osseous metastases: A multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989–97. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcy PY, Palussière J, Descamps B, Magné N, Bondiau PY, Ciais C, et al. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer. 2000;8:500–3. doi: 10.1007/s005200000138. [DOI] [PubMed] [Google Scholar]
- 49.Hoffmann RT, Jakobs TF, Trumm C, Weber C, Helmberger TK, Reiser MF. Radiofrequency ablation in combination with osteoplasty in the treatment of painful metastatic bone disease. J Vasc Interv Radiol. 2008;19:419–25. doi: 10.1016/j.jvir.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Callstrom MR, Atwell TD, Charboneau JW, Farrell MA, Goetz MP, Rubin J, et al. Painful metastases involving bone: Percutaneous image-guided cryoablation – Prospective trial interim analysis. Radiology. 2006;241:572–80. doi: 10.1148/radiol.2412051247. [DOI] [PubMed] [Google Scholar]
- 51.Callstrom MR, Dupuy DE, Solomon SB, Beres RA, Littrup PJ, Davis KW, et al. Percutaneous image-guided cryoablation of painful metastases involving bone: Multicenter trial. Cancer. 2013;119:1033–41. doi: 10.1002/cncr.27793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li C, Zhang W, Fan W, Huang J, Zhang F, Wu P. Noninvasive treatment of malignant bone tumors using high-intensity focused ultrasound. Cancer. 2010;116:3934–42. doi: 10.1002/cncr.25192. [DOI] [PubMed] [Google Scholar]
- 53.Hurwitz MD, Ghanouni P, Kanaev SV, Iozeffi D, Gianfelice D, Fennessy FM, et al. Magnetic resonance-guided focused ultrasound for patients with painful bone metastases: Phase III trial results. J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Catane R, Beck A, Inbar Y, Rabin T, Shabshin N, Hengst S, et al. MR-guided focused ultrasound surgery (MRgFUS) for the palliation of pain in patients with bone metastases – Preliminary clinical experience. Ann Oncol. 2007;18:163–7. doi: 10.1093/annonc/mdl335. [DOI] [PubMed] [Google Scholar]
- 55.Gianfelice D, Gupta C, Kucharczyk W, Bret P, Havill D, Clemons M. Palliative treatment of painful bone metastases with MR imaging – Guided focused ultrasound. Radiology. 2008;249:355–63. doi: 10.1148/radiol.2491071523. [DOI] [PubMed] [Google Scholar]
- 56.Liberman B, Gianfelice D, Inbar Y, Beck A, Rabin T, Shabshin N, et al. Pain palliation in patients with bone metastases using MR-guided focused ultrasound surgery: A multicenter study. Ann Surg Oncol. 2009;16:140–6. doi: 10.1245/s10434-008-0011-2. [DOI] [PubMed] [Google Scholar]
- 57.Kastler A, Alnassan H, Aubry S, Kastler B. Microwave thermal ablation of spinal metastatic bone tumors. J Vasc Interv Radiol. 2014;25:1470–5. doi: 10.1016/j.jvir.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 58.Filippiadis DK, Tutton S, Mazioti A, Kelekis A. Percutaneous image-guided ablation of bone and soft tissue tumours: A review of available techniques and protective measures. Insights Imaging. 2014;5:339–46. doi: 10.1007/s13244-014-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pusceddu C, Sotgia B, Fele RM, Melis L. Treatment of bone metastases with microwave thermal ablation. J Vasc Interv Radiol. 2013;24:229–33. doi: 10.1016/j.jvir.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics. 2005;25(Suppl 1):S69–83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 61.Nierman E, Zakrzewski K. Recognition and management of preoperative risk. Rheum Dis Clin North Am. 1999;25:585–622. doi: 10.1016/s0889-857x(05)70088-0. [DOI] [PubMed] [Google Scholar]
- 62.Houston MC, Ratcliff DG, Hays JT, Gluck FW. Preoperative medical consultation and evaluation of surgical risk. South Med J. 1987;80:1385–97. doi: 10.1097/00007611-198711000-00013. [DOI] [PubMed] [Google Scholar]
- 63.Wolfe BM, Phillips GJ, Hodges RE. Evaluation and management of nutritional status before surgery. Med Clin North Am. 1979;63:1257–69. doi: 10.1016/s0025-7125(16)31639-x. [DOI] [PubMed] [Google Scholar]
- 64.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 65.Ewer MS, Ali MK. Surgical treatment of the cancer patient: Preoperative assessment and perioperative medical management. J Surg Oncol. 1990;44:185–90. doi: 10.1002/jso.2930440312. [DOI] [PubMed] [Google Scholar]
- 66.Barwood SA, Wilson JL, Molnar RR, Choong PF. The incidence of acute cardiorespiratory and vascular dysfunction following intramedullary nail fixation of femoral metastasis. Acta Orthop. 2000;71:147–52. doi: 10.1080/000164700317413111. [DOI] [PubMed] [Google Scholar]
- 67.Choong PF. Cardiopulmonary complications of intramedullary fixation of long bone metastases. Clin Orthop Relat Res. 2003:S245–53. doi: 10.1097/01.blo.0000093058.96273.1f. [DOI] [PubMed] [Google Scholar]
- 68.Pietak S, Holmes J, Matthews R, Petrasek A, Porter B. Cardiovascular collapse after femoral prosthesis surgery for acute hip fracture. Can J Anaesth. 1997;44:198–201. doi: 10.1007/BF03013009. [DOI] [PubMed] [Google Scholar]
- 69.Duncan JA. Intra-operative collapse or death related to the use of acrylic cement in hip surgery. Anaesthesia. 1989;44:149–53. doi: 10.1111/j.1365-2044.1989.tb11168.x. [DOI] [PubMed] [Google Scholar]
- 70.Byrick RJ, Bell RS, Kay JC, Waddell JP, Mullen JB. High-volume, high-pressure pulsatile lavage during cemented arthroplasty. J Bone Joint Surg Am. 1989;71:1331–6. [PubMed] [Google Scholar]
- 71.Sherman RM, Byrick RJ, Kay JC, Sullivan TR, Waddell JP. The role of lavage in preventing hemodynamic and blood-gas changes during cemented arthroplasty. J Bone Joint Surg Am. 1983;65:500–6. [PubMed] [Google Scholar]
- 72.Wheelwright EF, Byrick RJ, Wigglesworth DF, Kay JC, Wong PY, Mullen JB, et al. Hypotension during cemented arthroplasty. Relationship to cardiac output and fat embolism. J Bone Joint Surg Br. 1993;75:715–23. doi: 10.1302/0301-620X.75B5.8376426. [DOI] [PubMed] [Google Scholar]
- 73.Müller C, McIff T, Rahn BA, Pfister U, Weller S. Intramedullary pressure, strain on the diaphysis and increase in cortical temperature when reaming the femoral medullary cavity – A comparison of blunt and sharp reamers. Injury. 1993;24(Suppl 3):S22–30. doi: 10.1016/0020-1383(93)90003-o. [DOI] [PubMed] [Google Scholar]
- 74.Martin R, Leighton RK, Petrie D, Ikejiani C, Smyth B. Effect of proximal and distal venting during intramedullary nailing. Clin Orthop Relat Res. 1996:80–9. doi: 10.1097/00003086-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 75.Molloy WD, Lee KY, Girling L, Schick U, Prewitt RM. Treatment of shock in a canine model of pulmonary embolism. Am Rev Respir Dis. 1984;130:870–4. doi: 10.1164/arrd.1984.130.5.870. [DOI] [PubMed] [Google Scholar]
- 76.Vlahakes GJ, Turley K, Hoffman JI. The pathophysiology of failure in acute right ventricular hypertension: Hemodynamic and biochemical correlations. Circulation. 1981;63:87–95. doi: 10.1161/01.cir.63.1.87. [DOI] [PubMed] [Google Scholar]
- 77.Galasko CS. Incidence and distribution of bone metastases. Clin Orthop. 1986;210:14–21. [Google Scholar]
- 78.Posner JB, editor. Vol. 45. Philadelphia: FA Davis Company; 1995. Neurologic complications of cancer. [Google Scholar]
- 79.Laufer I, Sciubba DM, Madera M, Bydon A, Witham TJ, Gokaslan ZL, et al. Surgical management of metastatic spinal tumors. Cancer Control. 2012;19:122–8. doi: 10.1177/107327481201900206. [DOI] [PubMed] [Google Scholar]
- 80.Klimo P, Jr, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7:64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366:643–8. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 82.Bilsky MH, Laufer I, Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976) 2009;34:S101–7. doi: 10.1097/BRS.0b013e3181bac4b2. [DOI] [PubMed] [Google Scholar]
- 83.George R, Jeba J, Ramkumar G, Chacko AG, Leng M, Tharyan P. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database Syst Rev. 2008:CD006716. doi: 10.1002/14651858.CD006716.pub2. [DOI] [PubMed] [Google Scholar]
- 84.Wang JC, Boland P, Mitra N, Yamada Y, Lis E, Stubblefield M, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: Results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:287–98. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 85.Rades D, Huttenlocher S, Evers JN, Bajrovic A, Karstens JH, Rudat V, et al. Do elderly patients benefit from surgery in addition to radiotherapy for treatment of metastatic spinal cord compression? Strahlenther Onkol. 2012;188:424–30. doi: 10.1007/s00066-011-0058-z. [DOI] [PubMed] [Google Scholar]
- 86.Rades D, Huttenlocher S, Dunst J, Bajrovic A, Karstens JH, Rudat V, et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. J Clin Oncol. 2010;28:3597–604. doi: 10.1200/JCO.2010.28.5635. [DOI] [PubMed] [Google Scholar]
- 87.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 88.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–91. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 89.Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: The Cancer Care Ontario Practice Guidelines Initiative’s Neuro-Oncology Disease Site Group. J Clin Oncol. 2005;23:2028–37. doi: 10.1200/JCO.2005.00.067. [DOI] [PubMed] [Google Scholar]
- 90.Ahn SG, Lee HM, Cho SH, Lee SA, Hwang SH, Jeong J, et al. Prognostic factors for patients with bone-only metastasis in breast cancer. Yonsei Med J. 2013;54:1168–77. doi: 10.3349/ymj.2013.54.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Niikura N, Liu J, Hayashi N, Palla SL, Tokuda Y, Hortobagyi GN, et al. Treatment outcome and prognostic factors for patients with bone-only metastases of breast cancer: A single-institution retrospective analysis. Oncologist. 2011;16:155–64. doi: 10.1634/theoncologist.2010-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baloch KG, Grimer RJ, Carter SR, Tillman RM. Radical surgery for the solitary bony metastasis from renal-cell carcinoma. J Bone Joint Surg Br. 2000;82:62–7. doi: 10.1302/0301-620x.82b1.9995. [DOI] [PubMed] [Google Scholar]
- 93.Hirano Y, Oda M, Tsunezuka Y, Ishikawa N, Watanabe G. Long term survival cases of lung cancer presented as solitary bone metastasis. Ann Thorac Cardiovasc Surg. 2005;11:401–4. [PubMed] [Google Scholar]
- 94.Fuchs B, Trousdale RT, Rock MG. Solitary bony metastasis from renal cell carcinoma: Significance of surgical treatment. Clin Orthop Relat Res. 2005:187–92. doi: 10.1097/01.blo.0000149820.65137.b4. [DOI] [PubMed] [Google Scholar]
- 95.Fottner A, Szalantzy M, Wirthmann L, Stähler M, Baur-Melnyk A, Jansson V, et al. Bone metastases from renal cell carcinoma: Patient survival after surgical treatment. BMC Musculoskelet Disord. 2010;11:145. doi: 10.1186/1471-2474-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weber KL, O’Connor MI. Operative treatment of long bone metastases: Focus on the femur. Clin Orthop Relat Res. 2003:S276–8. doi: 10.1097/01.blo.0000093850.72468.54. [DOI] [PubMed] [Google Scholar]
- 97.Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983:297–302. [PubMed] [Google Scholar]
- 98.Harrington KD. The management of acetabular insufficiency secondary to metastatic malignant disease. J Bone Joint Surg Am. 1981;63:653–64. [PubMed] [Google Scholar]
- 99.Johnson JT. Reconstruction of the pelvic ring following tumor resection. J Bone Joint Surg Am. 1978;60:747–51. [PubMed] [Google Scholar]
- 100.Damron TA, Sim FH. Operative treatment for metastatic disease of the pelvis and the proximal end of the femur. J Bone Joint Surg Am. 2000;82:114–26. [PubMed] [Google Scholar]
- 101.Satcher RL, Jr, O’Donnell RJ, Johnston JO. Reconstruction of the pelvis after resection of tumors about the acetabulum. Clin Orthop Relat Res. 2003:209–17. doi: 10.1097/01.blo.0000057791.10364.7c. [DOI] [PubMed] [Google Scholar]
- 102.Marco RA, Sheth DS, Boland PJ, Wunder JS, Siegel JA, Healey JH. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. J Bone Joint Surg Am. 2000;82:642–51. doi: 10.2106/00004623-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 103.Clarke HD, Damron TA, Sim FH. Head and neck replacement endoprosthesis for pathologic proximal femoral lesions. Clin Orthop Relat Res. 1998:210–7. doi: 10.1097/00003086-199808000-00024. [DOI] [PubMed] [Google Scholar]
- 104.Morris HG, Capanna R, Del Ben M, Campanacci D. Prosthetic reconstruction of the proximal femur after resection for bone tumors. J Arthroplasty. 1995;10:293–9. doi: 10.1016/s0883-5403(05)80177-9. [DOI] [PubMed] [Google Scholar]
- 105.Herrenbruck T, Erickson EW, Damron TA, Heiner J. Adverse clinical events during cemented long-stem femoral arthroplasty. Clin Orthop Relat Res. 2002:154–63. doi: 10.1097/00003086-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 106.Patterson BM, Healey JH, Cornell CN, Sharrock NE. Cardiac arrest during hip arthroplasty with a cemented long-stem component. A report of seven cases. J Bone Joint Surg Am. 1991;73:271–7. [PubMed] [Google Scholar]
- 107.Wedin R, Bauer HC. Surgical treatment of skeletal metastatic lesions of the proximal femur endoprosthesis or reconstruction nail? J Bone Joint Surg Br. 2005;87:1653–57. doi: 10.1302/0301-620X.87B12.16629. [DOI] [PubMed] [Google Scholar]
- 108.Frankel VH, Burstein AH. Philadelphia: Lea and Febiger; 1970. Orthopaedic biomechanics: The application of engineering to the musculoskeletal system. [Google Scholar]