Abstract
Recent evidence suggests that natural killer (NK) cells are typically defective in infiltrating solid tumors, with the exception of gastrointestinal stromal tumors (GIST). Interestingly, however, infrequently infiltrating NK cells do not appear to have a direct effect on tumor progression. Here, prompted by the recent evidence that NK cell and T cell crosstalk may trigger, or enhance, tumor antigen-specific immune responses, we have tested the clinical significance of this reciprocal signaling. To this end, a tissue microarray constructed with 1410 colorectal carcinoma (CRC) patient specimens was stained with NK and T cell antigen-specific monoclonal antibodies, utilizing the immunoperoxidase staining technique. Cut-off scores for positive (>4 NK cells) and negative (≤4 NK cells) NK cell CRC patient samples were determined using receiver operating characteristic curve analysis. Using this approach, NK cells were detected in 423 (30%) of the 1410 CRC specimens evaluated. The number of NK cells was >4 in only 132 (9%) of CRC samples. Correlation of the immunohistochemical staining results together with analysis of the clinical course of the disease revealed that the infiltration of colorectal tumors with both NK cells and CD8+ T cells is associated with prolonged patient survival. In contrast, infiltration of tumors with NK cells in combination with CD3+ and CD4+ T lymphocytes had no detectable effect on the clinical course of the disease. These results suggest that NK cell and CD8+ T cell crosstalk in the tumor microenvironment may benefit patient outcome and further, that the enumeration of infiltrating NK and CD8+ T cells in CRC tumors may provide useful prognostic information.
Keywords: CD8 T cell, colorectal carcinoma, cooperation, lymphocyte, NK cell, survival, tumor
Abbreviations: ADCC, antibody dependent cellular cytotoxicity; BC, breast cancer; CRC, colorectal carcinoma; CTL, cytotoxic T lymphocytes; DC, dendritic cells; FGFR, fibroblast growth factor receptor; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HLA, human leukocyte antigen; IDO, indoleamine-2, 3-dioxygenase; IFNγ, interferon γ; IRB, Institutional Review Board; LFA-1, lymphocyte function-associated antigen-1; MICA/B, the major histocompatibility complex (MHC) Class I polypeptide-related sequence A/B; MHC, the major histocompatibility complex; MMPs, matrix metalloproteinases; NK, natural killer; PGE2, prostaglandin E2; RCC, renal cell carcinoma; ROC, receiver operating characteristics; TAMs, tumor-associated macrophages; TGF-β1, transforming growth factor β1; TILs, tumor-infiltrating lymphocytes
Introduction
Convincing evidence suggests that the nature and function of inflammatory cells infiltrating the tumor microenvironment play a pivotal role in the clinical course of colorectal cancer (CRC). The tumor microenvironment can potentially be infiltrated by a variety of immune cells including T helper (Th) and cytotoxic T lymphocytes (CTLs), mast cells, plasma cells and macrophages. In contrast, NK cells and neutrophils are among the most scarce immune cell populations within the tumor.1 Often, colorectal tumors are markedly infiltrated with tumor-associated macrophages (TAMs).2,3 On the basis of FcγRIII (CD16) expression, 2 populations of TAMs (CD16+ versus CD16−) have been identified. Only CD16+ TAMs are associated with a favorable clinical course of the disease.4 Additional studies have shown that a sub-population of CD16+TAMs is myeloperoxidase positive, CD15+ and CD66b+, an expression profile consistent with a granulocyte phenotype.5,6 In addition to TAM infiltration, the presence of effector memory CD8+ T cells and T helper type 1 (Th1)-related cytokine products (includingIL-18),7 as well as CD4+ regulatory T cell infiltration, are also associated with a favorable prognosis 8,9 in CRC patients.
NK cells mediate a strong antitumor activity in murine models and human myeloid leukemia.9-11 In contrast, conflicting information is available about the role of NK cells in human solid tumors.9 According to Gulubova et al., Marechal et al., and Menon et al.,12-14 NK infiltration of neoplastic lesions is associated with a reduced risk of relapse and prolonged survival, whereas according to Sandel et al., and Halama et al.,15,16 the presence of infiltrating NK cells has no effect on the clinical course of the disease.15,16 We have also examined NK cell infiltration in a large number of solid tumors including 1414 CRC, 385 breast carcinomas (BC), 117 renal cell carcinomas (RCC), 336 hepatocellular carcinomas, (HCC) and 284 melanomas. In most of these solid malignancies, NK cell infiltration was barely detectable.4,17,18
Although NK cells have a limited ability to infiltrate the CRC microenvironment, there is a subpopulation of CRC patients with lesions infiltrated with a sufficient number of NK cells suitable for statistical analysis. In prior studies, NK cell infiltration was detected in about 30% of colorectal tumor specimens. Importantly, NK cell infiltration was not found to be associated with the clinical course of the disease.4
Studies both in vitro and in vivo in animal model systems19,20 have shown that NK cells can interact with CD8+ T cells, and, that this crosstalk may trigger, or enhance, a tumor antigen-specific T cell immune response and epitope spreading of the T-cell immune response. These findings have provided the rationale for our studies to determine whether infiltration of colorectal tumors by both NK cells and CD8+ T cells has a beneficial effect on the clinical course of the disease.
Results
Expression of CD56 in CRC tumors
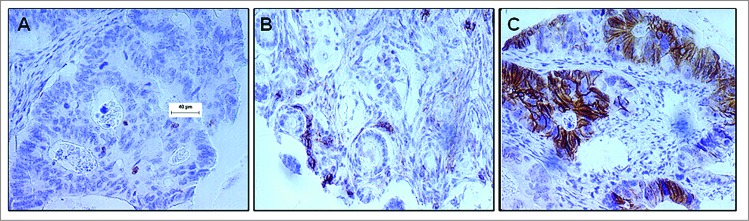
Since the majority of immunohistochemical studies investigating the presence of NK cells in the colorectal tumor microenvironment have utilized CD569 as an antigenic biomarker, we first assessed for the presence of NK cell infiltration in CRC patient tumors by staining the CRC tissue microarray with the anti-CD56 antigen-specific mAb, 123C3. We found positive NK cell infiltration (>4 positive cells per tumor) in only 132 (31%) of the 423 CRC patient tumor specimens analyzed. Representatives of a NK cell negative colorectal tumor punch with CD56+ cell infiltration ≤4 and a NK cell positive tumor punch with CD56+ cell infiltration >4 are shown in Figures 1A and 1B, respectively. Interestingly, CD56 antigen was not restricted to inflammatory cells but was also expressed by tumor cells in 2% of the CRC lesions evaluated (Fig. 1C).
Figure 1.
CD56 expression in the colorectal carcinoma microenvironment. Formalin-fixed paraffin-embedded tissue blocks of colorectal cancer (CRC) patient tumor specimens (n = 1410) were sectioned and stained with an anti–CD56 mAb. Following detection with a chromogenic substrate, the brown color shows CD56+ cells. (A) Representative example of CD56− CRC tumor punch with ≤4 CD56+ cells. (B) Representative example of CD56+ CRC patient tumor punch with chains of CD56+ cells >4. (C) IHC analysis detects CD56+ colorectal carcinoma cells.
We next sought to investigate the potential functional significance of NK cell infiltration in CRC patient tumors. To this end, we tested CRC cells for the expression of the major histocompatibility complex (MHC) Class I polypeptide-related sequence A/B (MICA/B). The latter is the ligand of the NK cell activating receptor, killer cell lectin-like receptor subfamily K, member 1 (KLRK1, also known as NKG2D). As already shown in other solid malignancies, most of the CRC cells (>90%) over-expressed MICA/B (data not shown) suggesting that CRC cells are good targets for locally infiltrating NK cells.4,17,18
Cooperation between NK cells and CD8+ T cells in the tumor microenvironment
To test the hypothesis that NK cells may improve the anticancer immune response of T lymphocytes and thus improving the clinical course of CRC patients, we assessed whether there was a correlation between NK cell infiltration (CD56) and infiltrating CD8+, CD3+, and CD4+ T lymphocytes,with CRC patient survival.
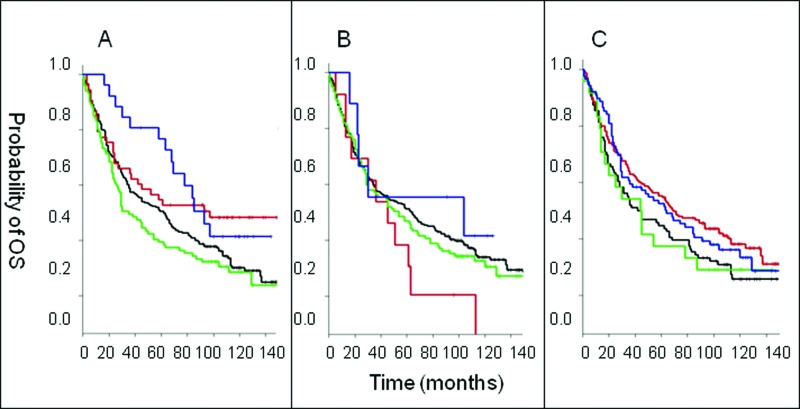
After more than 11 years of follow-up, patients with lesions marked by CD56+CD8− and CD56−CD8− cell infiltration profiles had significantly lower overall survival than CRC patients with CD56−CD8+ infiltrated lesions while the latter had an overall survival significantly lower than that of patients with CD56+CD8+ cell infiltration profiles. Interestingly, in the univariate analysis, within the first 5 years of follow-up, CRC patients with CD56+CD8+ CRC lesions survived significantly longer (p = 0.007) than CRC patients with CD56−CD8+ cell infiltration. Indeed, ∼80% of CRC patients with CD56+ and CD8+ cell infiltration remained alive while only ∼55% of CRC patients with only T cell infiltration (i.e., CD56−CD8+ cell infiltration profile) survived during the first 5 years of follow-up. However, following a 5-year follow up, the survival benefit of CRC patients with both CD56+ and CD8+ immune cell infiltration declined (p = 0.039; Fig. 2A).
Figure 2.
Association of CD8+ T cell and CD56+ natural killer cell infiltration in colorectal tumors with survival. (A—C) Colorectal cancer (CRC) patient samples were analyzed by immunohistochemical analysis to determine the presence of the indicated immune cell phenotypes. Overall survival (OS) is plotted among patients with the indicated immune cell marker profile over time. Statistical analysis was performed by log-rank test. (A) Analysis of CD8 and CD56. “Dotted/dashed” reads “Blue” line: CD56+CD8+ lesions (N = 26), “dashed” reads “red” line: CD56−CD8+ (N = 53), “unbroken” reads “black” line: CD56−CD8− (N = 231), “dotted” reads “green” line: CD56+CD8− (N = 101). (B) Analysis of CD4 and CD56. “Dotted/dashed” reads “Blue” line: CD56+CD4+ (N = 9), “dashed” reads “red” line: CD56−CD4+ (N = 13), “unbroken” reads “black” line: CD56−CD4− (N = 259), “dotted” reads “green” line: CD56+CD4− (N = 117). (C) Analysis of CD3 and CD56. “Dotted/dashed” reads “Blue” line: CD56+CD3+ (N = 89), “dashed” reads “red” line: CD56−CD3+ (N = 170), “unbroken” reads “black” line: CD56−CD3− (N = 96), “dotted” reads “green” line: CD56+CD3− (N = 24).
In regards to other T cell subsets, the overall survival of CRC patients with lesions exhibiting CD4+CD56− cell infiltration profiles did not differ from that of CRC patients with both CD4+ and CD56+ immune cell infiltration. In both cases, the survival of patients with either profile (CD4+CD56− or CD4+CD56+) was not significantly different from that of CRC patients with CD56−CD4− and CD56+CD4− cell infiltrations (p = 0.49; Fig. 2B). In addition, the overall survival of CRC patients with CD56+CD3+ cell infiltration was not significantly different from that of patients with CD56-CD3+ CRC tumors (p = 0.42; Fig. 2C).
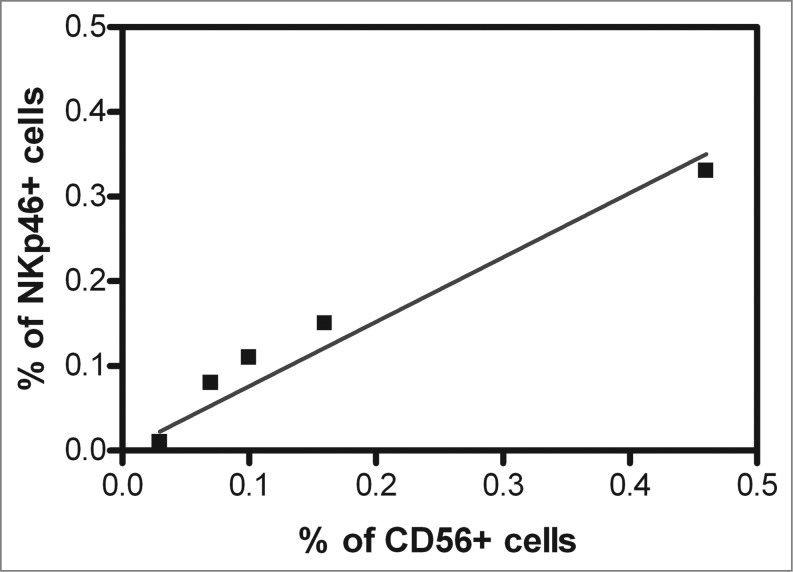
CD56 (NCAM1), albeit a broad marker of NK cells,9 has a low expression on ∼90% of NK cells, referred to as NK cellsCD56low and a high expression on ∼10% of NK cells, referred to as NK cellsCD56bright.The latter subpopulation represents the regulatory NK cell subset, while the former represents the cytotoxic NK cell subset. However, CD56 is also abundantly expressed on a small subset of T cells termed natural killer T (NK-T) cells. To test whether NK cells or NK-T cells collaborate with CD8+ T lymphocytes in improving the clinical course of CRC, we next investigated whether CD56high cell infiltration correlated with CD8+ T cell infiltration in CRC tumors. No CD8+ T lymphocytes expressing high levels of CD56 antigen were detected in a collective of 423 CRC patient tumors (r = 0.02; p = 0.58). Finally, in an additional study, we analyzed 15 enzymatically dissociated CRC specimens by immunofluorescence staining and flow cytometry for the expression of CD56 and an independent NK cell marker, NKp46. Positive cells were found to be present among the cell populations from 5 CRC tumors. Spearman Rank analysis showed a strong association of CD56+ cell infiltration with NKp46+ cell infiltration (R = 0.97; p = 0.005; Fig. 3), further evincing the NK cell identity of the infiltrating CD56+ cells.
Figure 3.
Relationship between CD56 and NKp46 positive cell infiltration in the CRC tumor microenvironment. Fifteen freshly, resected colorectal cancer (CRC) patient tumors were enzymatically digested to a single cell suspension and directly labeled using fluorescence-conjugated antibodies against CD56 and NKp46. CD56+ and NKp46+ cells were detected by cytofluorimetric analysis cytometry in 5 CRC patient specimens. Spearman Rank correlation analysis revealed that the percentage of CD56+ cells significantly correlated with the percentage of NKp46+ cells in the colorectal tumor microenvironment (correlation coefficient, R = 0.97, p = 0.005).
Discussion
The results we have shown indicate that the infiltration of CRC patient tumors with both NK cells and CD8+ T cells is associated with a favorable course of the disease. This association is likely to reflect the NK cell-T cell crosstalk which has been shown to trigger, or enhance, a tumor antigen-specific T cell-based immune response as a result of increased antigen presentation, epitope spreading and HLA Class I antigen processing machinery up regulation in target cells.19,20 It is of interest that CRC is not the only solid tumor in which NK cell-T cell cooperation appears to have a beneficial effect on the clinical course of the disease. T cells have been recently shown to support the antitumor activity of NK cells against mouse mastocytoma.19 In addition, NK cells activated by cetuximab have been shown to cooperate with dendritic cells to trigger tumor antigen-specific T cell immunity in head and neck cancer patients.20
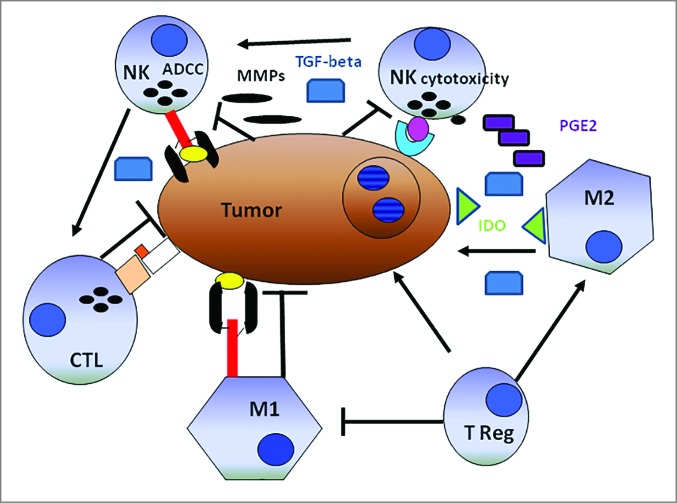
The beneficial effect of NK cell-T cell cooperation on the clinical course of CRC is attenuated following a 5-year follow up. A mechanism underlying this phenomenon is not readily apparent. One might speculate that over time, tumor-infiltrating NK cells lose their helper function because of altered activities induced by cancer cells via various routes. These include malignant cell production of indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2), metalloproteinases (MMPs) transforming growth factor β1, (TGFβ1) and integrin β2 (ITGB2, also known as LFA1) 9,17,21-28 all of which may affect NK cell function.26 An additional potential mechanism is suggested by the cancer cell-induced apoptosis of NK cells, since both the myeloid leukemia cell line, K562, and the RCC cell line, Caki-1, have been shown to stimulate cytotoxic NK cell depletion. The potential mechanisms by which cancer cells induce NK cell apoptosis are obscure. Cancer cell induced NK cell apoptosis may be mediated by the interaction of NK cell surface localized CD16 with its yet to be identified ligand presumably expressed on malignant cells. An alternative possibility is the induction of apoptosis in NK cells following interaction with MICA/B expressed on CRC cells and/or with CRC cells lacking cell surface HLA-Class I antigen expression. At any rate, the mechanisms we have listed may also account for the typical paucity of NK cell tumor infiltration (Fig. 4).
Figure 4.
Schematic representation of the alleged relationship between the immune system and solid tumors. Cancer cells cooperating with anti-inflammatory macrophages (M2) contribute to creating an immunosuppressive tumor microenvironment including indoleamine-2,3-dioxygenase (IDO) production, which induces tryptophan depletion and L-kynurenine accumulation (not shown), as well as prostaglandin E2 (PGE2) and transforming growth factor β1 (TGF-β1) accumulation. Tumor cells are also capable of activating tissue matrix metalloproteinases (MMPs), which cleave CD16 from the natural killer (NK) cell surface, reducing their ability to mediate antibody-dependent cellular cytotoxicity (ADCC). T lymphocytes and M1-macrophages are expected to work more efficiently than NK cells in mediating direct cellular cytotoxicity and ADCC, respectively. Both T lymphocytes and M1-macrophages exert indirect antitumor effects through the production of pro-inflammatory cytokines. In this context, NK cells could increase the antitumor activity of cytotoxic CD8+ T lymphocytes (CTLs). Symbols: → = cell activation, ┬ = cell inhibition.
To the best of our knowledge, this is the first demonstration of CD56 antigen expression by colorectal carcinoma cells in a subset of patients. However, this is not unique of CRC cells, since CD56 antigen has been found to be heterogeneously expressed by RCC, melanoma, myeloid leukemia cells and bone marrow progenitor cells.17,18,29 Furthermore, in acute myelod leukemia, CD56 expression has been associated with unfavourable cytogenetic abnormalities, reduced probability to achieve complete remission, and poor survival.30 We could not test this possibility in CRC since the number of CD56+ CRC patient tumors we identified was too low and, therefore, not suitable for statistical analysis.
The functional role of CD56 in tumors is not fully understood, however, there is evidence that it is involved in the pathogenesis of epithelial tumors. CD56 interaction with fibroblast growth factor receptor (FGFR) promotes ovarian cancer development, pancreatic tumor cell adhesion to the extracellular matrix, and invasiveness of epithelial tumors.31-33 In addition, CD56 is involved in a variety of cell functions including cell proliferation, migration and, epithelial-mesenchymal transition. Whether CD56 expression on CRC cells has a role in the pathogenesis of CRC tumor development and is a potential marker of poor prognosis in a rare subset of CRC patient tumors remains to be determined.
Materials and Methods
Antibodies
Anti-CD3, anti-CD4, anti-CD8, anti-CD16, anti-CD57, and anti-CD56 isotype matched mouse mAbs were purchased from DAKO while anti-CD68 mouse mAb was purchased from Novocastra.
Tissue microarray (TMAs)
The tissue microarrays (TMAs) of CRC patient tumors were composed of 1410 tissue biopsies.4 Briefly, TMAs were constructed using formalin-fixed, paraffin-embedded tissue blocks from CRC resections. All biopsies were collected and stored in the Biobank of the Institute of Pathology at the University Hospital Basel. The study was approved by the hospital Internal Review Board (IRB) and in accordance with the Helsinki Declaration of 1975.
Immunohistochemistry
TMAs were evaluated by a 2-step immunostaining approach using anti-CD56, anti-CD3, anti-CD4, anti-CD8, anti-CD16, anti-CD57, anti-CD68 and anti-MICA/B mAbs as primary antibodies and a peroxidase-labeled rabbit-anti-mouse IgG antibody as the secondary antibody. Following dewaxing and rehydration of the slides in distilled water, endogenous peroxidase activity was blocked with 0.5% H2O2 and colorectal tissue sections were incubated with the aforementioned mAbs for 1 hour at room temperature. Subsequently, tissues were incubated with peroxidase-labeled secondary antibody for 30 min at room temperature. For antigen visualization, colorectal tissues were soaked in 3-amino-9-ethylcarbazole (DAKO) supplemented with substrate-chromogen for 30 min and counterstained with Gill's hematoxylin (DAKO).
Cytofluorimetric analysis of cell suspension from CRC surgical specimens
Surgically removed CRC tumors were minced, centrifuged and resuspended in RPMI 1640 medium supplemented with 5%, fetal bovine serum. Minced CRC tumors were incubated overnight, at room temperature, in the presence of 2 mg/mL collagenase IV, 0.1 mg/mL hyaluronidase V and 0.2 mg/mL DNAse I (Sigma Aldrich). Single cell suspensions were isolated by Ficoll-Hypaque gradient separation, stained with fluorochrome-conjugated mAbs with specificity for CD56 and NKP46 antigens and analyzed utilizing a 2-laser BD FACSCalibur (Becton Dickinson) flow cytometer. Propidium iodide (PI) positive cells were excluded from the study. Results were analyzed by Cell Quest (Becton Dickinson) and Flow Jo (Tree Star) computer software programs.
Statistical analyses
Available clinicopathological data included pT, pN stage, tumor grade, vascular invasion, tumor budding, tumor location and patient survival. Four hundred and 20 3 CRC lesions were evaluable for CD56 staining. Cut-off scores for protein marker positivity were determined on the Test Group using receiver operating characteristic (ROC) curve analysis with the endpoint of survival/death and the 0,1-criterion to select the most discriminating cut-off score from the ROC curve. Results were validated by 3 independent investigators achieving an optimal concordance rate of 80–90%.
We considered CRC lesions positive for NK cell infiltration when there was an absolute number of CD56+ cells >4, per punch, in the presence or absence of CD16+ cells. Then, CD56+ cell infiltration was compared to the punch content of CD3+ and CD8+ T lymphocytes, CD4+ and CD57+ cells and CD16+ TAMs. CRC patient tumors were considered positive for CD3, CD8, and CD4 cell infiltration when CRC tumor punches contained more than 20, 10, and 20 positive cells, respectively. CRC was considered infiltrated by CD16+ TAMs when colorectal tumor punches were negative for CD56 and showed an infiltration of CD16+ cells >30. We considered CD57+ cell infiltration when, regardless the number of CD56+ cells, we detected >4 positive cells in the CRC patient tumor punches.
Survival time differences were determined using the Kaplan-Meier method and the Log-rank test. Strength of correlations between different cell types was assessed using the Spearman Rank correlation coefficient.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Giuseppe Sconocchia was supported by the AIRC grant: IG10555 and the Ministry of Education, University, and Research (PRIN), grant 2010AX2JX7_005. Soldano Ferrone was supported in part by PHS grants RO1CA138188, RO1CA110249 and P50CA121973 awarded by the National Cancer Institute.
References
- 1.McLean MH, Murray GI, Stewart KN, Norrie G, Mayer C, Hold GL, Thomson J, Fyfe N, Hope M, Mowat NAG, et al. The inflammatory microenvironment in colorectal neoplasia. PLoS One 2011; 6:e15366; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0015366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Algars A, Irjala H, Vaittinen S, Huhtinen H, Sundstrom J, Salmi M, Ristamaki R, Jalkanen S.Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer 2012; 131:864-73; PMID:; http://dx.doi.org/ 10.1002/ijc.26457 [DOI] [PubMed] [Google Scholar]
- 3.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R.High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin.Cancer Res 2007; 13:1472-9; PMID: [DOI] [PubMed] [Google Scholar]
- 4.Sconocchia G, Zlobec I, Lugli A, Calabrese D, Iezzi G, Karamitopoulou E, Patsouris ES, Peros G, Horcic M, Tornillo L, et al. Tumor infiltration by FcgammaRIII (CD16) +myeloid cells is associated with improved survival in patients with colorectal carcinoma. Int J Cancer 2011; 128:2663-72; PMID:; http://dx.doi.org/ 10.1002/ijc.25609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Droeser RA, Hirt C, Eppenberger-Castori S, Zlobec I, Viehl CT, Frey DM, Nebiker CA, Rosso R, Zuber M, Amicarella F, et al. High myeloperoxidase positive cell infiltration in colorectal cancer is an independent favorable prognostic factor. PLoS One 2013; 8:e64814; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0064814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirt C, Eppenberger-Castori S, Sconocchia G, Iezzi G, Tornillo L, Terracciano L, Spagnoli GC, Droeser R.Colorectal cancer infiltration by myeloperoxidase positive neutrophil granulocytes is associated with favorable prognosis. Oncoimmunology 2013; 2:e25990; PMID:; http://dx.doi.org/ 10.4161/onci.25990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; http://dx.doi.org/ 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 8.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L.High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer 2010; 126:2635-43; PMID: [DOI] [PubMed] [Google Scholar]
- 9.Desbois M, Rusakiewicz S, Locher C, Zitvogel L, Chaput N.Natural killer cells in non-hematopoieticmalignancies. Front Immunol 2012; 3:395; PMID:; http://dx.doi.org/ 10.3389/fimmu.2012.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savani BN, Rezvani K, Mielke S, Montero A, Kurlander R, Carter CS, Leitman S, Read EJ, Childs R, Barrett AJ.Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood 2006; 107:1688-95; PMID:; http://dx.doi.org/ 10.1182/blood-2005-05-1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savani BN, Mielke S, Adams S, Uribe M, Rezvani K, Yong AS, Zeilah J, Kurlander R, Srinivasan R, Childs R, et al. Rapid natural killer cell recovery determines outcome after T-cell-depleted HLA-identical stem cell transplantation in patients with myeloid leukemias but not with acute lymphoblastic leukemia. Leukemia 2007; 21:2145-52; PMID:; http://dx.doi.org/ 10.1038/sj.leu.2404892 [DOI] [PubMed] [Google Scholar]
- 12.Gulubova M, Manolova I, Kyurkchiev D, Julianov A, Altunkova I.Decrease in intrahepatic CD56 +lymphocytes in gastric and colorectal cancer patients with liver metastases. APMIS 2009; 117:870-9; PMID:; http://dx.doi.org/ 10.1111/j.1600-0463.2009.02547.x [DOI] [PubMed] [Google Scholar]
- 13.Marechal R, De SJ, Nagy N, Demetter P, Lemmers A, Deviere J, Salmon I, Tejpar S, Van Laethem JL.Putative contribution of CD56 positive cells in cetuximab treatment efficacy in first-line metastatic colorectal cancer patients. BMC Cancer 2010; 10:340; PMID:; http://dx.doi.org/ 10.1186/1471-2407-10-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon AG, Janssen-van Rhijn CM, Morreau H, Putter H, Tollenaar RA, van de Velde CJ, Fleuren GJ, Kuppen PJ.Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest 2004; 84:493-501; PMID: [DOI] [PubMed] [Google Scholar]
- 15.Sandel MH, Speetjens FM, Menon AG, Albertsson PA, Basse PH, Hokland M, Nagelkerke JF, Tollenaar RA, van de Velde CJ, Kuppen PJ.Natural killer cells infiltrating colorectal cancer and MHC class I expression. Mol Immunol 2005; 42:541-6. [DOI] [PubMed] [Google Scholar]
- 16.Halama N, Braun M, Kahlert C, Spille A, Quack C, Rahbari N, Koch M, Weitz J, Kloor M, Zoernig I, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res 2011; 17:678-89. [DOI] [PubMed] [Google Scholar]
- 17.Sconocchia G, Spagnoli GC, Del Principe D, Ferrone S, Anselmi M, Wongsena W, Cervelli V, Schultz-Thater E, Wyler S, Carafa V, et al. Defective infiltration of natural killer cells in MICA/B-positive renal cell carcinoma involves beta(2)-integrin-mediated interaction. Neoplasia 2009; 11:662-71; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sconocchia G, Arriga R, Tornillo L, Terracciano L, Ferrone S, Spagnoli GC.Melanoma cells inhibit NK cell functions–letter. Cancer Res 2012; 72:5428-9; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1181 [DOI] [PubMed] [Google Scholar]
- 19.Shanker A, Verdeil G, Buferne M, Inderberg-Suso EM, Puthier D, Joly F, Nguyen C, Leserman L, Auphan-Anezin N, Schmitt-Verhulst AM.CD8 T cell help for innate antitumor immunity. J Immunol 2007; 179:6651-62; http://dx.doi.org/ 10.4049/jimmunol.179.10.6651 [DOI] [PubMed] [Google Scholar]
- 20.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson C, Lopez-Albaitero A, Gibson SP, Gooding WE, Ferrone S, et al. Cetuximab-activated natural killer (NK) and dendritic cells (DC) collaborate to trigger tumor antigen-specific T cell immunity in head and neck cancer patients. Clin. Cancer Res 2013; 19:1858-72; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grzywacz B, Kataria N, Verneris MR.CD56(dim)CD16(+) NK cells downregulate CD16 following target cell induced activation of matrix metalloproteinases. Leukemia 2007; 21:356-9; PMID:; http://dx.doi.org/ 10.1038/sj.leu.2404499 [DOI] [PubMed] [Google Scholar]
- 22.Harrison D, Phillips JH, Lanier LL.Involvement of a metalloprotease in spontaneous and phorbol ester-induced release of natural killer cell-associated Fc gamma RIII (CD16-II). J Immunol 1991; 147:3459-65; PMID: [PubMed] [Google Scholar]
- 23.Jewett A, Bonavida B.Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol 1996; 156:907-15; PMID: [PubMed] [Google Scholar]
- 24.Ortaldo JR, Mason AT, O'Shea JJ.Receptor-induced death in human natural killer cells: involvement of CD16. J Exp Med 1995; 181:339-44; PMID:; http://dx.doi.org/ 10.1084/jem.181.1.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietra G, Vitale M, Manzini C, Balsamo M, Moretta L, Mingari MC.Melanoma cells inhibit NK cell functions–response. Cancer Res 2012; 72:5430; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2526 [DOI] [Google Scholar]
- 26.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res 2012; 72:1407-15; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2544 [DOI] [PubMed] [Google Scholar]
- 27.Poggi A, Massaro AM, Negrini S, Contini P, Zocchi MR.Tumor-induced apoptosis of human IL-2-activated NK cells: role of natural cytotoxicity receptors. J Immunol 2005; 174:2653-60; PMID:; http://dx.doi.org/ 10.4049/jimmunol.174.5.2653 [DOI] [PubMed] [Google Scholar]
- 28.Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, Andre P, Romagne F, Thibault G, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121:3609-22; PMID:; http://dx.doi.org/ 10.1172/JCI45816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sconocchia G, Fujiwara H, Rezvani K, Keyvanfar K, El Ouriaghli F., Grube M, Melenhorst J, Barrett AJ.G-CSF-mobilized CD34+ cells cultured in interleukin-2 and stem cell factor generate a phenotipically novel monocyte. J Leuk Biol 2004; 76:1214-9; http://dx.doi.org/ 10.1189/jlb.0504278 [DOI] [PubMed] [Google Scholar]
- 30.Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, Sestigiani C, Mariotti C, Birtolo S, Tozzi M, et al. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 2001; 15:1161-4; PMID:; http://dx.doi.org/ 10.1038/sj.leu.2402174 [DOI] [PubMed] [Google Scholar]
- 31.Zecchini S, Bombardelli L, Decio A, Bianchi M, Mazzarol G, Sanguinetti F, Aletti G, Maddaluno L, Berenzin V, Bock E, et al. The adhesion molecule NCAM promotes ovarian cancer progression via FGFR signaling. EMBO Mol Med 2011; 3:480-94; PMID:; http://dx.doi.org/ 10.1002/emmm.201100152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavallaro U, Niedermeyer J, Fuxa M, Christofori G.N-CAM modulates tumor-cell adhesion to matrix by inducing FGF-receptor signaling. Nat Cell Biol 2001; 3:650-57; PMID:; http://dx.doi.org/ 10.1038/35083041 [DOI] [PubMed] [Google Scholar]
- 33.Cirovic S, Vjestica J, Mueller CA, Tatic S, Vasiljevic J, Milenkovic S, Mueller GA, Markovic-Lipkovski J.NCAM and FGFR1 coexpression and colocalization in renal tumors. Int J Clin Exp Pathol 2014; 7:1402-14; PMID: [PMC free article] [PubMed] [Google Scholar]