Abstract
Tumor cells undergo molecular evolution under immune pressure. Using a murine metastatic lung cancer model, we recently reported that evolutionary pressure enforced through vaccination incites gain of Nanog, a master transcription factor that mediates both emergence of a stem-like cancer cell state and immune evasion. Thus, therapeutic strategies aiming to blunt NANOG's expression in patient tumors may improve the clinical management of cancer.
Keywords: cytotoxic T lymphocytes, immune-resistant, immune surveillance, Nanog, stem-like phenotype
Emergence of a stem-like state in the tumor and adaptation to host immune defenses have become established as hallmarks of cancer, responsible in large part for disease progression and recurrence in patients.1 Thus, future cancer therapy should aim to conquer both of these major obstacles. Currently, however, the molecular cues that give rise to either a stem-like state or immune adaptation remain shrouded in mystery.
In order to study tumor adaptation to host immune defenses, we previously devised an approach in which mice were first vaccinated with DNA encoding the E7 antigen from human papillomavirus type-16 linked to an endosome sorting signal, and then inoculated with TC-1 tumor cells, a murine metastatic lung cancer cell line which carries E7 antigen.2 In this system, 80% of mice rejected the tumor challenge, while the remaining 20% succumbed to the challenge. We explanted the tumor from these mice, expanded the tumor cells in vitro, and then re-inoculated them into another set of pre-vaccinated mice. This time, 40% of mice succumbed to the tumor challenge, and we again expanded these tumor cells in vitro and re-inoculated them into a third set of pre-vaccinated mice. After 3 rounds of this immune selection process, we were left with a line of TC-1 cells (termed P3) that would invariably form tumor in 100% of pre-vaccinated mice.
Curiously, we noticed that the P3 tumor cells, in contrast to their parental counterparts (P0), exhibited cardinal features of stem cells.3 In particular, these P3 cells had the capacity to form spheres in vitro, could efficiently give rise to tumor in immune-deficient mice (even at low challenge dose), and possessed markers characteristic of cancer stem cells (e.g., CD133, CD44, ALDH).3 To discover the molecular basis of this ‘stemness’ of P3 cells, we probed for their expression of a panel of proteins reported to be important for pluripotency of stem cells. Strikingly, we found that the homeobox transcription factor Nanog was about 10 times more abundant in P3 cells compared to P0 cells.3 Nanog—named after the fabled Tír na nÓg, or ‘land of the young’ in Irish mythology—is pivotal for the self-renewal of embryonic stem cells at the earliest stages of pluripotency.4
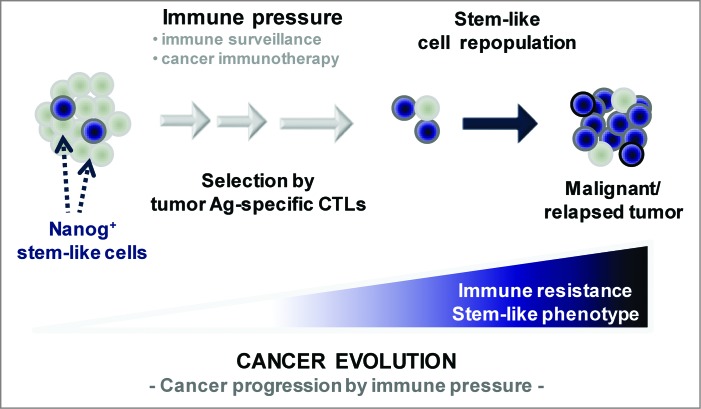
Considering its role in maintenance of pluripotency, we reasoned that Nanog could contribute to the stem-like state of tumor cells remaining after immune selection. To test this premise, we silenced Nanog expression in P3 cells with siRNA and found that this Nanog knockdown reversed the stem-like state. In other words, the Nanog-depleted tumor cells lost each of the cardinal features of stem cells mentioned above, such as sphere formation and tumorigenicity.3 Conversely, when we introduced Nanog cDNA into P0 tumor cells, they acquired a stem-like state.3 These data clearly implicate a role for Nanog in the control of tumor stemness, but—perhaps more crucially—they also demonstrate that immune selection may modulate the expression of key pluripotency factors in the tumor and therefore delineate a link between emergence of a stem-like state in the tumor and immune escape (Fig. 1).
Figure 1.
Vaccination-mediated selection and enrichment of Nanog+ tumor cells resistant to antigen-specific cytotoxic T lymphocytes. In this model, the tumor initially comprises a major population of Nanog− cells and a minor population of Nanog+ cells. Immune selection—first instigated by natural host immunosurveillance and then reinforced by vaccination—drives the preferential survival and expansion of the cytotoxic T lymphocyte (CTL)-resistant Nanog+ cancer cells. These Nanog+ malignant cells, in turn, mediate the immune-resistant and stem-like phenotype of the tumor and drive therapeutic failure and disease progression.
To test the role of Nanog in immune evasion, we examined the sensitivity of TC-1 cells with varying Nanog expression status to lysis by E7-specific CD8+ T cells (CTLs). We found that whereas P3 cells were resistant to CTLs, P3 cells with Nanog knockdown by siRNA could be killed by these CTLs.3 Conversely, P0 cells transduced with NANOG cDNA were more resistant to CTLs relative to the vector-transduced control P0 cells.3 Consistently, while the tumor regressed in mice inoculated with control P0 cells following adoptive transfer of E7-specific CTLs, tumor growth was rampant in mice inoculated with NANOG-transduced P0 cells despite adoptive therapy.3 These data demonstrate that Nanog plays a central role in tumor immune escape.
Since Nanog is critical for immune escape, we thought that blockade of the Nanog pathway in vivo would restore the success of adoptive therapy. To test this, we administered P3-bearing mice with nanoparticles loaded with siRNA against either Nanog or GFP control. While GFP siRNA-treated mice exhibited rapid tumor growth, we observed control of disease in Nanog siRNA-treated mice.3 Furthermore, Nanog siRNA-treated mice that also received adoptive therapy with E7-specific CTLs exhibited long-term tumor regression.3 These data prove the principle that components of the stemness transcription network may be rationally targeted within the tumor to overcome the hurdle of immune escape. It is also remarkable though that we saw retarded tumor growth in mice treated with Nanog siRNA alone (without adoptive therapy). This implies that perhaps—in response to immune selection—the tumor cells acquired Nanog expression and then became dependent on Nanog for survival and propagation, similar to the phenomenon of ‘oncogene addiction’. We propose that the antitumor effect of Nanog blockade occurs through a reversal of the stem-like state within the tumor, based on our prior discovery that knockdown of Nanog in vitro forces tumor cells to lose their stem-like features. Thus, strategies that impede the Nanog signaling pathway may not only conquer the problem of immune escape but also that of the stem-like state in cancer.
But would these strategies be effective in human cancer? We believe the answer is ‘yes’ based on several lines of evidence. First, we recently observed gain of NANOG in human tumor cells subjected to immune selection.5 Second, we found that NANOG induces a stem-like and immune-resistant phenotype across a wide spectrum of human cancer types, including those of the cervix, breast, lung, liver, ovary, and colon.5 And third, perhaps most importantly, we found that NANOG expression correlates with disease stage and worse prognosis in cervical cancer patients.5 Notably, we have also found that NANOG confers an immune-resistant and stem-like phenotype to tumor cells through transcriptional induction of TCL1A and subsequent activation of the Akt signaling pathway. Since the Akt pathway regulates the apoptosis machinery in cells6,7 and has been implicated as a central channel to a emergence of refractory disease in response to conventional therapy (e.g., chemotherapy, radiotherapy)8, we believe that gain of NANOG may also underlie the failure of a wide spectrum of clinical interventions. Altogether, these data argue that blockade of the NANOG pathway may be a promising approach for cancer therapy in the clinical setting.
Funding Statement
This work was supported by funding from the National Research Foundation of Korea (NRF-2013M3A9D3045881, NRF-2012R1A2A2A01007527) and the Korea Healthcare technology R&D Project (A062260).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74; PMID:; http://dx.doi.org/10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2. Lin KY, Lu D, Hung CF, Peng S, Huang L, Jie C, Murillo F, Rowley J, Tsai YC, He L, et al. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer Res 2007; 67:1832-41; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-06-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noh KH, Lee YH, Jeon JH, Kang TH, Mao CP, Wu TC, Kim TW. Cancer vaccination drives Nanog-dependent evolution of tumor cells toward an immune-resistant and stem-like phenotype. Cancer Res 2012; 72:1717-27.; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-11-3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003; 113:643-55; PMID:; http://dx.doi.org/10.1016/S0092-8674(03)00392-1 [DOI] [PubMed] [Google Scholar]
- 5. Noh KH, Kim BW, Song KH, Cho H, Lee YH, Kim JH, Chung JY, Kim JH, Hewitt SM, Seong SY, et al. Nanog signaling in cancer promotes stem-like phenotype and immune evasion. J Clin Invest 2012; 122:4077-93; PMID:; http://dx.doi.org/10.1172/JCI64057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 1999; 96:857-68; PMID:; http://dx.doi.org/10.1016/S0092-8674(00)80595-4 [DOI] [PubMed] [Google Scholar]
- 7. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997; 91:231-41; PMID:; http://dx.doi.org/10.1016/S0092-8674(00)80405-5 [DOI] [PubMed] [Google Scholar]
- 8. Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature 2004; 428:332-7; PMID:; http://dx.doi.org/10.1038/nature02369 [DOI] [PubMed] [Google Scholar]