Abstract
Focal tumor cell PD-L1 expression adjacent to TIL can be used as a surrogate marker of an ongoing antitumor host response, which may be unleashed by PD-1 blockade. Tumor cell PD-L1 expression is superior to TIL PD-1 expression and the presence of TIL alone, when predicting response to anti-PD-1 therapy.
Keywords: cancer immunotherapy, PD-1, PD-L1, PD-L2, tumor microenvironment
Abbreviations: CRPC, castration-resistant prostate cancer; FFPE, formalin-fixed paraffin-embedded; NSCLC, non-small cell lung carcinoma; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; PD-L2, programmed death-ligand 2; RCC, renal cell carcinoma; TIL, tumor-infiltrating lymphocytes
In addition to exciting durable tumor regressions, one of the more provocative findings associated with PD-L1/PD-1 pathway blockade involves the potential predictive value of pre-treatment specimen PD-L1 expression. We first reported a small series of nine patients from the MDX-1106/BMS-936558 trial, suggesting that tumor cell surface (membranous) PD-L1 expression may be associated with responsiveness to PD-1 blockade.1 These findings were supported in a larger series of 42 patients in the follow-up trial.2 Specifically, of the 25 patients who had a formalin-fixed paraffin-embedded (FFPE) pre-treatment specimen that was PD-L1(+), 36% had an objective response to anti-PD-1. In contrast, no patients whose tumors were PD-L1(−) demonstrated a clinical response (p = 0.006).
More recently, our group published the results of an expanded analysis conducted on 68 FFPE pre-treatment specimens from 41 patients with advanced cancers who were treated with anti-PD-1. The cohort included 16 patients with melanoma, 12 with non-small cell lung carcinoma (NSCLC), 6 with kidney cancer, 5 with colorectal carcinoma (CRC), and 2 with castration-resistant prostate cancer (CRPC).3 Fifty-three of these 68 specimens had previously been assessed for PD-L1 expression.2 The extended analysis included additional histologic and immune features in the pre-treatment tumor microenvironment, and how they related to each other and to patient outcomes. This involved a focus on infiltrating immune cell subsets, PD-1, PD-L1 and PD-L2 expression.
We found that tumor PD-L1 expression varied significantly by tumor type. Approximately 60% of the melanoma, NSCLC, and kidney cancer specimens tested demonstrated PD-L1 expression, in contrast to only one of 12 (8%) colorectal and CRPC specimens (p = 0.005). When tumor cell PD-L1 expression was observed, it was focal and seen in immediate geographic association with tumor infiltrating lymphocytes (TIL) in all but one case (33/34). Such constancy supports our hypothesis that PD-L1 expression by tumor is a mechanism of adaptive immune resistance.4 We also observed PD-L1 expression on infiltrating immune cells in the absence of tumor cell expression. For example, even though only 1 of 8 CRC cases demonstrated PD-L1+ tumor cells, 4 of the CRC cases (50%) had PD-L1 displayed on TIL and associated macrophages.
Additional histologic and immunoarchitectural features were also assessed for their relationship to PD-L1 expression. We found that the presence of TIL expressing PD-1 as well as CD20+ B-cells were both significantly associated with tumor and TIL PD-L1 expression. Features such as whether the specimen was from the primary tumor vs. a metastasis, the CD4+:CD8+ ratio, the presence or lymphoid aggregates, or tumor cell necrosis did not demonstrate a significant association. PD-L2, the second known ligand for PD-1 on T-cells, was observed to a lesser degree than PD-L1. When present, PD-L2 was observed in geographic association with PD-L1 at the interface of tumor and TIL (p = 0.05).
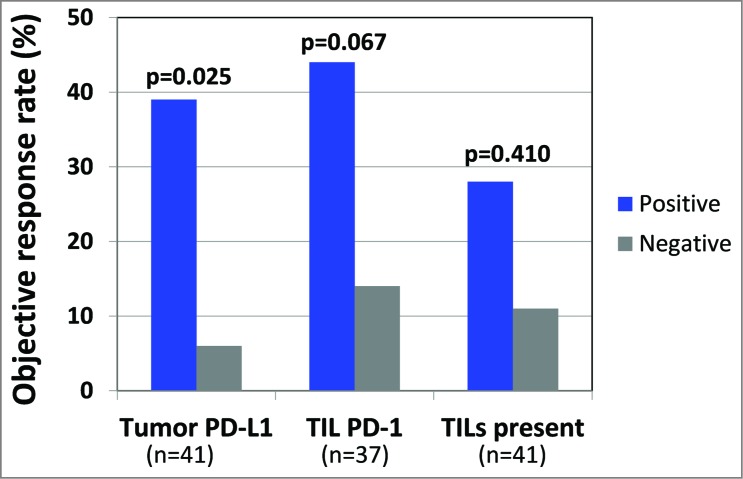
The finding that TIL PD-1 was displayed adjacent to PD-L1 (and sometimes with PD-L2) suggests an immunosuppressive microenvironment that may be altered by the administration of anti-PD-1 therapy. Accordingly, we examined how these factors in pre-treatment tumor specimens predicted response to anti-PD-1. We found that PD-L1 expression by tumor was the strongest single factor predicting objective response (Fig. 1), when compared to TIL PD-1 expression, or the presence of TIL alone. This is likely because focal tumor cell PD-L1 expression adjacent to TIL reflects an ongoing antitumor immune response, which may be protected by anti-PD-1. While PD-1 is the direct target of anti-PD-1, it only demonstrated a borderline association with response in our series. Similarly, the presence of TIL alone is not a significant factor predicting response to anti-PD-1. This latter finding suggests various functional states of TIL. Future studies will undoubtedly focus on further characterizing lymphocyte subsets, including regulatory T-cells, as well as other immunoactive cell types, such as myeloid-derived suppressor cells, and how these populations relate to response to anti-PD-1.
Figure 1.
Tumor PD-L1 is the strongest single predictor of response to anti-PD-1. When analyzing either the highest scoring sample among multiple biopsies from individual patients or the specimen obtained closest to therapy, tumor cell PD-L1 expression correlated with objective response to anti-PD-1 therapy. This association was stronger than the borderline association with PD-1 expression. Simply the presence of intratumoral immune cell infiltrates did not correlate with response. Additional features examined that did not predict response to anti-PD-1 in this limited cohort included PD-L2 expression by tumor or immune cells, CD4+:CD8+ ratio, CD20+ B−cells, or the presence of lymphoid aggregates or tumor necrosis (data not shown).
A proportion of the patients in our cohort had multiple pre-treatment specimens available for testing. PD-L1 expression was also heterogeneous across different pathologic specimens from a single patient. For the purpose of the aforementioned analysis where PD-L1 expression was correlated with response to anti-PD-1, a patient was considered PD-L1(+) if any of their specimens demonstrated tumor cell PD-L1 expression. For example, one melanoma patient who demonstrated a complete response had three different pre-treatment specimens available for study. The primary melanoma was PD-L1(+), and the lymph node and subsequent subcutaneous metastases were both PD-L1(−). By our methodology, the patient was considered PD-L1(+), due to PD-L1 expression of the primary tumor. Notably, if only one of the patient's latter specimens had been tested, and PD-L1 status was used as a selection criteria for PD-1/PD-L1 blockade, the patient would have been considered ‘PD-L1(−)’ and may have missed the opportunity to receive anti-PD-1.
Identifying and validating markers that could enrich for clinical response would have great significance for optimal therapeutic development. We, and now others 5-8 have demonstrated that PD-L1 expression in the tumor microenvironment enriches for response to anti-PD-1, though the association is not absolute. Uncertainty remains as to whether PD-L1 expression in a single pathologic specimen will routinely be used to pre-select individual patients for anti-PD1 therapy. Features such as the temporal and geographic heterogeneity of PD-L1 expression across specimens from a single patient call this approach into question. Our findings support the proposed mechanism of action of anti-PD-1 and suggest that study of the pre-treatment pathologic specimens may be used to help identify tumor types likely to respond to this therapy. Pre-treatment pathologic specimens will also likely be useful in identifying additional dominant or co-dominant pathways that may be targeted in combination with anti-PD-1 to further increase the proportion of patients who benefit from these exciting agents.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167-75; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New Engl J Med 2012; 366:2443-54; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1. Clin Can Res 2014; 20:5064-74. [Epub ahead of print]; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grosso J, Horak C, Inzunza D, Cardona D, Simon J, Gupta A, Sankar V, Park JS, Kollia G, Taube JM, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients with advanced solid tumors treated with nivolumab. J Clin Oncol 2013; 31 (suppl):Abstr nr 3016 [Google Scholar]
- 6. Daud HI, Hamid O, Ribas A, Hodi FS, Hwu WJ, Kefford R, Wolchok J, Hersey P, Weber JS, Joseph R, et al. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma: correlation of tumor PD-L1 expression with outcome. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5.9; San Diego, CA. Philadelphia (PA): AACR; 2014. Abstract nr CT104
- 7. Gandhi L, Balmanoukian A, Hui R, Hamid O, Rizvi NA, Leigh N, Gubens M, Goldman JW, Lubiniecki GM, Emancipator K, et al. MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): antitumor activity and association with tumor PD-L1 expression. In: Proceedings of the 105th Annual Meeting of the American Association for Cancer Research; 2014 Apr 5.9; San Diego, CA. Philadelphia (PA): AACR; 2014. Abstract nr CT105
- 8. Gettinger SN, Shepherd FA, Antonia SJ, Brahmer JR, Chow LQM, Juergens RA, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al. First line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: safety, efficacy, and correlation of outcomes with PD-L1 Status. J Clin Oncol 2014; 32(suppl):8024 [Google Scholar]