Abstract
Legionella pneumophila is a waterborne pathogen, and survival in the aquatic environment is central to its transmission to humans. Therefore, identifying genes required for its survival in water could help prevent Legionnaires' disease outbreaks. In the present study, we investigate the role of the sigma factor RpoS in promoting survival in water, where L. pneumophila experiences severe nutrient deprivation. The rpoS mutant showed a strong survival defect compared to the wild-type strain in defined water medium. The transcriptome of the rpoS mutant during exposure to water revealed that RpoS represses genes associated with replication, translation, and transcription, suggesting that the mutant fails to shut down major metabolic programs. In addition, the rpoS mutant is transcriptionally more active than the wild-type strain after water exposure. This could be explained by a misregulation of the stringent response in the rpoS mutant. Indeed, the rpoS mutant shows an increased expression of spoT and a corresponding decrease in the level of (p)ppGpp, which is due to the presence of a negative feedback loop between RpoS and SpoT. Therefore, the lack of RpoS causes an aberrant regulation of the stringent response, which prevents the induction of a successful response to starvation.
INTRODUCTION
Legionella pneumophila is the causative agent of Legionnaires' disease. It is widely distributed in natural freshwater systems (1) and readily colonizes man-made water systems such as cooling towers and water distribution systems (2). L. pneumophila persists in aquatic environments thanks to its ability to adapt to a variety of different ecological niches, either as an intracellular parasite of amoebae or ciliate protozoans, as a free-living member of complex biofilm communities, or as planktonic cells (3, 4). Amoebae support intracellular multiplication of L. pneumophila, and protect against suboptimal growth conditions and exposure to chlorine (5–7). Infection of HeLa cells and Tetrahymena tropicalis leads to the differentiation of L. pneumophila into mature infectious forms (MIFs), characterized by ultrastructural changes and accumulation of poly-β-hydroxybutyrate (8–10). MIFs are highly infectious and are more resistant to antibiotics and detergent than other forms (9, 11). Increased resistance to antibiotics was also described after incubation of L. pneumophila in Acanthamoeba castellanii buffer for 16 h (12). MIFs are able to resist starvation better than stationary-phase forms in encystment buffer (11), but both forms show similar levels of resistance in distilled water (8).
In the free-living state outside the amoebal host, L. pneumophila encounters stressful conditions, such as limited nutrient availability in aquatic systems (1, 13). Nevertheless, previous studies have shown that L. pneumophila is able to survive for long periods (up to 1.5 years) in sterilized tap, surface and estuarine waters (14–17). The genetic determinants underlying the ability of L. pneumophila to survive in its natural habitat of low-nutrient water for a long period are currently not well understood. Nevertheless, it was shown that the type II secretion system is required for survival in cold water (15).
In general, one important adaptive strategy to deal with changing conditions is the reorganization of the transcriptome in order to express genes necessary to cope with a new condition and to repress genes that are no longer required or whose expression would be deleterious (18). The sigma factor (σ) is a subunit of RNA polymerase that confers promoter specificity to the core enzyme for the initiation of transcription (19). Thus, the pool of σ factors within the cell is critical to modulate transcription patterns in response to a particular cell state and condition (18, 20, 21). The rpoS gene encodes the alternative σ factor RpoS, which is the master regulator of the general stress response in Escherichia coli (22). RpoS is involved in resistance to high osmolarity, acid, heat, and oxidative stress and starvation and in the expression of virulence factors (22). Activation of genes associated with survival under adverse conditions is often required for pathogenesis. Indeed, RpoS homologs have been identified in several pathogens, and yet their roles are variable among organisms (23, 24). Hales and Shuman (25) reported that RpoS expression increases in stationary phase in L. pneumophila similar to E. coli; however, they found that RpoS is not required for stationary phase-dependent resistance to different exogenous stresses in L. pneumophila (acid, oxidative stress, and high osmolarity). Nevertheless, RpoS is required for efficient intracellular multiplication in the protozoan hosts Acanthamoeba castellanii (25) and Acanthamoeba polyphaga (26), in murine bone marrow-derived macrophages (27), and in human monocyte-derived macrophages (26). RpoS is not required for replication in cultured human macrophage-like HL-60 and THP-1-derived cells, probably because of increased permissiveness of cultured mammalian host cells (25). RpoS, together with LetA/S and integration host factor, regulate the differentiation of L. pneumophila into MIFs (10, 28, 29). Comparison of the global transcriptional pattern between the wild type (WT) and the rpoS mutant in rich broth showed that RpoS controls multiple pathways associated with intracellular multiplication of L. pneumophila such as pathogenic functions, motility, transcriptional regulators, and Icm/Dot effectors (30). Interestingly, RpoS has a notable negative effect on genes associated with translation and metabolism during the postexponential phase (30).
Here, we examine the role played by the σ factor RpoS in promoting the survival of L. pneumophila in water in a free-living, planktonic state.
MATERIALS AND METHODS
Bacterial strains.
Media and antibiotics were used as previously described (31). The L. pneumophila strains used are derivatives of JR32, a streptomycin-resistant variant of the L. pneumophila strain Philadelphia-1 (31). All of the bacterial strains and plasmids are described in Table 1. The construction of the rpoS mutant strain was previously described in detail (25). The JR32 strain was used as the wild-type (WT) control. In experiments testing the relA spoT double mutant, the KS79 strain was used as the WT control, together with the JR32 strain.
TABLE 1.
Strains used in this study
| Strain or plasmid | Relevant genotypea | Source or reference |
|---|---|---|
| Strains | ||
| Legionella pneumophila | ||
| JR32 (WT) | Philadelphia-1; Smr; r− m+ | 31 |
| LM1376 (rpoS) | JR32 rpoS::Tn903dGent; Gmr | 25 |
| SPF176 (prpoS) | LM1376(pSF49) | This study |
| KS79 | JR32 ΔcomR | 78 |
| SPF197 (relA) | KS79 relA::aacC1; Gmr | This study |
| SPF204 (relA spoT) | SPF197 spoT::aptII; Gmr Kmr | This study |
| Escherichia coli | ||
| CF1648 (MG1655) | F− λ− ΔilvG rfb-50 rph-1 | 34 |
| CF1693 (MG1655 relA spoT) | ΔrelA251 ΔspoT207 | 34 |
| Plasmids | ||
| pMMB207c | Cmr; ΔmobA | 79 |
| pSF49 | rpoS in pMMB207c | This study |
Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Smr, streptomycin resistance; Kmr, kanamycin resistance.
Construction of complemented strains and the relA spoT double mutant.
The plasmid used for complementation of the rpoS mutation was constructed by cloning the rpoS gene in pMMB207c (32). The rpoS gene was first amplified by PCR using Taq polymerase (Invitrogen) and primers rpoS F and rpoS R that contain XbaI and EcoRI restriction sites, respectively. The rpoS amplicon and pMMB207c were digested with XbaI and EcoRI. The fragments were ligated using T4 DNA ligase (NEB) and transformed into E. coli DH5α. The resulting plasmid was transformed into the rpoS mutant strain by electroporation as previously described (33).
For the construction of the relA spoT double-mutant strain, the relA gene was deleted first, since a single spoT mutant is unviable due to its inability to degrade (p)ppGpp (34, 35). Deletion of relA was performed using primers described in Table 2. Briefly, primers relA5 and relA2 (containing EcoRI), and primers relA4 (containing PvuII) and relA6 were used to amplify a 1-kb fragment homologous to the upstream and downstream regions of the target gene, respectively. Primers relA1 and relA3 that contain EcoRI and PvuII, respectively, were used to amplify a gentamicin resistance cassette from the plasmid pBBR1MCS-5 (36). The three fragments were ligated by T4 DNA ligase (NEB) and the ligation mix (3 kb) was amplified by PCR using Phusion high-fidelity DNA polymerase (NEB). The 3-kb amplified fragment was then introduced into KS79 as described previously (30). The spoT mutation was constructed as described in the protocol above with three modifications: (i) primers spoT4 and spoT3 both contain XbaI restriction sites, (ii) primers spoT1 and spoT3 that contain EcoRI and XbaI restriction sites, respectively, were used to amplify a kanamycin resistance cassette from the plasmid pBBR1MCS-2 (36), and (iii) the resulting 3-kb fragment was introduced into the relA mutant background, resulting in the relA spoT double mutant. Allelic exchange was confirmed by PCR. The restriction sites described above are underlined in Table 2.
TABLE 2.
Primers used in this study
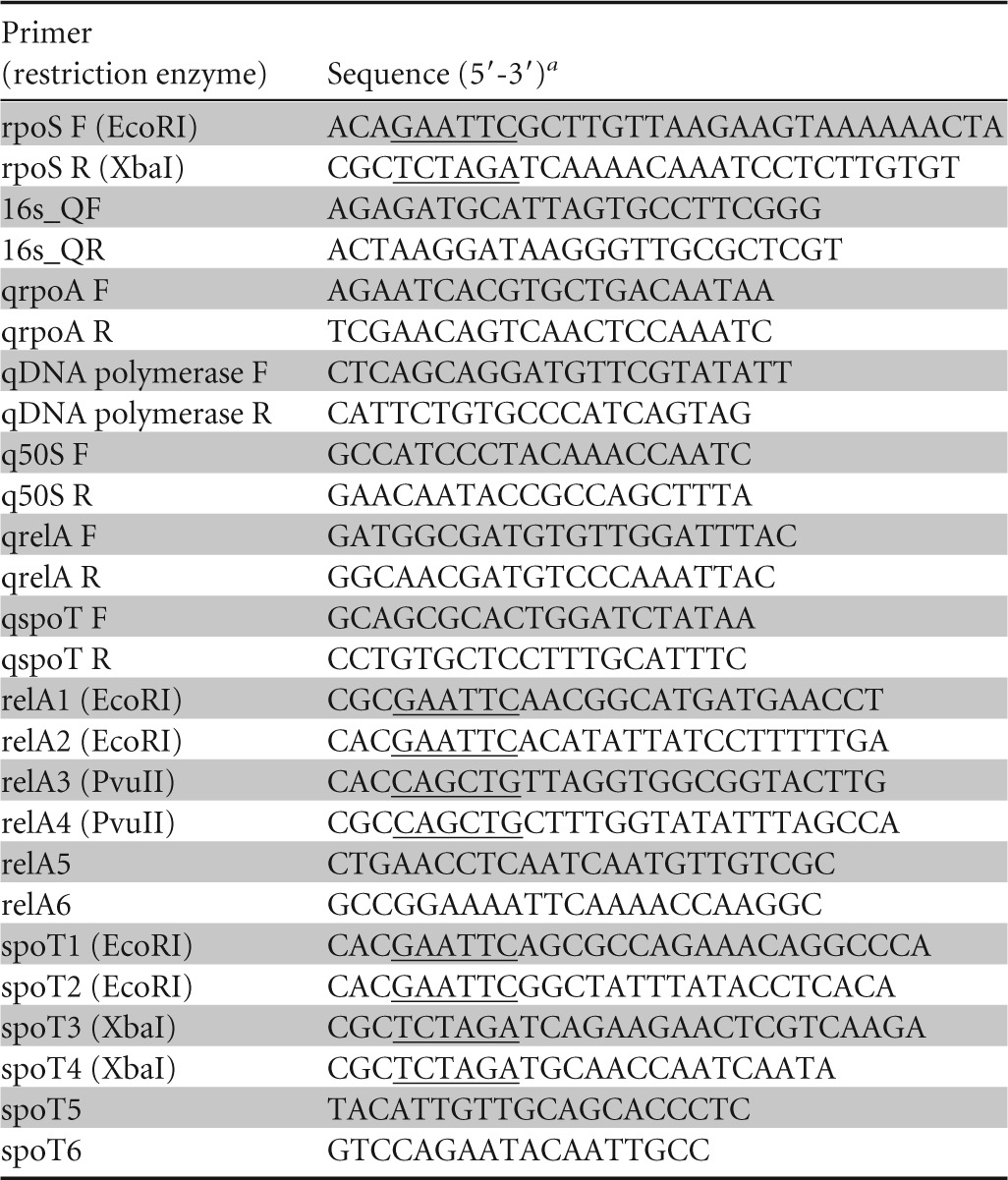
Restriction sites are indicated by underlining.
Survival in water.
The strains used in the present study were tested for survival in water. Since the composition of tap water is variable (14–17, 37, 38), experiments were carried out in the chemically defined water medium (DFM [NaCl, 50 mg/liter; KH2PO4, 20 mg/liter; KCl, 50 mg/liter]; pH 6.9) to ensure reproducibility. Survival was also tested in Fraquil [0.004% CaCl2, 0.004% MgSO4, 0.001% NaHCO3, 0.0002% K2HPO4, 0.004% NaNO3, 10 nM FeCl3, 1 nM CuSO4, 0.22 nM (NH4)6Mo7O24, 2.5 nM CoCl2, 23 nM MnCl2, 4 nM ZnSO4] and in tap water. Bacteria were suspended in DFM at an optical density at 600 nm (OD600) of 0.1, diluted 1:5 further with DFM, and then incubated for 1 month at 25°C. The CFU were determined every 7 days unless noted otherwise.
Live/Dead assay.
Bacteria were suspended in DFM at an OD600 of 0.1 and then incubated for 1 month at 25°C. Every 7 days, unless noted otherwise, bacterial viability was determined by using a Live/Dead BacLight kit in combination with flow cytometry. Cells were stained with 6 μM red fluorescing propidium iodide (PI) and 1 μM green fluorescing SYTO9. Stained cells were incubated for 15 min at room temperature in the dark. The fluorescence of each strain was then analyzed by flow cytometry (Guava easyCyte; 488-nm laser).
Transcriptomic study by microarray: samples and labeling.
The WT and the rpoS mutant strains were grown at 37°C in AYE to exponential phase (OD600 = 1), washed three times with DFM and suspended in 100 ml of DFM at a final OD600 of 0.1 in a tissue culture flask. After 24 h at 25°C, 100 ml of cells was pelleted by centrifugation, resuspended in 40 μl of Tris-EDTA, and lysed by the addition of 1 ml of TRIzol reagent. RNA extraction was performed with TRIzol reagent according to the manufacturer's protocol. The RNA was subsequently treated with Turbo DNase (Ambion) and purified by acid phenol extraction. The purity and concentration of RNA were determined by UV spectrophotometry and its integrity was confirmed on a formaldehyde-agarose gel. RNA from the replicates was pooled, and 15 μg of RNA was labeled with amino-allyl dUTP (Sigma) during reverse transcription (Superscript II; Invitrogen) using random hexamers (Invitrogen) as previously described (30, 39). Genomic DNA was used as a reference channel and labeled by random priming using Klenow fragments, amino-allyl dUTP, and random primers as described previously (39). DNA was subsequently coupled to the succinimidyl ester fluorescent dye (Invitrogen) Alexa Fluor 546 (for cDNA) or Alexa Fluor 647 (for gDNA) according to the manufacturer's protocols.
DNA microarray design.
One 50-mer oligonucleotide for each L. pneumophila gene was designed using the OligoWiz software version 2.2.0 (40, 41). The prokaryotic setting was used with default parameters. The microarray was then produced by photolithography by Mycroarray (Ann Arbor, MI). Each probe is replicated six times on the array, and negative and positive probes designed by Mycroarray were also included. The platform is available under GEO accession number GPL18472.
Hybridization, washing, and data analysis.
Hybridization was performed as previously described (39), and data were acquired using an InnoScan 710 microarray scanner. Statistical analysis between the rpoS mutant strain and the wild-type control was performed using an unpaired one-tailed Student t test. Genes were considered differentially expressed if they demonstrated a ratio-to-control value of ±2-fold with a P value of <0.05.
qPCR.
For analysis of gene expression by reverse transcription-quantitative PCR (RT-qPCR), RNA was extracted from strains exposed to DFM as described above. One microgram of RNA was then converted to cDNA by using random primers and Superscript II (Invitrogen) according to the manufacturer's instructions. For each sample, a negative control without reverse transcriptase was used. qPCRs were then performed with 1 μl of cDNA using iTaq Universal SYBR green Supermix (Bio-Rad). Primer sets used for real-time PCR analysis are listed in Table 2. A relative quantification strategy was used to perform analysis of the qPCR data. Transcript levels were normalized to 16S rRNA in each sample, and the fold change was calculated as previously described (42).
Rifampin sensitivity.
Different concentrations (0, 1, 12, and 25 μg ml−1) of rifampin were added to 24-h-old DFM-exposed bacteria, followed by incubation at 25°C. After 24 h, the CFU counts were determined.
De novo RNA synthesis.
To quantify newly transcribed RNA in each strain, cells exposed to DFM for 24 h were fed with 1 mM the uridine analog 5-ethynyl uridine (EU) for 1 h. Cells were fixed with 3.7% formaldehyde for 15 min at room temperature and permeabilized with 0.5% Triton X-100 for 15 min at room temperature. The modified RNA was then detected in situ with the Alexa Fluor 488 dye according to the manufacturer's instructions for the Click-iT RNA imaging assay (Invitrogen) (43). Cells fed with EU but not labeled with Alexa Fluor 488 served as a negative control to measure the autofluorescence of L. pneumophila cells. Stained cells were then analyzed by flow cytometry (FACSCanto II; 488-nm laser) by comparing the amounts of fluorescently labeled cells for each strain. The data were collected and analyzed using the FlowJo software. A total of 50,000 cells were analyzed for each sample.
(p)ppGpp quantification.
The presence of (p)ppGpp was detected with a fluorescent chemosensor, PyDPA, selective for (p)ppGpp (44). To ensure that PyDPA can be used with L. pneumophila, the WT and the relA spoT double mutant were grown to exponential phase in rich medium (AYE). The cultures were then split in two, and the stringent response was induced in half of the cultures by the addition of 500 μM serine hydroxamate (SHX), followed by incubation for 1 h. L. pneumophila strains, as well as the E. coli control strains (WT and relA spoT double mutant), were also exposed to DFM for 24 h (OD600 = 1). Next, 2 ml of the cells from AYE (with or without the addition of SHX) or cells from DFM was harvested by centrifugation at 8,000 × g for 5 min at 4°C and resuspended in 0.5 ml of ice-cold 13 M formic acid (45). After incubation for 30 min at 4°C, cellular debris was pelleted by centrifugation at 8,000 × g for 15 min at 4°C. An equal volume of phenol-chloroform-isoamyl alcohol (50:49:1 [vol/vol/vol]), saturated with 1 M Tris-HCl (pH 6.6), was added to the resulting supernatant, and the mixture was agitated and then centrifuged at 8,000 × g for 15 min at 4°C (46). This supernatant was concentrated by freeze-drying (47). The dried extracts were resuspended in 200 μl of 1 mM HEPES buffer (pH 7.4) containing 16% (vol/vol) dimethyl sulfoxide (47). PyDPA was added to a final concentration of 20 μM to 100 μl of supernatant. In the presence of (p)ppGpp, PyDPA emits a strong pyrene-excimer fluorescence (excitation, 344 nm; emission, 470 nm), while in the presence of nucleotides (ATP, GTP, CTP, TTP, UTP, cAMP, or cGMP) and inorganic pyrophosphate (PPi), PyDPA forms a pyrene monomer fluorescence (excitation, 344 nm; emission, 380 nm). The ratio of fluorescence emissions (I470 nm/I380 nm) was detected by using a Tecan microplate reader. For each sample, a negative control without PyDPA was carried out to determine the background fluorescence emissions at 470 and 380 nm.
Nucleotide sequence accession number.
The sequence data are available from the GEO database (GSE56258 ).
RESULTS
Survival of L. pneumophila in water.
Numerous studies have shown that L. pneumophila is able to survive in tap water for extended periods of time; however, differences in the survival times were reported, presumably because of local differences in the composition of tap water (14–17, 37, 38). One of the projects conducted in our group consisted in developing an artificial freshwater medium to increase the reproducibility of the survival assay. One such medium, here called DFM, is based on previous publications reporting the use of defined freshwater recipes and on the salt and buffer content of the chemically defined medium developed for L. pneumophila (48, 49). As part of the project, we tried to identify genetic determinants underlying the ability of L. pneumophila to survive in water by exposing several mutant strains from our collection to DFM and determined survivability by CFU counts. A strain carrying a transposon insertion in rpoS showed reduced survival in DFM compared to the WT strain (Fig. 1). To confirm that the observed phenotype was due to inactivation of RpoS, the rpoS gene was cloned downstream of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter (Ptac) of the pMMB207c vector (prpoS). Partial recovery of the WT phenotype was observed in the complemented strain induced with 0.1 mM IPTG (prpoS ON). Survival of the complemented strain not exposed to IPTG (prpoS OFF) was significantly lower than that of the WT and approximated that of the rpoS mutant (Fig. 1). prpoS OFF exhibited a slightly higher survival rate than the rpoS mutant, probably because of partial complementation due to leakage of the Ptac promoter (32). These results confirm that the survival defect observed is rpoS dependent. The reduction in CFU counts could be due to the death of the rpoS mutant cells or their premature entry into a viable but nonculturable state (reviewed in reference 50). Therefore, we assessed bacterial viability by using a Live/Dead BacLight kit in combination with flow cytometry (51). The viability of the rpoS mutant strain decreases over time proportionally to the reduction in CFU (Fig. 1B), showing that in the absence of rpoS, L. pneumophila cells die in DFM. To determine whether this survival defect could be seen in other types of water or if it is specific for DFM, the survival of the WT and the rpoS mutant strain was tested in Fraquil, a defined freshwater medium that approximates the composition of freshwater of North America (52), and tap water. The rpoS mutant was defective for survival in both Fraquil and tap water (Fig. 1C), although the reduction in tap water was slower than that in Fraquil and DFM. Therefore, it seems that the rpoS mutant is defective for survival in water in general.
FIG 1.

RpoS is necessary for survival in water. (A) L. pneumophila wild-type (WT), rpoS mutant (rpoS), and the complemented strains induced with 0.1 mM IPTG (prpoS ON) and not induced with IPTG (prpoS OFF) were tested for survival in defined freshwater medium (DFM) for 28 days at 25°C. CFU were counted every week on CYE plates. (B) Cells retrieved from DFM were stained with 6 μM PI and 1 μM green fluorescing SYTO9 and then analyzed for cell viability with flow cytometry. (C) The wild-type (WT) and the rpoS mutant (rpoS) were tested for survival in Fraquil and in tap water as described in panel A. DL, detection limit. Error bars represent the standard deviations from three independent biological replicates. We used an unpaired Student t test to assess statistical significance for each time point. *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005 (versus WT).
rpoS deficiency leads to overexpression of numerous genes.
We hypothesized that the lack of rpoS could lead to a misregulation of genetic factors involved in survival in water, causing the observed defect. DNA microarray technology was used to analyze the regulatory network of the rpoS mutant in water. RNA samples used for microarray analysis were isolated from three replicates of both the WT and the rpoS mutant after exposure to DFM for 24 h. Since the mutant strain dies in DFM, we chose this early time point to ensure that enough RNA could be extracted (Fig. 1). The normalized signal intensity of differentially expressed genes is shown in Fig. 2A (see also Table S1 in the supplemental material). Microarray analysis showed that, compared to the WT, the lack of RpoS significantly affects 668 genes, 506 positively (induced in the mutant) and 162 negatively (repressed in the mutant).
FIG 2.
Transcriptional response of the rpoS mutant after 24 h in water. The global gene expression profile of the rpoS mutant was compared to the WT strain after exposure to DFM for 24 h. (A) Hierarchical clustering of genes showing a ratio to control value of ±2-fold and a P value of ≤0.005. The normalized signal intensity is shown. The cluster of genes induced in the rpoS mutant is shown by a red line; the cluster of genes repressed is shown by a green line. (B) Cluster of orthologous group (COG) analysis of the microarray data. (C) RT-qPCR was used to confirm the expression of genes related to translation (50S ribosomal protein L25 gene, lpg0002), replication (DNA polymerase III gene, lpg2652), and transcription (rpoA, lpg0354). Strains are described in Fig. 1. The data are presented as means of three biological replicates, and the error bars represent the standard deviations. Since the data represent a ratio against the WT strain, we used the nonparametric Mann-Whitney test to assess statistical significance. *, P ≤ 0.05.
An analysis of the Clusters of Orthologous Groups (COG) protein database revealed that, in most categories, more genes were induced in the rpoS mutant than repressed, such as in the “icm/dot effector,” “toxin production/other pathogen function,” “energy metabolism,” “transcription,” “translation,” and “replication and repair” categories shown in Fig. 2B.
The expression of three genes was tested by RT-qPCR, including the RNA polymerase gene rpoA (lpg0354), the DNA polymerase III beta chain gene (lpg0002), and the 50S ribosomal protein L25 gene (lpg2652) involved in transcription, replication, and translation, respectively. Consistent with the microarray data, qPCR analysis showed that the expression of these genes increased in the rpoS mutant strain compared to the WT (Fig. 2C). Their expression in the complemented strain (prpoS ON) was similar to that of the WT. The increased expression of genes associated with transcription, replication, and translation, in the absence of RpoS, indicates that the expression of these genes is negatively controlled by RpoS, possibly through gene products that it regulates, such as transcriptional regulators or small regulatory RNAs (53).
Lack of RpoS results in a higher level of transcription.
The overexpression of several genes in the rpoS mutant, together with the overexpression of many genes involved in transcription, led us to hypothesize that the rpoS mutant suffers from an increase in the overall level of transcription, wasting metabolites and energy, and leading to the premature reduction in CFU counts observed in Fig. 1. In addition, the total RNA quantification revealed that an ∼2-fold increase was observed in the amount of RNA extracted by TRIzol from the rpoS mutant over that from the WT after exposure to DFM for 24 h (Fig. 3A). The sensitivity of the WT strain and the rpoS mutant to rifampin, which blocks RNA synthesis (54), was then tested. Since most antibiotics exert their effects on processes that are required for cell growth, their effect is usually proportional to the level of activity of their target (55), and thus rifampin is more potent against transcriptionally active bacteria. After 24 h of exposure to DFM, the rpoS mutant was significantly more sensitive to rifampin compared to the WT (Fig. 3B). The complemented strain showed a WT phenotype (Fig. 3B). These results are consistent with the hypothesis that the rpoS mutant undergoes more transcription than the WT. Nevertheless, there is a possibility that the lack of rpoS may affect cell wall synthesis or resistance mechanisms leading to increased lysis by TRIzol and increased sensitivity to antibiotics and therefore to the apparent increase in RNA level and rifampin sensitivity.
FIG 3.

The rpoS mutant shows a higher transcription level. (A) Total RNA was extracted from the WT and the rpoS mutant strain after 24 h in DFM and quantified by spectrophotometry. Error bars represent the standard deviations from three independent biological replicates. (B) Comparison of rifampin sensitivities of the rpoS mutant and the WT in DFM. Four concentrations of rifampin (0, 1, 12, and 25 μg/ml) were added to the DFM, which were then incubated at 25°C for 24 h. The data are presented as the means of three biological replicates, and the error bars represent the standard deviations. We used an unpaired Student t test to assess statistical significance. *, P = 0.05; **, P ≤ 0.005 (versus WT). DL, detection limit.
To confirm our hypothesis, we quantified the RNA synthesized de novo in the WT, the rpoS mutant, and the complemented strains by using the Click-iT RNA Alexa Fluor 488 imaging kit (Molecular Probes). Cells were exposed to water and fed for 1 h with 5-ethynyl uridine (EU), which is incorporated into nascent RNA. The cells were then fixed and permeabilized before the modified RNA was labeled with Alexa Fluor 488. To ensure that the difference in signals would be due to a difference in transcription and not to a difference in permeability, the cells were also stained with PI, which only enters cells with damaged cytoplasmic membranes (56). There were no differences in PI staining between the strains tested, whether they were stained before or after the fixation and permeabilization step (see Fig. S1 in the supplemental material). After 24 h in water, the rpoS mutant showed more Alexa Fluor 488 fluorescence than did the WT strain, which indicates a higher level of EU incorporation into new RNA molecules and, consequently, a higher level of transcription (Fig. 4A and B). The complemented strain showed a WT phenotype. Interestingly, the strains grown in rich broth to exponential phase showed no significant difference in staining, confirming that the increased level of transcription in the rpoS mutant strain is specific to water exposure and, consequently, to nutrient starvation (Fig. 4C and D). Taken together, our results clearly show that the rpoS mutant strain displays an increased transcription level compared to the WT when exposed to nutrient-poor water.
FIG 4.
The rpoS mutant shows higher de novo RNA synthesis in DFM. Cells exposed to DFM for 24 h or grown in broth (AYE) to exponential phase were fed for 1 h with the uridine analog 5-ethynyl uridine (EU), which is actively incorporated into nascent RNA. The cells were then fixed and permeabilized and the modified RNA was labeled with Alexa Fluor 488 dye using a Click-iT RNA imaging assay (Invitrogen). Stained cells were analyzed by flow cytometry (FACSCanto II; 488-nm laser). A total of 50,000 cells were analyzed for each sample. The fluorescence histograms of typical experiments performed in DFM (A) and in broth AYE (C) are shown. The horizontal bar represents the signal of Alexa Fluor 488 considered positive (above autofluorescence noise). The geometric mean from three independent experiments performed in DFM (B) and in broth (AYE) (D) was calculated for Alexa Fluor 488 positive cells. Cells fed with EU but not labeled with Alexa Fluor 488 served as a negative control to assess autofluorescence noise (Autofluo). Error bars represent the standard deviations from three independent biological replicates. We used a paired Student t test to assess statistical significance. Strains are described in Fig. 1.
(p)ppGpp is necessary for survival in water.
Traxler et al. (57) showed that in response to amino acid starvation, an E. coli mutant strain lacking (p)ppGpp shared a similar phenotype as our rpoS mutant strain in producing significantly more RNA than the WT strain. Based on this result and knowing that RpoS is one of the key effectors of the stringent response (58), we sought to further investigate the involvement of the stringent response in the RpoS regulatory network associated with survival in water. In most bacteria, the alarmone (p)ppGpp lies at the top of the network that governs global gene expression in response to nutrient limitation (59). In most gammaproteobacteria, including L. pneumophila, the (p)ppGpp synthetase RelA monitors amino acid availability through its association with the ribosome, whereas the bifunctional synthetase and hydrolase SpoT responds to a variety of stimuli, including fatty acid starvation, which requires direct interaction with an acyl-carrier protein (26, 35, 45, 60, 61).
In the present study, the DFM water represents a nutrient-poor condition; thus, we expected that cells unable to produce (p)ppGpp would be unable to survive in water. Indeed, as shown in Fig. 5A, the relA spoT double-knockout mutant showed a quick decrease in survival in water compared to the WT strain. The viability of the relA spoT double-mutant strain decreases over time proportionally to the reduction in CFU (Fig. 5B), showing that in the absence of (p)ppGpp, L. pneumophila cells die in water.
FIG 5.

The stringent response is required for survival in water and is affected by mutation of rpoS. (A) The stringent response mutant strain (relA spoT) and the wild-type strain (WT) were tested for survival in defined water (DFM) for 30 days at 25°C. CFU were counted every week on CYE plates. DL, detection limit of the CFU. (B) Cells retrieved from DFM were stained with 6 μM PI and 1 μM green fluorescing SYTO9 and then analyzed for cell viability with flow cytometry. We used an unpaired Student t test to assess statistical significance for each time point. **, P ≤ 0.005 (versus WT). (C) RT-qPCR was used to determine the level of expression of spoT and relA compared to the WT strain. The microarray data for the expression of spoT and relA (rpoS/WT) are indicated by black bars. The data are presented as the means of three biological replicates, and the error bars represent the standard deviations. Since the data represent a ratio against the WT strain, we used the nonparametric Mann-Whitney test to assess statistical significance. *, P ≤ 0.05 (versus WT).
Lack of RpoS results in higher expression of spoT and a reduction of the (p)ppGpp level.
At this point, we hypothesized that the lack of RpoS in water somewhat influences the stringent response circuitry in L. pneumophila which would, in turn, disturb the level of (p)ppGpp. The transcriptome of the rpoS mutant during exposure to water showed an increased expression of spoT, whereas the levels of expression of relA were similar in both strains (Fig. 5C, black bar). We used RT-qPCR to monitor the expression of the spoT and relA genes in the rpoS mutant, the WT, and the complemented strain after 24 h of exposure to DFM. In agreement with the microarray analysis, spoT was overexpressed in the rpoS mutant, and complementation restored the WT expression level (Fig. 5C). No significant difference was observed for the relA gene. Taken together, these observations strongly support our hypothesis that the stringent response is affected when RpoS is absent. The upregulation of the spoT gene in the rpoS mutant suggests that the (p)ppGpp level would, in turn, be affected. An increased expression of spoT in the rpoS mutant strain might decrease the level of (p)ppGpp through its hydrolase activity. To test this hypothesis, we determined the level of (p)ppGpp in the rpoS mutant. The (p)ppGpp assay was performed using PyDPA, a selective fluorescent chemosensor for (p)ppGpp (44). Extraction of nucleotides from bacterial cells was carried out with 13 M formic acid as described previously (45, 46), further purified with phenol-chloroform (46), and concentrated by freeze-drying (47). The production of (p)ppGpp after exposure of exponential-phase L. pneumophila to SHX for 1 h was measured first to ensure that PyDPA can be used for L. pneumophila. SHX is a serine analog that causes amino acid starvation and induces (p)ppGpp synthesis (62). Exposure of the WT strain to SHX leads to an increase in the signal, but exposure of the relA spoT double mutant did not (Fig. 6A). This shows that production of (p)ppGpp from L. pneumophila can be successfully detected with PyDPA. Then, the production of (p)ppGpp was measured after exposure to DFM for 24 h, at which point the rpoS mutant strain showed increased transcription (Fig. 2, 3, and 4). The E. coli WT and relA spoT double mutant, as well as the L. pneumophila WT and relA spoT double mutant, were used as a control for this assay. As expected, the relA spoT double mutants showed significantly less (p)ppGpp than did the WT strain (Fig. 6B). As hypothesized, we found that the rpoS mutant strain has a significantly lower level of (p)ppGpp than the WT strain (Fig. 6B). The complemented strain (prpoS ON) was found to produce more (p)ppGpp than the WT (Fig. 6B). Taken together, our results clearly show a link between the repression of spoT by RpoS and a higher level of (p)ppGpp in the WT. Therefore, it seems likely that a lower level of (p)ppGpp accumulation upon starvation in water is causing the survival defect of the rpoS mutant strain.
FIG 6.

Production of ppGpp in the rpoS mutant is reduced. Quantification of the (p)ppGpp level was performed by using PyDPA, a selective fluorescent chemosensor for (p)ppGpp. (A) The WT and relA spoT double-mutant strains were grown to exponential phase in AYE broth in triplicate. The cultures were split in two, and SHX was added to one tube. Samples were collected after 1 h, and (p)ppGpp was quantified by using PyDPA. The data are presented as the ratio between SHX-treated cells (+SHX) and the untreated control (−SHX). (B) Cells were exposed to DFM for 24 h. The (p)ppGpp was then extracted and quantified with PyDPA. The strains are described in Fig. 1 and Fig. 5, except for the E. coli wild-type (E. coli WT) and the E. coli stringent response mutant (E. coli relA spoT). The data are presented as ratios against the wild-type strain of three replicates, and the error bars represent the standard deviations. Since the data represent a ratio, we used the nonparametric Mann-Whitney test to assess statistical significance. *, P ≤ 0.05 (versus WT L. pneumophila [L. pneumophila mutants] or WT E. coli [E. coli mutant]). DL, detection limit.
DISCUSSION
L. pneumophila is commonly found in natural and man-made water systems. Our goal was to find genetic determinants involved in the survival of L. pneumophila in water. Hence, in the present study, we report the role that the σ factor RpoS plays in promoting survival in water, where L. pneumophila experiences severe nutrient deprivation. As expected, the lack of RpoS led to a decreased survival in water (Fig. 1), indicating that RpoS is a key regulator in L. pneumophila adaptation to the starving conditions found in water. It is possible that RpoS is also necessary for survival in rich broth after the postexponential phase has been reached, but this was not tested here since this possibility does not have any implication for the transmission of L. pneumophila from the water environment to the human host.
Several metabolic systems are induced in the rpoS mutant, such as transcription, translation, and replication (Fig. 2B and C). Our results are in agreement with those of Hovel-Miner et al. (30), who examined the expression profile of the L. pneumophila rpoS mutant during postexponential-phase growth in rich broth. Their study also showed that RpoS has notable negative effects on the transcription of genes associated with translation and metabolism such as those encoding ribosomal proteins, tRNA synthesis genes, and tRNA genes. In addition, we found that the rpoS mutant displays an accumulation of de novo-synthesized RNA (Fig. 4A and B), which means that upon exposure to water, L. pneumophila reduces transcription through negative regulation by RpoS. Consequently, the rpoS mutant showed reduced resistance to rifampin (Fig. 2B). Similarly, MIFs show an increased resistance to rifampin, presumably because of the lower transcription rate (8), and RpoS is necessary for the differentiation of L. pneumophila into MIFs (10).
For the model organism E. coli, RpoS is essential for the transcription of genes important for cell survival under conditions leading to the switch from the fully active to the slow metabolic state, as in the case of nutrient starvation (63). Among the many genes in the RpoS regulon, some are directly regulated by RpoS and others might be indirectly regulated through transcription factors (activators and repressors) and other σ factors (64).
The signaling alarmone guanosine tetraphosphate [(p)ppGpp] is a key player in the reduction of the transcription level during the postexponential phase in E. coli (65, 66). In most bacteria, nutrient depletion prompts a restructuring of global transcription patterns known as the stringent response (60, 67). (p)ppGpp is the general indicator of the nutritional status of the cell, and it lies at the top of the regulatory network of the stringent response (59, 60). In Gram-negative bacteria, including L. pneumophila, (p)ppGpp is predominantly produced by RelA, and the balance of (p)ppGpp is regulated by SpoT (60, 68), which mediates (p)ppGpp turnover via its hydrolase activity and weak synthase activity (34, 45). In E. coli, a mutant lacking relA and spoT is completely devoid of (p)ppGpp (69), a state that disrupts repression of rRNA transcription (70) and results in the synthesis of stable RNA (69), similar to the phenotype observed here in the rpoS mutant of L. pneumophila. Therefore, we confirmed that (p)ppGpp is necessary for survival of L. pneumophila in water by testing the relA spoT double mutant, which lacks all (p)ppGpp synthetase activity (45) (Fig. 5A and B). This mutant also showed a survival defect after a 60 h of incubation in rich medium (45).
In E. coli, it was shown that RpoS is one of the key regulators activated in response to a high level of (p)ppGpp (58). Control exerted by (p)ppGpp on RpoS is observed at several levels: gene transcription (71), regulon induction (58), protein stabilization (72), and σ factor competition (73). As with E. coli, the transcription of rpoS in L. pneumophila is sensitive to the (p)ppGpp level, since rpoS is induced in response to (p)ppGpp accumulation (74). Although the stringent response and RpoS are major virulence regulators in L. pneumophila, the link between RelA, SpoT, and RpoS remains to be clarified. During infection of host cells, L. pneumophila goes through two distinct phases: the replicative phase, characterized by active replication, and the transmissive phase, characterized by inhibition of replication and activation of motility and cytotoxicity (75). RelA activity is essential in inducing the transmissive phase and is dispensable during the replicative phase (35). Two studies reported the role of RelA in L. pneumophila in the postexponential phase, which mimics the transmissive phase. Zusman et al. (35) showed that a relA mutant made in JR32, which is the WT strain used here, does not produce detectable levels of (p)ppGpp, whereas Dalebroux et al. (45) showed that a relA mutant made in Lp02 still produces an extremely small amount of (p)ppGpp. Genetic differences or experimental variation could explain this apparent discrepancy. It was established that, in L. pneumophila, RelA produces (p)ppGpp in response to limiting amino acids, while SpoT produces (p)ppGpp in response to perturbation in fatty acid biosynthesis (45, 76). SpoT also regulates (p)ppGpp degradation and thus, it is critical for transmissive cells to reenter the replicative phase (45). To our knowledge, the mechanism controlling the synthetase and hydrolase activity of SpoT is currently not understood but could involve interactions with acyl-carrier proteins or other proteins (26, 35, 45, 60).
In the present study, we showed a new connection between RpoS and the stringent response effector (p)ppGpp associated with survival of L. pneumophila in water. As shown in both microarray and RT-qPCR analyses (Fig. 5C), expression of the spoT gene is increased in the rpoS mutant strain. This phenotype is confirmed by complementation, which restored the WT expression level. As a result, we found that the rpoS mutant strain has a significantly lower level of (p)ppGpp compared to that of the WT, and complementation of the rpoS mutant restored the WT phenotype (Fig. 6B). Overexpression of spoT would presumably overcome the regulatory mechanism controlling the synthetase and hydrolase functions of SpoT, which, according to our data, will ultimately result in an increase in its hydrolase activity. This is supported by a recent study reporting that overexpression of spoT in Salmonella enterica serovar Typhimurium drastically reduces the amount of (p)ppGpp and rescues the growth defect of a tufA499 mutant, which produces high levels of (p)ppGpp (77). These findings highlight the negative effect of RpoS on the expression level of spoT that helps maintain a high level of (p)ppGpp to efficiently cope with the nutritional limitations encountered upon exposure to water. The molecular mechanism behind this negative feedback loop is currently unknown, but could involve RpoS-dependent regulation of a negative regulator of spoT.
In light of our data, we propose the following model that might explain the survival defect of the rpoS mutant strain. Upon exposure to water, L. pneumophila would accumulate (p)ppGpp at a level sufficient to induce the RpoS regulatory network which would, in turn, adjust the gene expression program by reducing metabolic processes in favor of survival. Then, RpoS reduces the expression of spoT, probably through an indirect mechanism such as the induction of a negative regulator of spoT. This negative feedback loop ensures that (p)ppGpp is not degraded by SpoT and that the alarmone level is high enough to fully induce the stringent response. Nevertheless, the manner in which (p)ppGpp relays information to RpoS and the manner in which RpoS-mediated regulation is integrated into the stringent response in L. pneumophila remain unclear and will require further study.
To conclude, our study challenges the current paradigm that RpoS is merely a messenger of the stringent response and instead supports the hypothesis that RpoS is an active player in the induction and maintenance of the stringent response by regulating the expression of SpoT and, consequently, the level of (p)ppGpp itself. This proposed pathway could be present in other bacterial species as well.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the NSERC Discovery grant 418289-2012 and John R. Evans Leaders Fund/Funding for Research Infrastructure from the Canadian Foundation for Innovation to S.P.F. The synthesis of PyDPA was supported by an NRF grant to J.-I.H. funded by the MEST (grant 2009-0080734).
We thank Nilmini Mendis for critical reading of the manuscript, Serge Sénéchal from the University of Montreal for technical assistance with the flow cytometry, and Robert Williams from McGill University for technical assistance with the freeze-drying. The E. coli relA spoT double-mutant strain was kindly provided by Dao Nguyen.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03132-14.
REFERENCES
- 1.Fliermans C, Cherry W, Orrison L, Smith S, Tison D, Pope D. 1981. Ecological distribution of Legionella pneumophila. Appl Environ Microbiol 41:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.States SJ, Conley LF, Towner SG, Wolford RS, Stephenson TE, McNamara AM. 1987. An alkaline approach to treating cooling towers for control of Legionella pneumophila. Appl Environ Microbiol 53:1775–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields BS. 1996. The molecular ecology of legionellae. Trends Microbiol 4:286–290. doi: 10.1016/0966-842X(96)10041-X. [DOI] [PubMed] [Google Scholar]
- 4.Rogers J, Keevil C. 1992. Immunogold and fluorescein immunolabeling of Legionella pneumophila within an aquatic biofilm visualized by using episcopic differential interference contrast microscopy. Appl Environ Microbiol 58:2326–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaree JM, Fields BS, Feeley JC, Gorman GW, Martin WT. 1986. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol 51:422–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker J, Brown M, Collier PJ, Farrell I, Gilbert P. 1992. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol 58:2420–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilvington S, Price J. 1990. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Microbiol 68:519–525. [DOI] [PubMed] [Google Scholar]
- 8.Garduno RA, Garduno E, Hiltz M, Hoffman PS. 2002. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infect Immun 70:6273–6283. doi: 10.1128/IAI.70.11.6273-6283.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faulkner G, Garduño RA. 2002. Ultrastructural analysis of differentiation in Legionella pneumophila. J Bacteriol 184:7025–7041. doi: 10.1128/JB.184.24.7025-7041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faulkner G, Berk SG, Garduño E, Ortiz-Jiménez MA, Garduño RA. 2008. Passage through Tetrahymena tropicalis triggers a rapid morphological differentiation in Legionella pneumophila. J Bacteriol 190:7728–7738. doi: 10.1128/JB.00751-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koubar M, Rodier MH, Garduño RA, Frère J. 2011. Passage through Tetrahymena tropicalis enhances the resistance to stress and the infectivity of Legionella pneumophila. FEMS Microbiol Lett 325:10–15. doi: 10.1111/j.1574-6968.2011.02402.x. [DOI] [PubMed] [Google Scholar]
- 12.Bandyopadhyay P, Xiao H, Coleman HA, Price-Whelan A, Steinman HM. 2004. Icm/Dot-independent entry of Legionella pneumophila into amoeba and macrophage hosts. Infect Immun 72:4541–4551. doi: 10.1128/IAI.72.8.4541-4551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.States SJ, Wadowsky RM, Kuchta JM, Wolford RS, Conley LF, Yee RB. 1994. Legionella in drinking water, p 340–367. In McFeters GA. (ed), Drinking water microbiology: progress and recent developments. Springer-Verlag, New York, NY. [Google Scholar]
- 14.Paszko-Kolva C, Shahamat M, Colwell RR. 1992. Long-term survival of Legionella pneumophila serogroup 1 under low-nutrient conditions and associated morphological changes. FEMS Microbiol Lett 102:45–55. doi: 10.1111/j.1574-6968.1992.tb05794.x. [DOI] [Google Scholar]
- 15.Söderberg MA, Dao J, Starkenburg SR, Cianciotto NP. 2008. Importance of type II secretion for survival of Legionella pneumophila in tap water and in amoebae at low temperatures. Appl Environ Microbiol 74:5583–5588. doi: 10.1128/AEM.00067-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skaliy P, Mceachern HV. 1979. Survival of the Legionnaires' disease bacterium in water. Ann Intern Med 90:662–663. doi: 10.7326/0003-4819-90-4-662. [DOI] [PubMed] [Google Scholar]
- 17.Schofield GM. 1985. A note on the survival of Legionella pneumophila in stagnant tap water. J Appl Microbiol 59:333–335. [DOI] [PubMed] [Google Scholar]
- 18.Ishihama A. 2000. Functional modulation of Escherichia coli RNA polymerase. Annu Rev Microbiol 54:499–518. doi: 10.1146/annurev.micro.54.1.499. [DOI] [PubMed] [Google Scholar]
- 19.Wösten M. 1998. Eubacterial sigma-factors. FEMS Microbiol Rev 22:127–150. doi: 10.1016/S0168-6445(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 20.Gruber TM, Gross CA. 2003. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol 57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- 21.Navarro Llorens JM, Tormo A, Martínez-García E. 2010. Stationary phase in gram-negative bacteria. FEMS Microbiol Rev 34:476–495. doi: 10.1111/j.1574-6976.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 22.Dong T, Schellhorn HE. 2010. Role of RpoS in virulence of pathogens. Infect Immun 78:887–897. doi: 10.1128/IAI.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsén A, Arnqvist A, Hammar M, Sukupolvi S, Normark S. 1993. The RpoS Sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol 7:523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- 24.Waterman SR, Small P. 1996. Identification of σs-dependent genes associated with the stationary-phase acid-resistance phenotype of Shigella flexneri. Mol Microbiol 21:925–940. doi: 10.1046/j.1365-2958.1996.00058.x. [DOI] [PubMed] [Google Scholar]
- 25.Hales LM, Shuman HA. 1999. The Legionella pneumophila rpoS gene is required for growth within Acanthamoeba castellanii. J Bacteriol 181:4879–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu-Zant A, Asare R, Graham JE, Kwaik YA. 2006. Role for RpoS but not RelA of Legionella pneumophila in modulation of phagosome biogenesis and adaptation to the phagosomal microenvironment. Infect Immun 74:3021–3026. doi: 10.1128/IAI.74.5.3021-3026.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachman MA, Swanson MS. 2001. RpoS co-operates with other factors to induce Legionella pneumophila virulence in the stationary phase. Mol Microbiol 40:1201–1214. doi: 10.1046/j.1365-2958.2001.02465.x. [DOI] [PubMed] [Google Scholar]
- 28.Morash MG, Brassinga AKC, Warthan M, Gourabathini P, Garduno RA, Goodman SD, Hoffman PS. 2009. Reciprocal expression of integration host factor and HU in the developmental cycle and infectivity of Legionella pneumophila. Appl Environ Microbiol 75:1826–1837. doi: 10.1128/AEM.02756-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitre CA, Tanner JR, Patel P, Brassinga AKC. 2013. Regulatory control of temporally expressed integration host factor (IHF) in Legionella pneumophila. Microbiology 159:475–492. doi: 10.1099/mic.0.062117-0. [DOI] [PubMed] [Google Scholar]
- 30.Hovel-Miner G, Pampou S, Faucher SP, Clarke M, Morozova I, Morozov P, Russo JJ, Shuman HA, Kalachikov S. 2009. σS controls multiple pathways associated with intracellular multiplication of Legionella pneumophila. J Bacteriol 191:2461–2473. doi: 10.1128/JB.01578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadosky AB, Wiater LA, Shuman HA. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun 61:5361–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales VM, Bäckman A, Bagdasarian M. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39–47. doi: 10.1016/0378-1119(91)90007-X. [DOI] [PubMed] [Google Scholar]
- 33.Chen D-Q, Huang S-S, Lu Y-J. 2006. Efficient transformation of Legionella pneumophila by high-voltage electroporation. Microbiol Res 161:246–251. doi: 10.1016/j.micres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Xiao H, Kalman M, Ikehara K, Zemel S, Glaser G, Cashel M. 1991. Residual guanosine 3′, 5′-bispyrophosphate synthetic activity of relA-null mutants can be eliminated by spoT-null mutations. J Biol Chem 266:5980–5990. [PubMed] [Google Scholar]
- 35.Zusman T, Gal-Mor O, Segal G. 2002. Characterization of a Legionella pneumophila relA insertion mutant and roles of RelA and RpoS in virulence gene expression. J Bacteriol 184:67–75. doi: 10.1128/JB.184.1.67-75.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 37.Hussong D, Colwell R, O'Brien M, Weiss E, Pearson A, Weiner R, Burge W. 1987. Viable Legionella pneumophila not detectable by culture on agar media. Nat Biotechnol 5:947–950. doi: 10.1038/nbt0987-947. [DOI] [Google Scholar]
- 38.Guerrieri E, Bondi M, Ciancio C, Borella P, Messi P. 2005. Micro- and macromethod assays for the ecological study of Legionella pneumophila. FEMS Microbiol Lett 252:113–119. doi: 10.1016/j.femsle.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 39.Faucher SP, Shuman HA. 2012. Methods to study Legionella transcriptome in vitro and in vivo, p 567–582. In Legionella. Springer-Verlag, New York, NY. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen HB, Wernersson R, Knudsen S. 2003. Design of oligonucleotides for microarrays and perspectives for design of multi-transcriptome arrays. Nucleic Acids Res 31:3491–3496. doi: 10.1093/nar/gkg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wernersson R, Nielsen HB. 2005. OligoWiz 2.0: integrating sequence feature annotation into the design of microarray probes. Nucleic Acids Res 33:W611–W615. doi: 10.1093/nar/gki399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livark K, Schmittgen T. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 43.Jao CY, Salic A. 2008. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc Natl Acad Sci U S A 105:15779–15784. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhee H-W, Lee C-R, Cho S-H, Song M-R, Cashel M, Choy HE, Seok Y-J, Hong J-I. 2008. Selective fluorescent chemosensor for the bacterial alarmone (p)ppGpp. J Am Chem Soc 130:784–785. doi: 10.1021/ja0759139. [DOI] [PubMed] [Google Scholar]
- 45.Dalebroux ZD, Edwards RL, Swanson MS. 2009. SpoT governs Legionella pneumophila differentiation in host macrophages. Mol Microbiol 71:640–658. doi: 10.1111/j.1365-2958.2008.06555.x. [DOI] [PubMed] [Google Scholar]
- 46.Nanamiya H, Kasai K, Nozawa A, Yun CS, Narisawa T, Murakami K, Natori Y, Kawamura F, Tozawa Y. 2008. Identification and functional analysis of novel (p)ppGpp synthetase genes in Bacillus subtilis. Mol Microbiol 67:291–304. [DOI] [PubMed] [Google Scholar]
- 47.Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, Holmes NE, Rhee H-W, Hong J-I, Hartland EL. 2010. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog 6:e1000944. doi: 10.1371/journal.ppat.1000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeves M, Pine L, Hutner S, George J, Harrell W. 1981. Metal requirements of Legionella pneumophila. J Clin Microbiol 13:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tourna M, Stieglmeier M, Spang A, Könneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A. 2011. Nitrososphaera viennensis, an ammonia-oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108:8420–8425. doi: 10.1073/pnas.1013488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. 2014. The importance of the viable but nonculturable state in human bacterial pathogens. Microbial Physiol Metab 5:258. doi: 10.3389/fmicb.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berney M, Hammes F, Bosshard F, Weilenmann H-U, Egli T. 2007. Assessment and interpretation of bacterial viability by using the Live/Dead BacLight kit in combination with flow cytometry. Appl Environ Microbiol 73:3283–3290. doi: 10.1128/AEM.02750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morel FMM, Westall JC, Rueter JG, Chapfick JP. 1975. Description of the algal growth media Aquil and Fraquil, technical note no. 16, p 1–33. Water Quality Laboratory, Laboratory for Water Resources and Hydrodynamics, Department of Civil Engineering, Massachusetts Institute of Technology, Cambridge, MA. [Google Scholar]
- 53.Triqui H, Mendis N, Li L, Saad M, Faucher SP. 2013. Facets of small RNA-mediated regulation in Legionella pneumophila. Curr Top Microbiol Immunol 376:53–80. doi: 10.1007/82_2013_347. [DOI] [PubMed] [Google Scholar]
- 54.Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, Goldfarb A, Darst SA. 2001. Structural mechanism for rifampicin inhibition of bacterial RNA polymerase. Cell 104:901–912. doi: 10.1016/S0092-8674(01)00286-0. [DOI] [PubMed] [Google Scholar]
- 55.Tomasz A, Albino A, Zanati E. 1970. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature 227:138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- 56.Gasol JM, Del Giorgio PA. 2000. Using flow cytometry for counting natural planktonic bacteria and understanding the structure of planktonic bacterial communities. Scientia Marina 64:197–224. [Google Scholar]
- 57.Traxler MF, Summers SM, Nguyen HT, Zacharia VM, Hightower GA, Smith JT, Conway T. 2008. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol Microbiol 68:1128–1148. doi: 10.1111/j.1365-2958.2008.06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traxler MF, Zacharia VM, Marquardt S, Summers SM, Nguyen HT, Stark SE, Conway T. 2011. Discretely calibrated regulatory loops controlled by ppGpp partition gene induction across the ‘feast to famine’ gradient in Escherichia coli. Mol Microbiol 79:830–845. doi: 10.1111/j.1365-2958.2010.07498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cashel MGD, Hernandez VJ, Vinella D. 1996. The stringent response, p 1458–1496. In Neidhardt NC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC. [Google Scholar]
- 60.Potrykus K, Cashel M. 2008. (p)ppGpp: still magical? Annu Rev Microbiol 62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 61.Battesti A, Bouveret E. 2009. Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J Bacteriol 191:616–624. doi: 10.1128/JB.01195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tosa T, Pizer LI. 1971. Effect of serine hydroxamate on the growth of Escherichia coli. J Bacteriol 106:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. 1993. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci U S A 90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maciąg A, Peano C, Pietrelli A, Egli T, De Bellis G, Landini P. 2011. In vitro transcription profiling of the σS subunit of bacterial RNA polymerase: re-definition of the σS regulon and identification of σS-specific promoter sequence elements. Nucleic Acids Res 39:5338–5355. doi: 10.1093/nar/gkr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dennis PP, Nomura M. 1974. Stringent control of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A 71:3819–3823. doi: 10.1073/pnas.71.10.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nomura M, Gourse R, Baughman G. 1984. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem 53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- 67.Magnusson LU, Farewell A, Nyström T. 2005. ppGpp: a global regulator in Escherichia coli. Trends Microbiol 13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 68.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. 2010. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stent GS, Brenner S. 1961. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A 47:2005. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paul BJ, Ross W, Gaal T, Gourse RL. 2004. rRNA transcription in Escherichia coli. Annu Rev Genet 38:749–770. doi: 10.1146/annurev.genet.38.072902.091347. [DOI] [PubMed] [Google Scholar]
- 71.Lange R, Fischer D, Hengge-Aronis R. 1995. Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J Bacteriol 177:4676–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bougdour A, Gottesman S. 2007. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc Natl Acad Sci U S A 104:12896–12901. doi: 10.1073/pnas.0705561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jishage M, Kvint K, Shingler V, Nyström T. 2002. Regulation of ς factor competition by the alarmone ppGpp. Genes Dev 16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dalebroux ZD, Yagi BF, Sahr T, Buchrieser C, Swanson MS. 2010. Distinct roles of ppGpp and DksA in Legionella pneumophila differentiation. Mol Microbiol 76:200–219. doi: 10.1111/j.1365-2958.2010.07094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Molofsky AB, Swanson MS. 2004. Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol Microbiol 53:29–40. doi: 10.1111/j.1365-2958.2004.04129.x. [DOI] [PubMed] [Google Scholar]
- 76.Edwards RL, Dalebroux ZD, Swanson MS. 2009. Legionella pneumophila couples fatty acid flux to microbial differentiation and virulence. Mol Microbiol 71:1190–1204. doi: 10.1111/j.1365-2958.2008.06593.x. [DOI] [PubMed] [Google Scholar]
- 77.Bergman JM, Hammarlöf DL, Hughes D. 2014. Reducing ppGpp level rescues an extreme growth defect caused by mutant EF-Tu. PLoS One 9:e90486. doi: 10.1371/journal.pone.0090486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Felipe KS, Glover RT, Charpentier X, Anderson OR, Reyes M, Pericone CD, Shuman HA. 2008. Legionella eukaryotic-like type IV substrates interfere with organelle trafficking. PLoS Pathog 4:e1000117. doi: 10.1371/journal.ppat.1000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Charpentier X, Faucher SP, Kalachikov S, Shuman HA. 2008. Loss of RNase R induces competence development in Legionella pneumophila. J Bacteriol 190:8126–8136. doi: 10.1128/JB.01035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




