Abstract
Head blight (HB) is one of the most damaging diseases on wheat, inducing significant yield losses and toxin accumulation in grains. Fungal pathogens responsible for HB include the genus Microdochium, with two species, and the toxin producer genus Fusarium, with several species. Field studies and surveys show that two or more species can coexist within a same field and coinfect the same plant or the same spike. In the current study, we investigated how the concomitant presence of F. graminearum and another of the HB complex species influences the spike colonization and the toxin production by the fungi. To study these interactions, 17 well-characterized isolates representing five species were inoculated alone or in pairs on wheat spikes in greenhouse and field experiments. The fungal DNA in the grains was estimated by quantitative PCR and toxin contents (deoxynivalenol and nivalenol) by ultraperformance liquid chromatography-UV detection-tandem mass spectrometry. The responses of the different isolates to the presence of a competitor were variable and isolate specific more than species specific. The development of the most aggressive isolates was either unchanged or a slightly increased, while the development of the less aggressive isolates was reduced. The main outcome of the study was that no trend of increased toxin production was observed in coinoculations compared to single inoculations. On the contrary, the amount of toxin produced was often lower than expected in coinoculations. We thus conclude against the hypothesis that the co-occurrence of several HB-causing species in the same field might aggravate the risk linked to fusarium toxins in wheat production.
INTRODUCTION
In addition to causing yield losses in wheat and other cereals, Fusarium species pose a serious threat to food safety because of their ability to produce mycotoxins. The most frequent toxin producer is Fusarium graminearum, which is able to produce deoxynivalenol (DON) or nivalenol (NIV). European regulation imposes a regulatory threshold on the DON toxin content above which wheat grain cannot be sold. In this context, a specific concern has been raised by the fact that several species causing head blight (HB) often co-occur in the same field (1, 2, 20), including toxin producers (Fusarium species) and non-toxin producers (Microdochium species). Should greater toxin contents be expected in wheat grain in the case of coinfection by several species? Is the presence of Microdochium spp. a factor aggravating the risk posed by Fusarium graminearum? These questions represent a major concern for advisors and producers. They also raise the more fundamental matter of species coexistence when competing for the same resource. We examine here the competition of F. graminearum isolates with isolates of other Fusarium and Microdochium species, and we test the hypothesis that coinfection of a wheat spike by two species increases the toxin production.
Most studies on interactions among Fusarium species were carried out under in vitro conditions (3, 4) and do not allow drawing a clear conclusion. Only a few ones were done in planta, but most often on seedlings. Simpson et al. (3) studied interactions between Fusarium culmorum, Microdochium nivale, and Microdochium majus in vitro and on wheat seedlings under different temperature regimes. The outcome of the competition depended on the temperature and the sequence in which the species were inoculated and the coinoculation led to a decrease in DON production. In another in vitro study, Cooney et al. (4) showed that DON production by F. graminearum was reduced in the presence of F. subglutinans. Velluti et al. (5) inoculated maize grains in vitro with single isolates of three Fusarium species and under different water and temperature conditions and showed that toxin production was enhanced or reduced, depending on the species in presence.
To our knowledge, only one study examined the competition of Fusarium species on wheat spikes (6). Interactions between single isolates of F. graminearum, F. poae, F. culmorum, and F. avenaceum were compared after inoculation on wheat spikes in controlled conditions. In all cases, F. graminearum was slightly affected or not affected by its competitor whereas the other species tended to decrease. A striking result was that the mycotoxin productivity (amount of toxin produced per unit of fungal DNA) appeared to increase considerably in coinfections compared to single inoculations.
A few studies performed in field conditions did not confirm this result. Miedaner et al. (7) found that interactions between four isolates of F. culmorum led to reduced visual symptoms and toxins levels, excepted when two highly aggressive isolates were inoculated together, which led to higher DON contents. In other field experiments and with different species, Reid et al. (8) and Picot et al. (9) showed that DON productivity did not vary much or was reduced in maize ear coinoculations of F. graminearum with F. verticillioides compared to single inoculations.
A major problem, when investigating the competition of HB-causing pathogens, is the ability to monitor the amount of each species, together with the toxin production on the same host individual. In an earlier study (6), as in most other studies on Fusarium, the toxin and DNA contents were measured on pooled samples, either for cost or technological limitations. It thus remains difficult to clearly relate toxin content and fungal DNA within an individual host unit (here, a spike). This is, however, the only way to clarify the question of how coinfections influence toxin production in wheat HB.
In the present study, we investigated how the concomitant presence of F. graminearum with another species causing wheat HB influences the spike colonization and the toxin production by the fungi. To this aim, we monitored the fungal development and the toxin production in individual wheat spikes in controlled conditions after single and mixed inoculations of F. graminearum with F. culmorum, F. poae, M. majus, and M. nivale. The use of precise quantification techniques (quantitative PCR [qPCR], liquid chromatography, and mass spectrometry) allowed quantifying both fungal DNA and toxin contents on the same individual spikes. The results were compared to those of a field experiment.
MATERIALS AND METHODS
Overview.
A set of 17 isolates belonging to five species was used in a greenhouse experiment carried out in 2010 and done again in 2011. The disease severity, the fungal colonization, and the toxin production were assessed in single and in coinoculations of these species on adult wheat plants. A subset of the isolates was used for performing a similar experiment in the field conditions. In the coinoculations, each isolate of F. graminearum was confronted to isolates of the other species. In addition, each isolate was inoculated alone in order to evaluate its aggressiveness level.
Plant material.
For the greenhouse experiment, seeds of winter wheat cv. Royssac, considered to be highly susceptible to HB, were sown in Jiffy peat pots kept for 2 weeks under greenhouse conditions for seedling emergence. Seedlings were vernalized in a growth chamber for 8 weeks at 8°C with a 10-h light period and a 14-h dark period. Then, they were individually transplanted into pots filled with 1 liter of commercial compost (Klasmann peat substrate 4; Klasmann France SARL, France) with 2 g of slow-release fertilizer (Osmocote Exact 16-11-11N-P-K 3MgO Te). Pots were then placed in a greenhouse compartment at 15 to 20°C and under a 15-h-day photoperiod. During plant growth, natural daylight was supplemented with 400-W sodium lights between 6:00 a.m. and 9:00 p.m. Plants were fertilized with Hydrokani C2 (Hydro Agri Spécialités, France) at a 1:100 dilution rate. In each pot, two or three tillers were maintained. For each spike, the flowering date was recorded. The plants were sprayed during growth with Metrafenone (Flexity at 1 ml liter−1; Bayer CropScience, Germany) and Lambda-cyhalothrine (Karaté Zéon at 0.2 ml liter−1; Syngenta Agro S.A.S., France) as a preventive measure to control powdery mildew (Blumeria graminis) and insects, respectively. These pesticides have been tested in preliminary experiments and have no detectable effect on HB. For each treatment (single inoculations and mixed inoculations), five pots with two or three spikes per pot were inoculated. The pots were randomized in the greenhouse.
For the field experiment, the same wheat cv. Royssac was sown at 275 kernels per square meter in Grignon (Yvelines, France) on 28 October 2011. Plants were grown in microplots (nine wheat rows, 1 m in length) laid out as a randomized block design with 12 treatment combinations (five inoculations with individual isolates, six coinoculations and a noninoculated control) and four replicates. Each plot was surrounded on its four sides by border plots of another variety to minimize the impact of unwanted cross-inoculations.
Fungal material.
The list of fungal isolates used in the experiments is given in Table 1. The 17 isolates belong to a larger set of isolates that were preliminary characterized for their chemotypes (on grain), for their in vitro growth rates and sporulation capacities (in petri dishes), and for their in planta aggressiveness (HB severity on spikes, grain weight, fungal biomass, and toxin production in grains). The isolates chosen presented different level of aggressiveness (Table 1). The F. graminearum and the F. culmorum isolates were provided by F. Forget (INRA Bordeaux, France), the F. poae isolates were provided by Bayer CropScience, and the Microdochium isolates were provided by A.-S. Walker (INRA Versailles-Grignon, France). They were all taken from field samplings in France.
TABLE 1.
Isolates used in the 2010 and 2011 greenhouse experiments and in the 2011 field experimenta
| Code | Species | Chemotypeb | Avg aggressivenessc | Isolates used |
||
|---|---|---|---|---|---|---|
| Greenhouse |
Field (2011) | |||||
| 2010 | 2011 | |||||
| fg91 | F. graminearum | NIV | ++ | × | × | × |
| fg159 | F. graminearum | DON | + | × | × | |
| fg165 | F. graminearum | DON | ++ | × | × | |
| fg178 | F. graminearum | DON | +++ | × | × | × |
| fg201 | F. graminearum | DON | +++ | × | ||
| fc124 | F. culmorum | DON | +++ | × | × | × |
| fc233 | F. culmorum | DON | +++ | × | × | |
| fc129 | F. culmorum | NIV | ++ | × | × | |
| fc337 | F. culmorum | NIV | +++ | × | × | × |
| fp3 | F. poae | NIV | + | × | × | |
| fp4 | F. poae | NIV | + | × | ||
| fp6 | F. poae | NIV | + | × | × | |
| fp14 | F. poae | NIV | + | × | ||
| mm58 | M. majus | – | × | × | ||
| mm221 | M. majus | – | × | |||
| mm229 | M. majus | – | × | |||
| mn227 | M. nivale | – | × | × | ||
In the coinoculations, each F. graminearum isolate was inoculated in a 50:50 mixture with each of the F. culmorum, F. poae, or Microdochium isolates.
That is, the main toxin produced.
The average aggressiveness was measured on wheat spikes in other experiments. +, ++, and +++ indicate low, moderate, and high aggressiveness, respectively, as determined by AUDPC measurements on wheat spikes in the greenhouse experiment; –, not tested. Because Microdochium isolates were inoculated by a different method, their aggressiveness cannot be directly compared to that of the Fusarium isolates.
The isolates were grown in petri dishes on potato dextrose agar (PDA, 39 g liter−1) at 19°C and kept in the light during 3 days. For Fusarium, four mycelial plugs were then transferred in 250 ml of carboxymethyl cellulose (CMC) broth (7.5 g of CMC, 0.5 g of yeast extract, 0.5 g of MgSO4, 0.5 g of NH4NO3, and 0.5 g of KH2PO4 per liter) with continual shaking. After 3 days, the medium was filtered through cheesecloth to collect the spores. For Microdochium, fungi were transplanted twice in petri dishes with PDA and kept at 19°C in the light during 5 days. Then, 5 ml of sterile water was added into the petri dish to collect the spores. For each isolate, a spore suspension in sterile-distilled water was adjusted to a content of 2 × 104 conidia ml−1 using a Malassez cell. For the coinoculations in a greenhouse, mixed inocula were prepared by mixing two spore suspensions at 4 × 104 spores ml−1 resulting in 2 × 104 spores ml−1 of each isolate. The suspensions were then stored at 4°C until they were used for inoculations, which took place within the same day. After inoculation, a few microliters of each spore suspension was deposited on PDA to check for spore viability.
Inoculation procedure.
In the greenhouse, the spikes were inoculated with either single isolates or a mixture of two isolates at the midflowering stage. In the coinoculation, each F. graminearum isolate was opposed to each isolate from the other species (Table 1). Ten (in 2011) or fifteen (in 2010) replicates were performed for each pair of fungal isolates. The inoculations were performed with an atomizer (Ecospray; Prolabo, France). An inoculation consisted in spraying each side of the entire spike with 1 ml of inoculum suspension. Due to very low infection efficacy, Microdochium isolates were inoculated differently, by injection of the spore suspension in each spikelet along the spike. After the inoculation, the wheat heads were enclosed during 3 days in a transparent polyethylene bag to maintain 100% humidity and promote infection.
In the field, the five central rows of each plot were inoculated with 60 ml of spore suspension at the midflowering stage with a portable sprayer (five inoculations with individual isolates, six coinoculations and a noninoculated control; Table 1). Coinoculations were done by successively spraying a spore suspension for a F. graminearum isolate (fg91 or fg178) and a F. culmorum (fc124 or fc337) or a F. poae isolate (fp3). A mist irrigation was applied for 2 h after inoculation and then every morning and evening of the three following days.
Disease assessment, fungal DNA, and mycotoxin quantification.
In the greenhouse, the number of spikelets with HB symptoms was visually assessed thrice a week from 5 days after inoculation (appearance of first symptoms) to 28 days after inoculation. Since the aspect of HB symptoms may vary, each symptomatic spikelet showing a premature bleaching or brown necrosis was putatively considered diseased. The percentage of diseased spikelets in each spike was calculated. The dynamics of the appearance of symptomatic spikelets on each spike was characterized by the area under the disease progression curve (AUDPC), which was normalized by dividing the AUDPC value by the number of observation days. At maturity, the spikes were cut and hand-threshed to separate the kernels from the chaff. Kernels were then weighed to estimate the thousand kernel weight (TKW). Then, the kernels of each spike were ground with a mixer mill (MM 400; Retsch, France) and stored for fungal DNA and toxin quantification.
In the field, HB was visually assessed once when symptoms were the most visible (33 days after inoculation). Disease incidence was estimated as the percentage of blighted spikes in each microplot. At maturity, all the spikes along three central rows per plot were harvested and hand-threshed. From these samples, the TKW was assessed, and 200-g subsamples were ground with a mixer mill and stored for fungal DNA and toxin quantification.
The methods for species-specific quantification of fungal DNA (extraction and qPCR) and toxin quantification (extraction and ultraperformance liquid chromatography-UV detection-tandem mass spectrometry [UPLC-UV-MS/MS] analysis) from all the tissue samples were developed in a previous study (10). The total DNA was extracted from around 50 mg of ground material using the DNeasy plant minikit (Qiagen). The amount of both fungal and wheat DNA was estimated by qPCR using species-specific primer pairs and species-specific TaqMan probes (Table 2; see also “Extraction and quantification of fungal DNA” in the supplemental material). The amount of fungal DNA was calculated from Cq values using the standard curves from F. graminearum, F. culmorum, F. poae, M. majus, and M. nivale, and these values were normalized with the estimated amount of plant DNA of the same sample, quantified by wheat-specific qPCR assay. The quantification of the plant DNA was performed using plant EF1 qPCR primers and SYBR green I (10, 11) (Table 2). A homogenized flour sample of around 1 g was used for toxin extraction. The UPLC-UV-MS/MS analysis of the toxin extract was performed with an Acquity Ultra-Performance liquid chromatograph coupled with a TQD tandem mass spectrometer (Waters Corp.), as described by Siou et al. (10). The mass spectrometer was operated in electrospray ionization (ESI negative-ion mode) under multiple-reaction monitoring mode. As confirmed by single inoculations, the isolates produced either DON or NIV, according to their chemotype. As established in other studies (see, for example, references 6 and 10), the amount of acetylated derivatives were expected to be highly correlated with the amount of the predominant DON or NIV toxin and only those two were quantified. For cost limitation, only eight replicates (spikes) were used for toxin quantification.
TABLE 2.
Primer and probe sequences and qPCR amplification conditions for each Fusarium and Microdochium species and wheat
| Target species | Name | Sequence | Reporter/quenchera (5′/3′) | Final concn (nM) | Annealing temp (°C) |
|---|---|---|---|---|---|
| Fusarium graminearum (EF1α) | EF1-FCFG_F | TCGATACGCGCCTGTTACC | 300 | 62 | |
| EF1-FG_R | ATGAGCGCCCAGGGAATG | 300 | 62 | ||
| grami2-EF1_rev | AGCCCCACCGGGAAAAAAATTACGACA | 6-FAM/TAMRA | 100 | 62 | |
| Fusarium culmorum (EF1α) | EF1-FC_F2 | CGAATCGCCCTCACACG | 300 | 62 | |
| EF1-FC-R2 | GTGATGGTGCGCGCCTAG | 300 | 62 | ||
| culmo2-EF1-R2 | ATGAGCCCCACCAGAAAAATTACGACAA | 6-FAM/TAMRA | 100 | 62 | |
| Fusarium poae (EF1α) | EF1-FP2_F | CTCGAGCGATTGCATTTCTTT | 300 | 60 | |
| EF1_FP2_R | GGCTTCCTATTGACAGGTGGTT | 300 | 60 | ||
| EF1-FP | CGCGAATCGTCACGTGTCAATCAGTT | 6-FAM/TAMRA | 100 | 60 | |
| Microdochium majus (β tubulin) | MN_Btub_F | TCAATAACCGACTCGCACTTCTT | 300 | 60 | |
| MN_Btub_R | GGTGCCGTTGTAGCTATTGACA | 300 | 60 | ||
| Mmajustub | AGGCCACTTCCCTGATGGCGTATCA | 6-FAM/TAMRA | 200 | 60 | |
| Microdochium nivale (β tubulin) | Mniv_Btub_F | TCTACTTCAACGAGGTATGTCACCAT | 300 | 62 | |
| Mniv_Btub_R | CCTAAGTTATGTGGGTGGTCAGTTAG | 300 | 62 | ||
| Mniv_Btub | TTCGGGCTTCACACATTCGGCC | 6-FAM/TAMRA | 150 | 62 | |
| Triticum aestivum (wheat) | Hor1f | TCTCTGGGTTTGAGGGTGAC | 250 | 62 | |
| Hor2r | GGCCCTTGTACCAGTCAAGGT | 250 | 62 |
6-FAM, 6-carboxyfluorescein; TAMRA, 6-carboxytetramethylrhodamine.
Data analyses.
An objective of this study was to assess the impact of species interaction on the amount of toxin produced. To this end, the toxin content in the grains was compared between spikes inoculated with a single species and spikes coinoculated with a mixture of two species. An expected toxin content was calculated for the coinoculations, based on the respective species-specific fungal DNA that were estimated by qPCR and on the toxin production in spikes inoculated with a single species. For each isolate, the expected toxin content was calculated as (TOX.S/DNA.S) × DNA.C, with TOX.S and DNA.S as the average toxin and fungal DNA contents, respectively, in single inoculations and DNA.C as the species-specific fungal DNA produced by each isolate in coinoculations. The expected toxin contents accounted for by each isolate was then added to give the total expected content in the spike. This expected toxin content was compared to the measured toxin content to establish whether the presence of another species in the same spike enhanced or reduced the toxin production. For each pair of isolates, we evaluated whether the difference between expected and measured toxin contents was significantly different from zero with Wilcoxon's tests. In order to produce synthetic results, the percentage of spikes for which the difference between measured and expected toxin contents was negative, null, or positive was established by pooling the data for each competing species and for the 2010 and 2011 experiments.
Analyses of variance (ANOVAs) were performed with the DON and NIV concentrations, the quantities of fungal DNA, and the HB severity (AUDPC) as variables to be explained and the year of experiment and the isolate as explanatory variables for the 2010 and 2011 greenhouse experiments. Multiple comparisons were done with the Tukey's honestly significant difference (HSD) test. Residuals distribution was checked for variance homogeneity and normality. Regression analyses were used to investigate the relationship between variables such as AUDPC and fungal DNA or fungal DNA and toxin contents.
RESULTS
Correlations between variables.
In the greenhouse experiment, the AUDPC was found highly correlated to the fungal DNA, as expected (r = 0.78, P < 0.001; Fig. 1a, all values pooled). As well, the toxin content was found highly correlated to the fungal DNA (r = 0.82, P < 0.001; Fig. 1b, all values pooled). In the field experiment, a similar correlation was found between the fungal DNA and the toxin contents (r = 0.78, P < 0.001; Fig. 1c, all values pooled).
FIG 1.
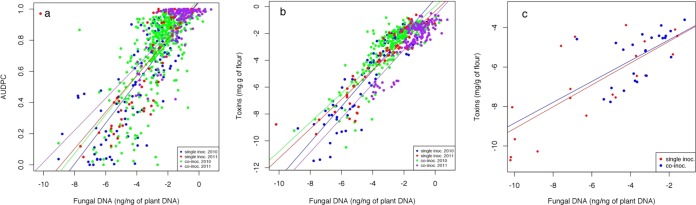
Relationship between AUDPC and fungal DNA and between toxin concentrations and fungal DNA in wheat samples inoculated by different species responsible for head blight in a greenhouse (a and b) or field (c) experiment. Regression lines were drawn separately for single inoculations and coinoculations in each experiment. The fungal DNA was quantified by qPCR. The toxin concentration was determined by using UPLC-UV-MS/MS. Fungal DNA and toxin concentration data are log transformed.
Effect of coinoculations on disease severity.
In both the 2010 and the 2011 greenhouse experiments and whatever the isolate considered, AUDPC was highly variable. An ANOVA analysis showed a significant effect of the year experiment (P < 0.001) that explained 61% of the variance in AUDPC (Table 3). The AUDPC was lower in 2010 (0.65 in average) than in 2011 (0.74 in average), except for fc337, for which it was the opposite in coinoculations and single inoculations. The isolate (or pair of isolates) significantly influenced the AUDPC. Multiple mean comparisons were performed separately for each year and showed that F. poae isolates and fg159 were always less aggressive than the other isolates (Tukey's HSD test, P < 0.001). Since Microdochium isolates were inoculated with a different method, their AUDPC values cannot be directly compared to those of the Fusarium isolates.
TABLE 3.
ANOVA results with the toxin concentration, the quantities of fungal DNA, and the HB severity as variables to be explained and the year of the experiment and the isolate as explanatory variables for the 2010 and 2011 greenhouse experimentsa
| Source of variationb | df | MS | F-value | P |
|---|---|---|---|---|
| On toxin concentrations | ||||
| Exp (A) | 1 | 0.552 | 135.56 | <0.001 |
| Iso (B) | 69 | 0.083 | 20.34 | <0.001 |
| A × B | 32 | 0.028 | 6.86 | <0.001 |
| Residuals | 615 | 0.004 | ||
| On fungal DNA | ||||
| Exp (A) | 1 | 5.362 | 143.15 | <0.001 |
| Iso (B) | 73 | 0.127 | 3.38 | <0.001 |
| A × B | 34 | 0.144 | 3.84 | <0.001 |
| Residuals | 1,336 | 0.037 | ||
| On AUDPC | ||||
| Exp (A) | 1 | 1.730 | 90.96 | <0.001 |
| Iso (B) | 73 | 0.846 | 44.51 | <0.001 |
| A × B | 34 | 0.239 | 12.55 | <0.001 |
| Residuals | 1,334 | 0.019 |
ANOVA was performed with the toxin concentration (either DON or NIV, depending on the isolates), the quantities of fungal DNA and the HB severity (AUDPC) as variables to be explained and the year of experiment (Exp) and the isolate (Iso) as explanatory variables for the 2010 and 2011 greenhouse experiments. In the coinoculation, each Fusarium graminearum isolate (fg91, fg159, fg165, and fg178) was opposed to each isolate of F. culmorum (fc124, fc129, fc233, and fc337), F. poae (fp3, fp4, fp6, and fp14), Microdochium majus (mm58, mm221, and mm229), and Majus nivale (mn227) (see Table 1). MS, mean square.
Exp (A), year of experiment (2010 or 2011); Iso (B), either a single isolate or pair of isolates.
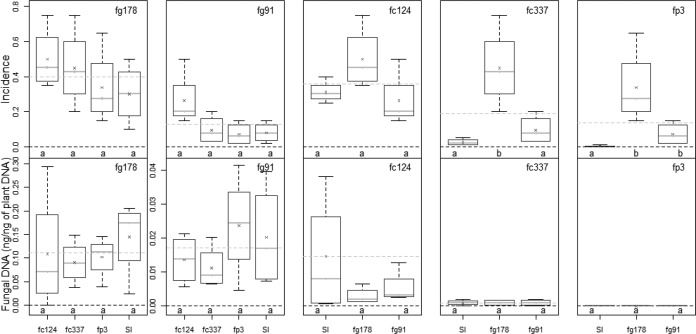
In the field experiment (Fig. 2), fp3 and fc337 developed less symptoms than the three other isolates (fg178, fg91, and fc124) when inoculated alone. Coinoculation with other isolates did not significantly affect severity compared to single inoculations with F. graminearum isolates.
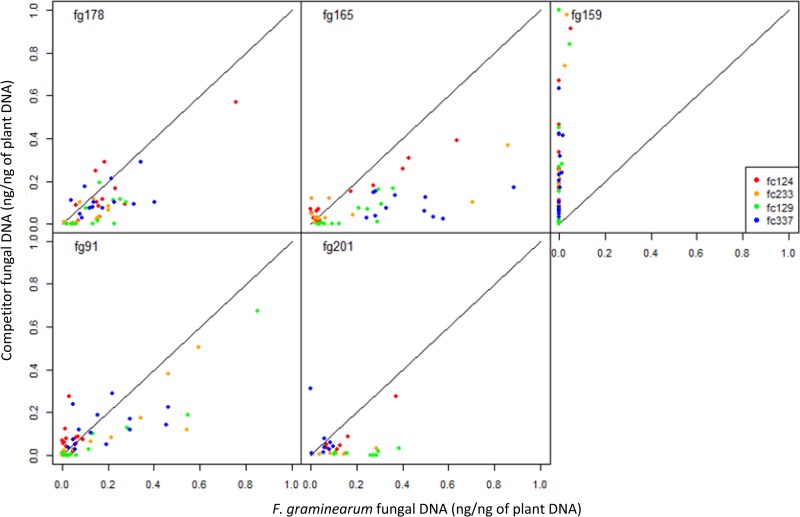
FIG 2.
Amounts of DNA of F. culmorum (on the ordinate) plotted against those of F. graminearum (on the abscissa) in each individual wheat spike in the 2010 and 2011 greenhouse experiments. Each graph corresponds to an F. graminearum isolate, and each color indicates its F. culmorum competitor. fg178 produced more DNA than the competing isolate in 69/75 of the spikes in 2010 and 26/30 in 2011. fg165 produced more DNA than the competing isolate in 50/65 in 2010 and in every spike in 2011. fg201 produced more DNA in 71/78 spikes in 2010, and fg91 produced more DNA in 50/85 spikes in 2010 and in 33/35 in 2011. Additional tables (means values for fungal DNA quantified in wheat spikes inoculated with different Fusarium and Microdochium isolates in the 2010 and 2011 experiments) are presented in Table S1 in the supplemental material.
Effect of coinoculations on fungal DNA.
In the coinoculations, the fungal DNA of each species was expected to decrease relative to single inoculations since both isolates had to share the same spike, viewed as a limited resource, but this was not always the case. For instance, the fungal DNA of fc124 was significantly greater when it was inoculated with fg159, compared to single inoculations, in the 2010 experiment (Table S1).
The experiment (year) significantly influenced the amounts of fungal DNA measured (P < 0.001; Table 3). For instance, in 2011, both Microdochium isolates mm58 and mn227 produced more fungal DNA when inoculated with fg178 than when inoculated alone, while this was not the case in 2010: mm58 produced less fungal DNA in the presence of fg178, and there was no significant difference for mn227 (see Table S1 in the supplemental material). More generally, depending on the isolates and on the year, the fungal DNA increased, decreased or remained stable in the coinoculations compared to the single inoculations (see Table S1 in the supplemental material). Several isolates appeared better competitor than others. In most cases, the fungal DNA of isolates fg159, fp6, and fp14 was less when confronted to a competitor. On the contrary, the DNA of fg201 was higher when confronted to a competitor. The situation was less clear with fg165, which seemed to be a good competitor in 2010 but not in 2011; as well, fg178 seemed to be a good competitor in 2011 but not in 2010. When fg159 was inoculated with another poorly aggressive isolate (such as fp3, fp4, fp6, or fp14), its development was not significantly affected. In general, it was difficult to infer any general trend from these data, regarding the outcome of isolate competition for spike colonization.
The difficulty to draw conclusion from the above analysis partly came from the high variability in the fungal colonization among the different replicates. We thus performed a different analysis, based on the direct comparison of the fungal DNA of each isolate within each spike. Figure 2 shows the amounts of DNA of F. culmorum plotted against those of F. graminearum in each individual spike. This gives a much clearer picture of the overall effect of coinoculation on spike colonization by each species. Only F. graminearum and F. culmorum isolates were considered in this analysis because the others isolates had a low aggressiveness (Table 1). A Wilcoxon test indicates that the F. graminearum isolates, except fg159, produced in average more fungal DNA than their competing F. culmorum isolates (P < 0.001). This analysis, based on the comparison of DNA amounts at the scale of single spikes, revealed that some isolates, such as fg178 and fg165, were better competitor than others, whereas fc129 and fc337 tended to be poor competitors (Fig. 2). In the field experiment, no significant difference in fungal DNA was observed between coinoculations and single inoculations (Fig. 3).
FIG 3.
Variations of HB severity (percentage of blighted spikes) and fungal DNA in field microplots inoculated with five isolates belonging to three Fusarium species (F. graminearum, F. culmorum, and F. poae; see Table 1) in single inoculations (SI) or in coinoculation. Treatments indicated by the same lowercase letter within the same graph were not significantly different; the six coinoculation treatments (fg178/fc337, fg178/fc337, fg178/fp3, fg91/fc337, fg91/fc337, and fg91/fp3) are presented twice in order to compare each of them with the two single inoculations (fg178 and fg91).
Effect of coinoculations on toxin production.
The amount of toxins produced (whether it was DON or NIV) depended on the year of experiment, the isolates and their interaction (Table 3). Since the toxin content in the spikes was highly correlated with the fungal DNA (Fig. 1), it is likely that the dependency of toxin content to the environmental effects was largely accounted for by differences in the fungus development.
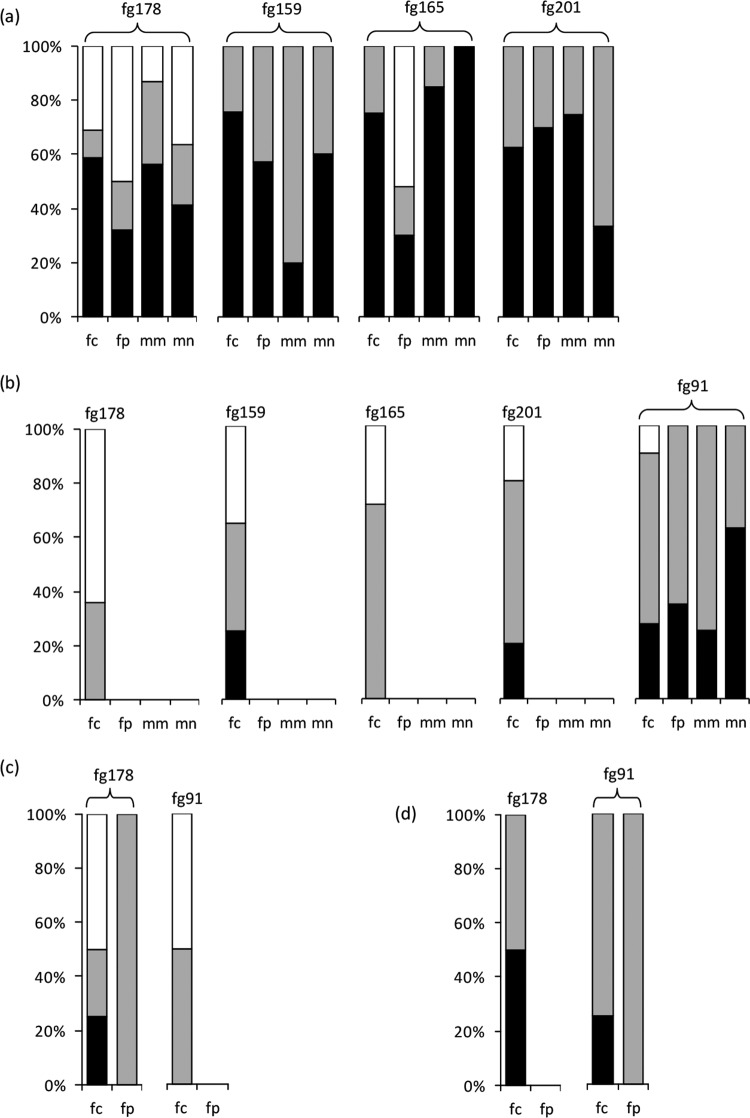
The greenhouse experiments did not reveal an increase in the toxin productivity in coinoculations, relative to single inoculations. The situations in which the toxin production was similar or lower in coinoculations were the most frequent (Fig. 4). In the greenhouse experiments, whatever the year and the isolate combination, significantly less DON was produced than expected in coinoculations for 57.4% of the spikes. The same trend was observed for NIV, with 64.2% of the spikes having a lower toxin content than expected. Figure S1 in the supplemental material shows the data in more detail: in the 2010 greenhouse experiment, the toxin productivity increased moderately in some spikes but remained unchanged or decreased in the majority of the replicates, and in 2011 the toxin productivity was either lower or unchanged in coinoculations relative to single inoculations. In the field experiment, expected and measured toxin values were not found significantly different.
FIG 4.
Differences between measured and expected contents of DON (a, greenhouse experiments; c, field experiment) and NIV (b, greenhouse experiments; d, field experiment) in individual wheat spikes in which Fusarium graminearum was coinoculated with F. culmorum, F. poae, or Microdochium nivale. Black, gray, and white bars indicate the percentages of spikes in which the measured toxin content was significantly lower, not different, or higher, respectively, than the level expected from single inoculation data (Wilcoxon test). The figures synthesize the coinoculation data for the 2010 and 2011 greenhouse experiments (DON; isolates fc124, fc129, fc233, fc337, fp3, fp4, fp6, fp14, mn58, mn221, mn227, and mn229) (a), the 2010 and 2011 greenhouse experiments (NIV; isolates fc124, fc129, fc233, fc337, fp4, fp6, fp14, mn58, mn221, mn227, and mn229) (b), the 2011 field experiment (DON; isolates fc124, fc337, and fp3) (c), and the 2011 field experiment (NIV; isolates fc124, fc337, and fp3) (d). The F. graminearum isolates are indicated on the graphs. Values for individual spikes are also presented in Fig. S1 in the supplemental material.
DISCUSSION
The main outcome of our study is that toxin productivity did not increase in coinoculations, relative to single inoculations. On the contrary, it was significantly reduced for several isolate combinations. This was clearly shown by analyzing each spike individually for both toxin production and fungal DNA. This result is confirmed by the fact that the relationship between toxin content and fungal DNA is linear and mainly accounted for by the quantity of DNA. The same conclusion was reached from the greenhouse experiment and from the field experiment.
This result is in accordance with other studies (7–9) that were realized in field conditions. Although these studies did not allow researchers to precisely relate fungal colonization and toxin production within the same host plant, the findings suggest that strain competition does not involve a significant increase in toxin production in natural conditions. Our own field experiment reaches the same conclusion and is in good accordance with the results obtained in the greenhouse experiments. Note, however, that competition was evaluated here in wheat heads but certainly takes place in other environments (e.g., crop debris), where the interactions and role of toxins may be different. In vitro studies (3, 12) also suggest that toxin production is proportional to the quantity of fungal DNA and does not increase when strains are in competition. The study from Xu et al. (6) yielded, however, a different conclusion and showed a strong increase in toxin productivity in the case of coinoculations. By working with several isolates belonging to four HB species and by measuring toxin contents in each spike, we never found such a strong increase in DON or NIV productivity in the case of coinoculations. In 2011, we only observed a slight but significant increase in the coinoculations (see, for instance, fg178). The increase in toxin productivity established by Xu et al. (6) may have resulted from specific conditions, such as high temperatures during initial infection periods. It may also be due to an isolate-specific response (the authors of that study only examined one isolate per species).
Our study was not designed to draw conclusions at the species scale. We simply attempted to take the pathogen diversity into account by working with different species and different strains for each species. The number of isolates we could handle was only limited by the practical constraints of working on adult plants in a greenhouse. We can nevertheless compare our results with published data on HB species. F. poae is generally believed to be less aggressive than the other Fusarium species (13, 14), which was found again in our experiment. We found that F. poae produced a lower disease severity and smaller amounts of fungal DNA in the grains compared to other Fusarium species in both greenhouse and field experiments. In contrast, no significant difference was found between F. graminearum and F. culmorum isolates for spike colonization, except for fg159, which showed a low aggressiveness and was not competitive against the F. culmorum strains. This illustrates the variability between isolates of the same species, as often observed in FHB studies.
Both our field and greenhouse experiments showed that isolates with a low aggressiveness are poor competitors against aggressive isolates, whereas the development of aggressive isolates (such as fg178, fg201, fc124, and fc233) was not or slightly altered by the presence of a poorly aggressive isolate. This is consistent with (6) and with the results of Velluti et al. (5), which established that the growth rate of F. graminearum was relatively unaffected by the presence of less aggressive species (F. proliferatum and F. moniliforme).
Environmental factors are known to influence the development of plant pathogens (15), and environmental effects are commonly found in experiments dealing with Fusarium and Microdochium (16, 17). This might explain the differences between the 2010 and the 2011 experiments. In 2010, the DON and NIV productivities in coinoculations were either lower or not significantly different from that in single inoculations, whatever the isolate combination. In 2011, even if most isolate combinations responded as in 2010, the toxin productivity sometimes slightly but significantly increased in the coinoculations (see, for instance, fg178).
For practical reasons, only one host variety could be used in our study. We cannot exclude an influence of the host genotype on species competition and the resulting toxin productivity, but this should be assessed in further experiments. Note, however, that in a different species (maize), Reid et al. (8) and Picot et al. (9) also found that DON production did not vary much or was reduced in coinoculations compared to single inoculations.
In the greenhouse experiments, the correlation between the fungal biomass (estimated by DNA quantification) and HB severity (AUDPC) was strong and positive, in agreement with other studies using similar techniques (6, 18, 19). A good correlation between fungal biomass and HB severity is expected when the inoculations are performed at the most receptive stage of the host plant, which was the case in both our greenhouse and our field study.
In most cases, the HB severity increased or remained stable in the coinoculations compared to the single inoculations. This is consistent with other studies (see, for example, reference 6). Some exceptions were however observed in 2010, with lower AUDPC values produced by coinoculations. A similar effect was observed in the field by Miedaner et al. (7), who measured a lower HB severity and a lower yield reduction in coinoculations than in single inoculations. The outcome of HB species competitions in terms of disease severity seems difficult to predict and may depend on the strains, as well as on environmental conditions (6). In all cases, however, the disease severity was strongly linked to the fungal biomass.
Our data suggest that DON and NIV productivity either does not increase in multiple infections by two HB species on the same wheat spike or increases to a very limited extent and for some strains only. This conclusion disagrees with that of another study (6) but is in accordance with several field experiments. According to these data, the DON and NIV toxins do not seem to be a key element in the competition between HB species for host colonization.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Bayer CropScience for their financial support and to the Ile-de-France region for their contribution to the UPLC-UV-MS/MS acquisition. Financial support was also provided by the ANR DON & Co project. A portion of the isolates was supplied by INRA MycSa Bordeaux.
We thank M. Dufresne for help in the methodological developments and B. Beauzoone and M. Willigsecker for technical support. The PCR tools were developed by S. Elbelt.
We are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this article.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02879-14.
REFERENCES
- 1.Xu XM, Nicholson P. 2009. Community ecology of fungal pathogens causing wheat head blight. Annu Rev Phytopathol 47:83–103. doi: 10.1146/annurev-phyto-080508-081737. [DOI] [PubMed] [Google Scholar]
- 2.Xu XM, Parry DW, Nicholson P, Thomsett MA, Simpson D, Edwards SG, Cooke BM, Doohan FM, Brennan JM, Moretti A, Tocco G, Mule G, Hornok L, Giczey G, Tatnell J. 2005. Predominance and association of pathogenic fungi causing Fusarium ear blight in wheat in four European countries. Eur J Plant Pathol 112:143–154. doi: 10.1007/s10658-005-2446-7. [DOI] [Google Scholar]
- 3.Simpson DR, Thomsett MA, Nicholson P. 2004. Competitive interactions between Microdochium nivale var. majus, M. nivale var. nivale, and Fusarium culmorum in planta and in vitro. Environ Microbiol 6:79–87. [DOI] [PubMed] [Google Scholar]
- 4.Cooney JM, Lauren DR, di Menna ME. 2001. Impact of competitive fungi on trichothecene production by Fusarium graminearum. J Agric Food Chem 49:522–526. doi: 10.1021/jf0006372. [DOI] [PubMed] [Google Scholar]
- 5.Velluti A, Marin S, Bettucci L, Ramos AJ, Sanchis V. 2000. The effect of fungal competition on colonization of maize grain by Fusarium moniliforme, F. proliferatum, and F. graminearum and on fumonisin B and zearalenone formation. Int J Food Microbiol 59:59–66. doi: 10.1016/S0168-1605(00)00289-0. [DOI] [PubMed] [Google Scholar]
- 6.Xu XM, Monger W, Ritieni A, Nicholson P. 2007. Effect of temperature and duration of wetness during initial infection periods on disease development, fungal DNA and mycotoxin contents on wheat inoculated with single, or combinations of, Fusarium species. Plant Pathol 56:943–956. doi: 10.1111/j.1365-3059.2007.01650.x. [DOI] [Google Scholar]
- 7.Miedaner T, Schilling AG, Geiger HH. 2004. Competition effects among isolates of Fusarium culmorum differing in aggressiveness and mycotoxin production on heads of winter rye. Eur J Plant Pathol 110:63–70. doi: 10.1023/B:EJPP.0000010136.38523.a9. [DOI] [Google Scholar]
- 8.Reid LM, Nicol RW, Ouellet T, Savard M, Miller JD, Young JC, Stewart DW, Schaafsma AW. 1999. Interaction of Fusarium graminearum and F. moniliforme in maize ears: disease progress, fungal DNA, and mycotoxin accumulation. Phytopathology 89:1028–1037. [DOI] [PubMed] [Google Scholar]
- 9.Picot A, Hourcade-Marcolla D, Barreau C, Pinson-Gadais L, Caron D, Richard-Forget F, Lannou C. 2012. Interactions between Fusarium verticillioides and Fusarium graminearum in maize ears and consequences for fungal development and mycotoxin accumulation. Plant Pathol 61:140–151. doi: 10.1111/j.1365-3059.2011.02503.x. [DOI] [Google Scholar]
- 10.Siou D, Gélisse S, Laval V, Repinçay C, Canalès R, Suffert F, Lannou C. 2013. Effect of wheat spike infection timing on Fusarium head blight development and mycotoxin accumulation. Plant Pathol 63:390–399. doi: 10.1111/ppa.12106. [DOI] [Google Scholar]
- 11.Nicolaisen M, Suproniene S, Nielsen LK, Lazzaro I, Spliid NH, Justesen AF. 2009. Real-time PCR for quantification of eleven individual Fusarium species in cereals. J Microbiol Methods 76:234–240. doi: 10.1016/j.mimet.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Ramakrishna N, Lacey J, Smith JE. 1996. The effects of fungal competition on colonization of barley grain by Fusarium sporotrichioides on T-2 toxin formation. Food Addit Contam 13:939–948. doi: 10.1080/02652039609374481. [DOI] [PubMed] [Google Scholar]
- 13.Brennan JM, Fagan B, Van Maanen A, Cooke BM, Doohan FM. 2003. Studies on in vitro growth and pathogenicity of European Fusarium fungi. Eur J Plant Pathol 109:577–587. doi: 10.1023/A:1024712415326. [DOI] [Google Scholar]
- 14.Stenglein SA. 2009. Offered review: Fusarium poae: a pathogen that needs more attention. J Plant Pathol 91:25–36. [Google Scholar]
- 15.Lannou C. 2012. Variation and selection of quantitative traits in plant pathogens. Annu Rev Phytopathol 50:319–338. doi: 10.1146/annurev-phyto-081211-173031. [DOI] [PubMed] [Google Scholar]
- 16.Doohan FM, Brennan J, Cooke BM. 2003. Influence of climatic factors on Fusarium species pathogenic to cereals. Eur J Plant Pathol 109:755–768. doi: 10.1023/A:1026090626994. [DOI] [Google Scholar]
- 17.Popovski S, Celar FA. 2013. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production: a review. Acta Agric Slov 101:105–116. doi: 10.2478/acas-2013-0012. [DOI] [Google Scholar]
- 18.Hestbjerg H, Felding G, Elmholt S. 2002. Fusarium culmorum infection of barley seedlings: correlation between aggressiveness and deoxynivalenol content. J Phytopathol 150:308–312. doi: 10.1046/j.1439-0434.2002.00760.x. [DOI] [Google Scholar]
- 19.Lemmens M, Buerstmayr H, Krska R, Schuhmacher R, Grausgruber H, Ruckenbauer P. 2004. The effect of inoculation treatment and long-term application of moisture on Fusarium head blight symptoms and deoxynivalenol contamination in wheat grains. Eur J Plant Pathol 110:299–308. doi: 10.1023/B:EJPP.0000019801.89902.2a. [DOI] [Google Scholar]
- 20.Audenaert K, Van Broeck R, Bekaert B, De Witte F, Heremans B, Messens K, Höfte M, Haesaert G. 2009. Fusarium head blight (FHB) in Flanders: population diversity, inter-species associations and DON contamination in commercial winter wheat varieties. Eur J Plant Pathol 125:445–458. doi: 10.1007/s10658-009-9494-3. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.