Abstract
Lowering the pH in bacterium-based succinate fermentation is considered a feasible approach to reduce total production costs. Newly isolated Enterobacter aerogenes strain AJ110637, a rapid carbon source assimilator under weakly acidic (pH 5.0) conditions, was selected as a platform for succinate production. Our previous work showed that the ΔadhE/PCK strain, developed from AJ110637 with inactivated ethanol dehydrogenase and introduced Actinobacillus succinogenes phosphoenolpyruvate carboxykinase (PCK), generated succinate as a major product of anaerobic mixed-acid fermentation from glucose under weakly acidic conditions (pH <6.2). To further improve the production of succinate by the ΔadhE/PCK strain, metabolically engineered strains were designed based on the elimination of pathways that produced undesirable products and the introduction of two carboxylation pathways from phosphoenolpyruvate and pyruvate to oxaloacetate. The highest production of succinate was observed with strain ES04/PCK+PYC, which had inactivated ethanol, lactate, acetate, and 2,3-butanediol pathways and coexpressed PCK and Corynebacterium glutamicum pyruvate carboxylase (PYC). This strain produced succinate from glucose with over 70% yield (gram per gram) without any measurable formation of ethanol, lactate, or 2,3-butanediol under weakly acidic conditions. The impact of lowering the pH from 7.0 to 5.5 on succinate production in this strain was evaluated under pH-controlled batch culture conditions and showed that the lower pH decreased the succinate titer but increased its yield. These findings can be applied to identify additional engineering targets to increase succinate production.
INTRODUCTION
There is an increasing interest in bio-based chemicals from renewable carbon sources because of the increasing price of petroleum and the negative impact of petrochemical production on the environment (1, 2). Succinate, a C4-dicarboxylic acid, which is an intermediate metabolite in the tricarboxylic acid cycle, is potentially useful as a chemical precursor for many commodity chemicals, such as γ-butyrolactone, tetrahydrofuran, and 1,4-butanediol. These chemicals can, in turn, be converted into a wide variety of products, such as green solvents, pharmaceuticals, and biodegradable plastics (3, 4). Lowering the pH of microbial cultures has been considered a feasible approach to reducing the total costs of succinate production by limiting the use of alkali and acids in the fermentation and recovery processes (5, 6). Although anaerobic succinate production by Escherichia coli, Corynebacterium glutamicum, Actinobacillus succinogenes, and Anaerobiospirillum succiniciproducens has been studied with pHs ranging from 6.0 to 7.0 (7–9), few studies have focused on the effect of weakly acidic pH (pH <6.0) on succinate production by bacteria. This is because these bacteria are sensitive to acidic stress and are unable to grow and assimilate carbon sources effectively under weakly acidic conditions (10, 11). One potential solution to this limitation is to develop a new platform for producing succinate by using bacteria that are inherently adapted to acidic conditions (12). Such studies have the potential to advance bacterium-based succinate production processes.
Enterobacter aerogenes can rapidly assimilate carbon sources, such as glucose and glycerol, under moderately acidic conditions (pH <6.0), and it effectively produces biofuels, such as 2,3-butanediol, hydrogen, and ethanol, under anaerobic conditions (13–16). Recently, the whole genome sequence of E. aerogenes KCTC2190 was determined (17). Thus, the anaerobic central pathways involved in ethanol, lactate, 2,3-butanediol, and succinate formation can be predicted. The availability of this information provided the incentive to evaluate the suitability of this organism as a platform for succinate production under weakly acidic and anaerobic conditions.
A newly isolated E. aerogenes strain, AJ110637, that more rapidly consumed glucose under anaerobic conditions (pH 5.0) than did the well-characterized ATCC 13048 strain was selected as the platform strain. This strain was used to construct the ΔadhE/PCK strain, which has inactivated alcohol dehydrogenase (ADH) and introduced Actinobacillus succinogenes phosphoenolpyruvate carboxykinase (PCK). The ΔadhE/PCK strain produced succinate from glucose with a 60% yield under weakly acidic (pH <6.2) and anaerobic conditions (18). Compared with previously constructed E. coli succinate producers, such as KJ122 and KJ134, whose yields are near theoretical (19), the succinate titer and yield from the ΔadhE/PCK strain is lower and needs to be improved with further metabolic engineering applications.
One metabolic modification often used to improve succinate production in various bacteria is the elimination of pathways that compete with succinate synthesis and yield undesirable products (19–21). Differences in anaerobic metabolism between E. aerogenes and well-studied succinate producers, such as E. coli and C. glutamicum, indicate that the target pathways that should be inactivated for improvement of succinate production in E. aerogenes are different from those in E. coli and C. glutamicum (22–25). An optimal strategy for improvement of succinate production by E. aerogenes remains to be determined but is important to better understand the anaerobic metabolism of this organism.
A general strategy to increase succinate synthesis is to enhance the carboxylation pathways (26–28). In particular, the introduction of two carboxylation pathways from phosphoenolpyruvate (PEP) and pyruvate to oxaloacetate (OAA) effectively stimulates succinate production (Fig. 1). For example, coexpression of Sorghum vulgare PEP carboxylase (PPC) and Lactococcus lactis pyruvate carboxylase (PYC) in E. coli increased succinate production to a greater extent than expression of either pathway alone (29). In the present study, based on these strategies, we generated strain ES04/PCK+PYC, with genes adhE, ldhA, pta, and budA deleted and with a new coexpression system involving A. succinogenes PCK and C. glutamicum PYC introduced. This strain produced succinate from glucose with over 70% yield without the formation (i.e., below 0.1 g/liter) of ethanol, lactate, or 2,3-butadiol under a weakly acidic condition. This strain was also used to investigate the impact on succinate production of lowering the pH from 7.0 to 5.5.
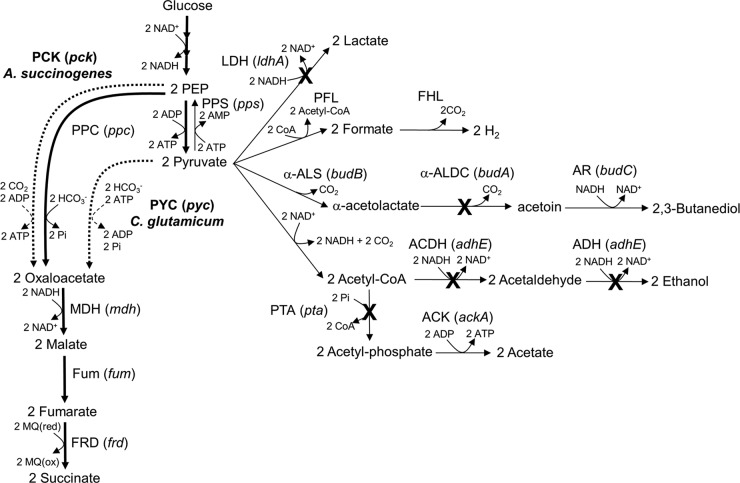
FIG 1.
Pathways involved in ethanol, 2,3-butanediol, lactate, acetate, and formate (thin arrows), as well as succinate synthesis (thick arrows), in E. aerogenes. Broken arrows indicate exogenous pyruvate carboxylase and PEP carboxykinase from C. glutamicum and A. succinogenes, respectively. The gene names or locus symbols are shown in parentheses. PCK, PEP carboxykinase; PYC, pyruvate carboxylase; MDH, malate dehydrogenase; Fum, fumarase; FRD, fumarate reductase; LDH, d-lactate dehydrogenase; PFL, pyruvate formate-lyase; FHL, formate hydrogen lyase; α-ALS, α-acetolactate synthase; α-ALDC, α-acetolactate decarboxylase; AR, acetoin reductase; ACDH, acetaldehyde dehydrogenase; ADH, alcohol dehydrogenase; PTA, phosphate acetyltransferase; ACK, acetate kinase; PPS, PEP synthetase.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Details of the strains and plasmids used in this study are summarized in Table 1. E. aerogenes AJ110637 was deposited at the International Patent Organism Depository, Agency of Industrial Science and Technology (Japan), under accession no. FERM P-21348 (45). The deposit was converted to an international deposit and assigned receipt no. FERM BP-10955 (18). Plasmids were introduced into E. coli and E. aerogenes by electrotransformation. Both E. coli and E. aerogenes were grown in Luria-Bertani (LB) medium at 37°C. When needed, 50 mg/liter kanamycin or 40 mg/liter chloramphenicol was added to select transformants and to maintain the plasmids.
TABLE 1.
Microbial strains and plasmids used in this study
| Strains or plasmid | Description | Antibiotic resistancea | Reference or source |
|---|---|---|---|
| Strains | |||
| AJ110637 | Isolated wild-type strain (FERM BP-10955) | 18 | |
| ΔadhE mutant | AJ110637 ΔadhE | Km | 18 |
| ES02 | AJ110637 ΔadhE ΔldhA | Km | This work |
| ES03 | AJ110637 ΔadhE ΔldhA Δpta | Km | This work |
| ES04 | AJ110637 ΔadhE ΔldhA Δpta ΔbudA | Km | This work |
| Plasmids | |||
| pKD46 | λ Red-expressing plasmid | Ap | 30 |
| pRSFRedTER | Broad-host-range λ Red-expressing plasmid | Cm | 31 |
| pMW-attLλ-Kmr-attRλ | Cassette for gene disruption containing kanamycin resistance gene | Km | 31 |
| pMW-intxis-ts | λ Int/Xis-expressing plasmid | Ap | 32, 33 |
| pRSF-Para-IX | Broad-host-range λ Int/Xis-expressing plasmid | Cm | This work |
| pSTV28 | Plasmid vector with a replication origin of pACYC184 | Cm | TaKaRa BIO |
| pSTV28-pyc | Plasmid for expression of pyc from C. glutamicum | Cm | 18 |
| pSTV28-pck | Plasmid for expression of pck from A. succinogenes | Cm | 18 |
| pSTV28-pck-pyc | Plasmid for coexpression of A. succinogenes pck and C. glutamicum pyc | Cm | This work |
Km, kanamycin; Ap, ampicillin; Cm, chloramphenicol.
Disruption of ldhA, pta, and budA genes.
To disrupt the ldhA, pta, and budA genes, the λ Red gene knockout system was used with the Red-recombineering helper plasmid pRSFRedTER (18, 30, 31) (see Fig. S1A in the supplemental material). A removable kanamycin resistance gene flanked by attLλ and attRλ was amplified with ΔldhA-attL/ΔldhA-attR, Δpta-attL/Δpta-attR, and ΔbudA-attL/ΔbudA-attR primers containing 60-nucleotide (nt) sequences homologous to the target region at the 5′ end of the chromosome. The pMW-attLλ-Kmr-attRλ plasmid was used as the DNA template (30, 31). Replacement of the target genes on the chromosome with the attLλ-Kmr-attRλ fragment was confirmed by PCR using ΔldhA-CF/ΔldhA-CR, Δpta-CF/Δpta-CR, and ΔbudA-CF/ΔbudA-CR as primers. To remove pRSFRedTER from the marker strains, the strains were spread on LB plates containing 10% sucrose and 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG) to obtain single colonies. Removal of pRSFRedTER was confirmed on LB plates containing 40 mg/liter chloramphenicol. The strain that could not grow under these conditions was designated the “pRSFRedTER-free” strain. All primer sequences are listed in Table 2.
TABLE 2.
Oligonucleotide primer sequences used in this study
| Primer | Primer sequence (5′→3′) |
|---|---|
| Disruption of adhE, budA, ldhA, and pta genes | |
| ΔbudA-attL | 5′-AACCTTTATTTAACCTTTCTTATATTTGTTGAACGAGGAAGTGGCTCATGTGAAGCCTGCTTTTTTATACTAAGTTGGC-3′ |
| ΔbudA-attR | 5′-GCGCCCACTGGCGCTGCGGATACTGTTTGTCCATGTGAACCTCCTAACTTCGCTCAAGTTAGTATAAAAAAGCTGAACGA-3′ |
| ΔldhA-attL | 5′-ATGTTAACGACGCATACGGCTTTGAACTGGAATTTTTCGACTTCCTGCTGACCGAAAAGATGAAGCCTGCTTTTTTATACTAAGTTGGC-3′ |
| ΔldhA-attR | 5′-GGCAGGCGGAGAGCCGGCGGAACACGTCATCCTGGATGACATCATTCGATTTATCTTCAACGCTCAAGTTAGTATAAAAAAGCTGAACGA-3′ |
| Δpta-attL | 5′-CTGGCGGTGCTGTTTTGTATCCCGCCTAAAACTGGCGGTAACGAAAGAGGATATATCGTGTGAAGCCTGCTTTTTTATACTAAGTTGGC-3′ |
| Δpta-attR | 5′-GCGTTAGAGCCATAAAAAAGGCAGCCATTTGGCTGCCTTTCTTGTCTCAGCGGGAGATTACGCTCAAGTTAGTATAAAAAAGCTGAACGA-3′ |
| ΔbudA-CF | 5′-TTTCTATATTGGAACTGTGAGCTGAATCG-3′ |
| ΔbudA-CR | 5′-GCTTCCAGTTGGCTGACGACCAGATC-3′ |
| ΔldhA-CF | 5′-TTTCTTAAGACTGCGATATGCTCTAG-3′ |
| ΔldhA-CR | 5′-TTCATTCGTACTCCAAAACATTTGTC-3′ |
| Δpta-CF | 5′-TCGTGAACTGTCCCTGGG-3′ |
| Δpta-CR | 5′-GTCAGCGAGGTATGCTGG-3′ |
| Construction of pRSF-Para-IX | |
| intxis-F | 5′-CATGGCGGCCGCTTATTTGATTTCAATTTTGTCCCAC-3′ |
| intxis-R | 5′-GATCCCGATCGAAGGAGGTTATAAAAAATGGAATTGAATTCGTGTAATTGC-3′ |
| PKD466199F | 5′-GCGCGGTCTCACTCTTCCTTTTTCAATA-3′ |
| PKD461243R | 5′-GGCCCGATCGTTTTTATAACCTCCTTAG-3′ |
Construction of pRSF-Para-IX.
pRSF-Para-IX was constructed to remove the kanamycin resistance gene on the chromosome in the marker strains. The pMW-intxis-ts plasmid carries the gene encoding integrase (Int) of the λ phage and the gene encoding excisionase (Xis) and provides temperature-sensitive replication (32, 33). The pKD46 plasmid carries the gene encoding the λ Red genes under the control of an arabinose promoter (30). DNA fragments containing the xis-int and arabinose promoter regions were amplified by PCR using the intxis-F/intxis-R and PKD466199F/PKD461243R primers and pMW-intxis-ts and pKD46 as the templates, respectively. The xis-int and arabinose promoter regions were cloned into the NotI/PvuI and BsaI/PvuI sites of the pRSFRedTER plasmid, respectively. This plasmid was designated “pRSF-Para-IX” (see Fig. S1B in the supplemental material).
Removal of antibiotic resistance genes from the chromosome.
pRSF-Para-IX was used to remove the kanamycin resistance gene from the genetically modified bacterial chromosome (see Fig. S1 in the supplemental material). pRSF-Para-IX was introduced into the marker strain via electrotransformation. Transformants harboring pRSF-Para-IX were selected on LB plates containing 40 mg/liter of chloramphenicol. Obtained transformants were spread on LB plates containing 40 mg/liter chloramphenicol and 1% l-arabinose. Clones that formed single colonies were identified, and elimination of the kanamycin resistance gene was confirmed by growth on LB medium containing 50 mg/liter kanamycin. Strains that could not grow on the kanamycin plates were utilized as marker-free strains. The procedure to remove pRSF-Para-IX from the marker-free strain was the same as that of pRSFRedTER (18).
Construction of plasmid to co-overexpress A. succinogenes PCK and C. glutamicum PYC.
DNA fragments obtained by SacI digestion, containing the thrL promoter and C. glutamicum pyc of pSTV28-pyc, were ligated into SacI-digested pSTV28-pck in order to obtain a plasmid that coexpressed PCK and PYC. The resultant plasmid was designated “pSTV28-pck-pyc.” The strains harboring pSTV28, pSTV28-pck, pSTV28-pyc, and pSTV28-pck-pyc are formatted as follows: “strain name/empty,” “strain name/PCK,” “strain name/PYC,” and “strain name/PCK+PYC,” respectively (e.g., the ΔadhE mutant harboring pSTV28-pck is represented as the “ΔadhE/PCK” strain).
Succinate fermentation in 1.5-ml microcentrifuge tubes.
To evaluate succinate production under weakly acidic (pH <6.2) and anaerobic conditions, we employed a convenient evaluation system utilizing 1.5-ml microcentrifuge tubes (18). Preculture for cell growth was performed on LB plates at 37°C for 10 h. The precultured cells were then incubated anaerobically at 37°C for 16 h in an AnaeroPack A-04 (Mitsubishi Gas Chemicals, Inc., Tokyo, Japan), washed twice with cold NaCl solution (0.8%), and inoculated into 1.5-ml microcentrifuge tubes containing 1.4 ml MS medium [40 g/liter glucose, 1 g/liter MgSO4·7H2O, 2 g/liter Bacto yeast extract, 1 g/liter (NH4)2SO4·7H2O, 1 g/liter KH2PO4, 10 mg/liter MnSO4·5H2O, 10 mg/liter FeSO4·7H2O, 1 mg/liter biotin] containing 50 g/liter precipitated CaCO3 sterilized by dry heat at 180°C for 3 h (Japanese Pharmacopoeia, Tokyo, Japan). After tight capping, succinate fermentation was performed using an Eppendorf Thermomixer comfort (Eppendorf, Hamburg, Germany) at 34°C and a rotation speed of 1,400 rpm. The initial biomass was adjusted to approximately 1.2 g dry cell weight (DCW) per liter. Using this system, the pH dropped from an initial 6.2 to approximately 5.4 due to the formation of acidic compounds, such as succinate, during fermentation.
pH-controlled succinate fermentation.
pH-controlled succinate fermentation was carried out in a 100-ml jar fermenter with 60 ml MS2 medium [50 g/liter glucose, 1 g/liter MgSO4·7H2O, 2 g/liter Bacto yeast extract, 1 g/liter (NH4)2SO4·7H2O, 1 g/liter KH2PO4, 10 mg/liter MnSO4·5H2O, 10 mg/liter FeSO4·7H2O, 1 mg/liter biotin, 0.05 g GD113 (antifoam reagent)]. The initial pH was adjusted to 5.5, 5.7, or 7.0 by KOH at 34°C. Each pH was maintained with 2 N NaOH during cultivation. Carbon dioxide gas was applied at 40 ml/min, and agitation was set at 700 rpm. Preculture was performed on LB agar at 37°C for 10 h. The cells were then incubated at 37°C for 16 h in an AnaeroPack A-04. Initial biomass was adjusted to approximately 1.2 g (DCW)/liter.
Analysis of metabolites.
Organic acids that accumulated in the medium were analyzed by high-performance liquid chromatography on a CDD-10AD system (Shimadzu Co., Ltd., Kyoto, Japan) after a suitable dilution (34). Acetoin, 2,3-butanediol, and ethanol were quantified on a GC4000 gas chromatograph (GL-Science Co., Ltd., Tokyo, Japan) equipped with a flame ionization detector. A TC-BOND Q (GL-Science) capillary column was used for separation (18). The glucose concentration was analyzed using an AS-310 Biotech analyzer (Sakura SI Co., Ltd., Tokyo, Japan). Yield is expressed as gram of product per gram of consumed glucose (g/g). Optical densities at 600 nm (OD600) were measured with a U-2001 spectrometer (Hitachi Co. Ltd., Tokyo, Japan). Broth containing CaCO3 was diluted with 0.1 N HCl before measurement of OD600. Dry cell weight was calculated according to a standard curve between the g (DCW)/liter and OD600. The formula used to calculate the dry cell weight of E. aerogenes was g (DCW)/liter = 0.291 × OD600.
RESULTS
Development of a λ Int/Xis-dependent selective marker excision system in E. aerogenes.
The λ Red-recombination system has been successfully used to rapidly generate chromosomal modifications in members of the Enterobacteriaceae family (14, 30, 31). In our previous study, we demonstrated λ Red-dependent integration using pRSFRedTER to disrupt the adhE gene in E. aerogenes (Table 1; see Fig. S1A in the supplemental material). To accelerate genome engineering, we modified a selective marker excision system for E. aerogenes. A λ Int/Xis-dependent site-specific recombination system is available to remove selective markers from the E. coli chromosome (see Fig. S1A) (32, 33). In this system, the pMW-intxis-ts plasmid contains β-lactamase as a selective marker and is used for the expression of λ Int/Xis protein in E. coli (Table 1). However, E. aerogenes exhibits inherently strong resistance toward ampicillin, and there are multiple copies of the β-lactamase gene on the E. aerogenes KCTC2190 chromosome. For this reason, we reconstructed a λ Int/Xis expression plasmid from pRSFRedTER that contains a different selection marker, chloramphenicol acetyltransferase (encoded by cat) and levansucrase (encoded by sacB) from Bacillus subtilis. This allows for efficient recovery of this plasmid from cells grown in a sucrose-containing medium (31). A newly constructed λ Int/Xis expression plasmid (pRSF-Para-IX) provided l-arabinose-inducible expression of the xis and int genes under the control of an arabinose promoter (Fig. S1B). A marker-free strain was obtained from the ΔadhE (Kmr) strain harboring pRSF-Para-IX after it was cultured in LB medium containing 1% l-arabinose (details may be found in Materials and Methods).
Effect of inactivation of lactate dehydrogenase on succinate production in the ΔadhE mutant.
Our previous study showed that lactate was a significant by-product in the ΔadhE/PYC and ΔadhE/PCK strains, which produced 7.0 and 1.6 g/liter lactate with yields of 45.5% and 18.6% (g/g), respectively (18). Lactate is formed from pyruvate by coupling with the oxidation of NADH. d-Lactate dehydrogenase (LDH) (encoded by ldhA) is the dominant catalyst for this reaction in E. aerogenes (14) (Fig. 1). The ldhA gene was, therefore, disrupted from the ΔadhE mutant (designated ES02) to reduce lactate formation (Table 1). Then, the heterogeneous carboxylation enzyme expression plasmids, pSTV28-pyc and pSTV28-pck, were introduced into ES02 to enhance succinate production (Table 1). The results of succinate fermentation in 1.5-ml microcentrifuge tubes using these strains are summarized in Table 3. Lactate formation was not observed in ES02/empty. However, the acetate titer and yield were increased in ES02/empty (1.8 g/liter corresponding to a 52.9% yield) compared to the ΔadhE/empty strain (0.5 g/liter corresponding to a 20.8% yield). The succinate titers in strains ES02/PYC and ES02/PCK were 4.8 g/liter and 4.6 g/liter, respectively. These values were approximately 0.9 times of those in the ΔadhE/PYC (5.4 g/liter) and ΔadhE/PCK (5.2 g/liter) strains (Table 3) (18). The succinate yield in ES02/PYC (51.6%) was increased compared with that in the ΔadhE/PYC strain (35.0%). In contrast, the succinate yield in ES02/PCK (63.9%) was similar to that in the ΔadhE/PCK strain (60.5%). Both ES02/PYC and ES02/PCK produced 3.3 g/liter acetate as a major by-product. The yields of acetate in ES02/PYC (35.5%) and ES02/PCK (45.8%) were 1.9 and 1.8 times those in the ΔadhE/PYC (18.8%) and ΔadhE/PCK (25.6%) strains, respectively. Thus, elimination of lactate formation in ES02/PYC and ES02/PCK leads to a slightly decreased succinate titer and enhanced acetate production.
TABLE 3.
Profiles of end pH, biomass, consumed glucose, and end products in multiple knockout mutants overexpressing PYC and/or PCKa
| Strainb | End pH | Biomass (g [DCW]/liter)c | Consumed glucose (g/liter) | End product (g/liter) |
Succinate yield (% [g/g])d | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyruvate | Malate | Formate | Succinate | Lactate | Acetate | Ethanol | 2,3-Butanediol | |||||
| ES02/empty | 5.8 ± 0.1 | 1.2 ± 0.1 | 3.4 ± 0.4 | 0.4 ± 0.1 | NDe | 0.7 ± 0.1 | 0.2 ± 0.1 | ND | 1.8 ± 0.1 | ND | 0.2 ± 0.1 | 5.9 ± 1.1 |
| ES02/PYC | 5.4 ± 0.1 | 1.6 ± 0.1 | 9.3 ± 0.2 | ND | ND | 0.2 ± 0.1 | 4.8 ± 0.1 | ND | 3.3 ± 0.1 | ND | 0.2 ± 0.1 | 51.6 ± 0.9 |
| ES02/PCK | 5.4 ± 0.1 | 1.5 ± 0.1 | 7.2 ± 0.4 | <0.1 | <0.1 | <0.1 | 4.6 ± 0.3 | <0.1 | 3.3 ± 0.1 | ND | 0.2 ± 0.1 | 63.9 ± 3.1 |
| ES03/empty | 5.7 ± 0.1 | 1.3 ± 0.1 | 3.7 ± 0.3 | 0.5 ± 0.1 | ND | 0.3 ± 0.1 | 0.3 ± 0.1 | ND | 0.5 ± 0.2 | <0.1 | 1.4 ± 0.2 | 9.1 ± 2.9 |
| ES03/PYC | 5.5 ± 0.1 | 1.5 ± 0.1 | 8.9 ± 0.5 | ND | ND | <0.1 | 3.8 ± 0.2 | <0.1 | 0.4 ± 0.1 | <0.1 | 3.1 ± 0.2 | 42.7 ± 2.5 |
| ES03/PCK | 5.4 ± 0.1 | 1.5 ± 0.2 | 7.4 ± 0.7 | 0.2 ± 0.1 | ND | 0.2 ± 0.1 | 4.2 ± 0.2 | ND | 0.5 ± 0.2 | <0.1 | 3.7 ± 0.2 | 56.8 ± 4.4 |
| ES04/empty | 5.7 ± 0.1 | 1.4 ± 0.1 | 3.1 ± 0.2 | 0.8 ± 0.1 | ND | 0.4 ± 0.1 | 0.5 ± 0.2 | ND | 0.8 ± 0.1 | <0.1 | <0.1 | 15.1 ± 2.8 |
| ES04/PYC | 5.5 ± 0.1 | 1.6 ± 0.1 | 6.2 ± 0.3 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 3.6 ± 0.3 | <0.1 | 0.4 ± 0.1 | <0.1 | <0.1 | 58.1 ± 4.2 |
| ES04/PCK | 5.5 ± 0.1 | 1.5 ± 0.1 | 5.8 ± 0.3 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 3.5 ± 0.3 | ND | 0.5 ± 0.1 | <0.1 | <0.1 | 60.3 ± 3.6 |
| ES02/PCK+PYC | 5.4 ± 0.1 | 1.5 ± 0.1 | 8.5 ± 0.4 | <0.1 | 0.2 ± 0.1 | <0.1 | 5.7 ± 0.3 | ND | 3.1 ± 0.1 | ND | 0.2 ± 0.1 | 67.0 ± 4.3 |
| ES03/PCK+PYC | 5.4 ± 0.1 | 1.5 ± 0.2 | 9.7 ± 0.9 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 5.9 ± 0.2 | ND | 0.7 ± 0.2 | <0.1 | 3.4 ± 0.2 | 60.8 ± 4.8 |
| ES04/PCK+PYC | 5.4 ± 0.1 | 1.6 ± 0.1 | 11.4 ± 0.9 | 0.3 ± 0.1 | 0.5 ± 0.1 | <0.1 | 8.1 ± 0.4 | <0.1 | 0.6 ± 0.1 | <0.1 | <0.1 | 71.0 ± 2.8 |
Data are expressed as means ± SD from four independent experiments.
All strains were cultivated for 48 h.
Initial biomass was adjusted to 1.2 g (DCW/liter).
Succinate yield is gram of product per gram of consumed glucose expressed as a percentage.
ND, not determined.
Effect of inactivation of PTA on succinate production in ES02.
Acetate was a significant by-product in strains ES02/PYC and ES02/PCK. Therefore, we next disrupted the pta gene in strain ES02 to reduce acetate production. The pta gene codes for phosphoacetyl transferase (PTA), which catalyzes the reversible conversion of acetyl coenzyme A (acetyl-CoA) to acetylphosphate in E. coli (Fig. 1). Because the level of pyruvate increases under anaerobic conditions and activates PTA toward acetylphosphate synthesis, anaerobic conditions favor the production of acetylphosphate. Therefore, PTA is a target of metabolic engineering to reduce acetate production and, subsequently, to increase succinate production in E. coli (25).
The Δpta mutant of strain ES02 was designated ES03 (Table 1). The strains ES03/empty, ES03/PYC, and ES03/PCK were constructed and evaluated for fermentative products (Table 3). The acetate titer and yield were decreased in ES03/empty (0.5 g/liter corresponding to a 13.5% yield) compared to ES02/empty (1.8 g/liter corresponding to a 52.9% yield). However, the 2,3-butanediol titer in ES03/empty (1.4 g/liter corresponding to a 37.8% yield) was approximately 7-fold higher than that of ES02/empty (0.2 g/liter corresponding to a 5.9% yield).
The strains ES03/PYC and ES03/PCK produced 3.8 and 4.2 g/liter succinate from 8.9 and 7.4 g/liter consumed glucose, respectively, resulting in succinate yields of 42.7% and 56.8%, respectively (Table 3). Although inactivation of PTA was effective in decreasing acetate formation, it also decreased succinate production because of the increased 2,3-butanediol production in ES03/PYC and ES03/PCK.
Effect of inactivating α-acetolactate decarboxylase on succinate production in ES03.
Our previous study showed that deletion of the budA gene encoding α-acetolactate decarboxylase eliminated both acetoin and 2,3-butanediol production (Fig. 1). The budA gene was therefore disrupted from strain ES03 to obtain ES04 (Table 1). The production of succinate in ES04/empty, ES04/PYC, and ES04/PCK is summarized in Table 3. Both the titer and yield of acetate and pyruvate in ES04/empty (pyruvate, 0.8 g/liter, corresponding to a 25.8% yield; acetate, 0.8 g/liter, corresponding to a 25.8% yield) were increased over 1.6 times compared to those in ES03/empty (pyruvate, 0.5 g/liter, corresponding to a 13.5% yield; acetate, 0.5g/liter, corresponding to a 13.5% yield).
Strains ES04/PYC and ES04/PCK produced 3.6 and 3.5 g/liter succinate from 6.2 and 5.8 g/liter consumed glucose, resulting in yields of 58.1% and 60.3%, respectively (Table 3). These succinate yield values were increased approximately 1.4- and 1.1-fold from those of ES03/PYC (42.7%) and ES03/PCK (56.8%), respectively. However, the succinate titers in ES04/PYC (3.6 g/liter) and ES04/PCK (3.5 g/liter) were slightly decreased from those in ES03/PYC (3.8 g/liter) and ES03/PCK (4.2 g/liter). Elimination of the 2,3-butanediol synthesis pathway slightly increased the succinate yield but decreased its titer because of increased pyruvate and acetate production in ES04/PYC and ES04/PCK.
A metabolic engineering approach based on the elimination of pyruvate node reactions involved in lactate, acetate, and 2,3-butanediol synthesis from the ΔadhE/PCK strain contributes to decreasing the total amount of by-products (Table 3). However, a decreased formation of by-products did not correlate with increased succinate production in strains overexpressing PYC or PCK (Fig. 2B).
FIG 2.
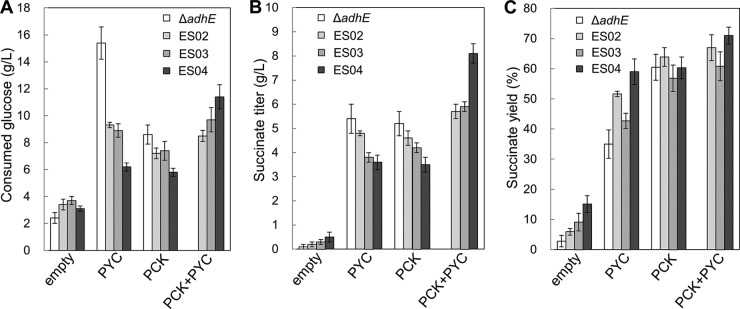
Consumed glucose, succinate titer, and succinate yield of all strains using 1.5-ml microcentrifuge tube scale fermentation. (A) Consumed glucose (g/liter); (B) succinate titer (g/liter); (C) succinate yield (%). The values from the ΔadhE/empty, ΔadhE/PYC, and ΔadhE/PCK strains were excerpted from our previous work (18). Data are expressed as means ± standard deviations (SD).
Coexpression of C. glutamicum pyc and A. succinogenes pck in ES02, ES03, and ES04.
To investigate the effect of coexpressing PCK and PYC on succinate production, the coexpression plasmid pSTV28-pck-pyc was introduced into strains ES02, ES03, and ES04. The succinate titer and yield in all strains was increased when either PYC or PCK was overexpressed but was greatest when they were coexpressed (Fig. 2B and C and Table 3). The highest production of succinate was observed in strain ES04/PCK+PYC. The succinate titer of this strain was 8.1 g/liter, which was approximately 2.3-fold higher than those of ES04/PCK (3.5 g/liter) and ES04/PYC (3.6 g/liter). The succinate yield in ES04/PCK+PYC reached 71.0% and was approximately 1.2-fold higher than those of ES04/PYC (58.1%) and ES04/PCK (60.3%). The yields of pyruvate (2.6%) and acetate (5.2%) in ES04/PCK+PYC were slightly decreased from those in ES04/PYC (pyruvate, 3.2%; acetate, 6.5%) and ES04/PCK (pyruvate, 3.4%; acetate, 8.6%). Thus, this new coexpression system was more effective in increasing both the succinate titer and yield than when PCK and PYC were individually expressed.
Succinate production in ES04/PCK+PYC under pH-controlled conditions.
To evaluate the impact on succinate production of lowering the culture pH from neutral to weakly acidic conditions, Strain ES04/PCK+PYC was cultured at pH 5.5, 5.7, or 7.0 in a 100-ml jar fermenter. The results are summarized in Fig. 3 and Table 4. At pH 5.7, the succinate titer and yield were 11.2 g/liter and 72.7%, respectively. These values and the profiles of end products were similar to those obtained in 1.5-ml microcentrifuge tubes (Table 3). Lowering the pH from 7.0 to 5.5 had negative effects on the succinate titer but positive effects on its yield (Fig. 3, Table 4). The succinate titer at pH 5.5 was 5.1 g/liter, which was only 22.6% of that at pH 7.0 (22.6 g/liter). In contrast, the succinate yield at pH 5.5 was 70.8%, 1.3 times higher than that at pH 7.0 (55.8%). At pH 7.0, the titers of pyruvate (5.1 g/liter) and malate (5.4 g/liter) were increased 10 and 27 times compared with those at pH 5.5, respectively. Thus, lowering the pH from 7.0 to a weakly acidic pH (pH 5.5 and 5.7) leads to a decreased succinate titer but increased yield.
FIG 3.
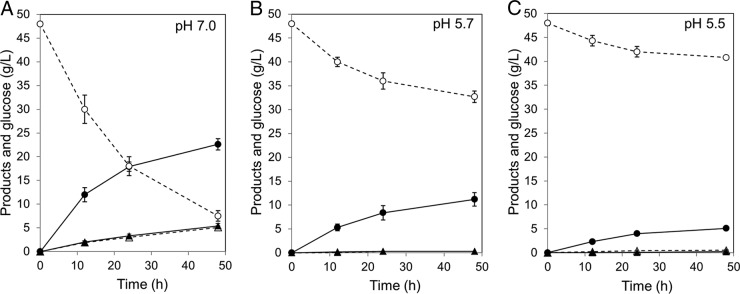
Profiles of main products and glucose of strain ES04/PCK+PYC under pH-controlled conditions. The pH was maintained at 7.0 (A), 5.7 (B), and 5.5 (C). Symbols indicate glucose (open circles), succinate (closed circles), pyruvate (open triangles), and malate (closed triangles). Data from three independent experiments are shown. Error bars indicate ±SD.
TABLE 4.
Profiles of biomass, consumed glucose, end products, succinate yield, and volumetric productivity in strain ES04/PCK+PYC under pH-controlled batch culture conditionsa
| Controlled pHb | Biomass (g [DCW]/liter)c | Consumed glucose (g/liter) | End product (g/liter) |
Succinate yield (% [g/g])d | Volumetric productivity (g/liter/h)e | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyruvate | Malate | Formate | Succinate | Lactate | Acetate | Ethanol | 2,3-Butanediol | |||||
| 7.0 | 1.5 ± 0.1 | 40.5 ± 1.1 | 5.1 ± 0.3 | 5.4 ± 0.5 | 1.0 ± 0.1 | 22.6 ± 1.2 | 0.3 ± 0.1 | 1.1 ± 0.4 | 0.2 ± 0.1 | <0.1 | 55.8 ± 3.8 | 0.47 ± 0.02 |
| 5.7 | 1.3 ± 0.1 | 15.4 ± 1.2 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.1 | 11.2 ± 1.4 | 0.1 ± 0.1 | 0.5 ± 0.1 | <0.1 | <0.1 | 72.7 ± 3.6 | 0.23 ± 0.03 |
| 5.5 | 1.2 ± 0.1 | 7.2 ± 0.4 | 0.5 ± 0.1 | 0.2 ± 0.1 | <0.1 | 5.1 ± 0.2 | 0.1 ± 0.1 | 0.4 ± 0.1 | <0.1 | <0.1 | 70.8 ± 0.5 | 0.11 ± 0.01 |
Data are expressed as means ± SD from three independent experiments.
pH was maintained at 7.0, 5.7, and 5.5 by 2 N NaOH.
Initial biomass was adjusted to 1.2 g (DCW)/liter.
Succinate yield is gram of product per gram of consumed glucose expressed as a percentage.
Volumetric productivity indicates the succinate titer (g/liter/h).
DISCUSSION
Two general metabolic engineering approaches have been used with various bacteria to increase carbon flux toward succinate synthesis: (i) elimination of pathways that compete for the carbon needed for succinate synthesis (19–21) and (ii) enhancement of carboxylation pathways from PEP and/or pyruvate to OAA (26–29). In the present study, both strategies were utilized, and the effects on succinate production in E. aerogenes were determined. As shown Fig. 2, an increased titer and yield of succinate were evident in strains coexpressing PCK and PYC. The extent of this increase depended upon which competing pathways were eliminated. These results strongly suggested that combination approaches, elimination of by-product pathways and this coexpression system, are necessary to maximally improve succinate production in E. aerogenes. Thus, strain ES04 (ΔadhE ΔldhA Δpta ΔbudA)/PCK+PYC produced succinate with small amounts of by-products and over a 70% yield at pH 5.5 (Fig. 3, Table 4).
Earlier studies showed that elimination of pyruvate node reactions effectively increased succinate production in E. coli and C. glutamicum strains overexpressing PYC (20, 27, 35–37). In E. aerogenes, this approach increased the succinate yield but decreased its titer (Fig. 2B and C) (Table 3). The succinate yield and titer in strain ES04/PYC were 1.7 and 0.8 times, respectively, those of the “parental” ΔadhE/PYC strain. Removing the pyruvate node reactions increased the flux of the PYC-dependent reaction, thereby increasing the succinate yield. However, removing these reactions also promoted redox imbalance, resulting in decreased glucose consumption. For example, a decrease in glucose consumption was observed in the PYC-overexpressing strain when the lactate and 2,3-butanediol synthesis pathways, which are involved in NADH reoxidation, were inactivated (Fig. 2A). These results strongly suggested that succinate production in the strain overexpressing PYC was not limited by the supply of NADH.
Interestingly, eliminating the pyruvate node reactions in E. aerogenes strains overexpressing PCK decreased the succinate titer but did not influence the succinate yield, which was approximately 60% in the ΔadhE, ES02, ES03, and ES04 PCK-overexpressing strains (Fig. 2C). This phenomenon can be explained by the balance of PEP and pyruvate when glucose is imported via phosphotransferase (PTS), which is the predominant glucose uptake system in E. aerogenes. In the PTS system, the import of 1 mol of glucose yields 1 mol of pyruvate and PEP. A PCK-dependent reaction converts most of the PEP to OAA, while most of the pyruvate is converted to by-products in the PCK-overexpressing strains (Fig. 1). Overall, succinate yields in these strains were similar and approximately half of the maximum theoretical yield (112.3%).
In E. aerogenes, pyruvate can be presumably converted to PEP by PEP synthetase (PPS) when coupled with the conversion of ATP to AMP (Fig. 1). However, we speculate that this reaction is performed in an ATP-dependent manner and thus is largely inactive under energy-insufficient anaerobic conditions. Thus, the production of succinate in the strains overexpressing PCK is limited by the supply of PEP.
To overcome these factors limiting succinate production in strains overexpressing PYC or PCK, two carboxylation pathways from PEP and pyruvate to OAA were introduced (29). This approach was feasible because overexpressing PYC concurrently with PCK can recapture the pyruvate generated by glucose-PTS and direct it back to OAA. Subsequently, the increased OAA pool contributes to improve redox balance by effectively consumption of NADH via malate dehydrogenase (MDH) in succinate synthesis pathways (Fig. 1). We used A. succinogenes PCK and C. glutamicum PYC to create a new coexpression system in strains ES02, ES03, and ES04. This coexpression resulted in strains with a succinate yield and titer that were higher than those when either PYC or PCK was overexpressed individually (Fig. 2, Table 3). Overall, this expression system effectively functions to increase succinate production in these strains with eliminated pyruvate node reactions.
There are only a few previous reports on the impact of low pH (below pH 6.0) on succinate production in bacteria, even though this is considered economically favorable compared to neutral pH (7). As shown Fig. 3 and Table 4, at pH 5.5 strain ES04/PCK+PYC produced 5.1 g/liter succinate with a 70.8% yield. Interestingly, the succinate yield at pH 5.5 and 5.7 was approximately 1.3 times higher than that at pH 7.0 (55.8%). This is because the formation of excess pyruvate and malate was only observed at pH 7.0. These data suggested that downstream reactions from malate, such as fumarase (FUM), fumarate reductase (FRD), and the succinate exporter, limited succinate production under neutral pH conditions (Fig. 1). Although enhanced MDH activity leads to increased succinate production in E. coli (38), other downstream reactions, such as FRD, regulate succinate production in ES04/PCK+PYC (Fig. 1). FRD activity is induced via FNR (a DNA-binding transcriptional dual regulator) under anaerobic conditions in E. coli (39). Therefore, if anaerobic conditions are insufficient to induce FRD activity, an excess level of malate can be formed. The effect of decreasing malate production on succinate production by continuously expressing a high level of FRD in ES04/PCK+PYC remains to be determined.
The results from pH-controlled fermentation showed that a decreased pH decreased the succinate titer in strain ES04/PCK+PYC, similar to previous reports in E. coli (7, 40) (Fig. 3, Table 4). Maintaining a constant pH in the cell under acidic conditions requires more energy than under neutral conditions because of the demands imposed by acid resistance mechanisms, such as the proton efflux pump. This can decrease overall energy availability, thereby limiting succinate production under acidic and anaerobic conditions. To improve the production of succinate under these conditions, genetic modifications based on an energy-conserving strategy may be effective. Previous studies indicated that replacing glucose uptake from PTS with glucose permease to increase the PEP supply resulted in an increase net yield of ATP because of the dominant PEP carboxylation by PCK with ATP generation in E. coli (41–44). In the near future, we will construct a further engineered strain based on the energy-conserving strategy and evaluate the impact of increase net ATP yield on succinate production under weakly acidic (pH <6.0) and anaerobic conditions. We believe that these studies will be useful to understand the relationship between the net yield of ATP and succinate production and to advance our understanding of bacterium-based succinate fermentation under acidic and anaerobic conditions.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. I. Katashkina for providing experimental materials and Kazue Kawamura for useful discussions.
This study was funded by Ajinomoto Co., Inc. No external funds were used.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03213-14.
REFERENCES
- 1.Buschke N, Schäfer R, Becker J, Wittmann C. 2013. Metabolic engineering of industrial platform microorganisms for biorefinery applications–optimization of substrate spectrum and process robustness by rational and evolutive strategies. Bioresour Technol 135:544–554. doi: 10.1016/j.biortech.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 2.Jang Y-S, Kim B, Shin JH, Choi YJ, Choi S, Song CW, Lee J, Park HG, Lee SY. 2012. Bio-based production of C2-C6 platform chemicals. Biotechnol Bioeng 109:2437–2459. doi: 10.1002/bit.24599. [DOI] [PubMed] [Google Scholar]
- 3.McKinlay JB, Vieille C, Zeikus JG. 2007. Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740. doi: 10.1007/s00253-007-1057-y. [DOI] [PubMed] [Google Scholar]
- 4.Zeikus JG, Jain MK, Elankovan P. 1999. Biotechnology of succinic acid production and markets for derived industrial products. Appl Microbiol Biotechnol 51:545–552. doi: 10.1007/s002530051431. [DOI] [Google Scholar]
- 5.Cheng K-K, Zhao X-B, Zeng J, Wu R-C, Xu Y-Z, Liu D-H, Zhang J-A. 2012. Downstream processing of biotechnological produced succinic acid. Appl Microbiol Biotechnol 95:841–850. doi: 10.1007/s00253-012-4214-x. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Wang D, Wu Y, Li W, Zhang Y, Xing J, Su Z. 2010. One step recovery of succinic acid from fermentation broths by crystallization. Sep Purif Technol 72:294–300. doi: 10.1016/j.seppur.2010.02.021. [DOI] [Google Scholar]
- 7.Lu S, Eiteman MA, Altman E. 2009. pH and base counterion affect succinate production in dual-phase Escherichia coli fermentations. J Ind Microbiol Biotechnol 36:1101–1109. doi: 10.1007/s10295-009-0594-z. [DOI] [PubMed] [Google Scholar]
- 8.Samuelov NS, Lamed R, Lowe S, Zeikus JG. 1991. Influence of CO(2)-HCO(3) levels and pH on growth, succinate production, and enzyme activities of Anaerobiospirillum succiniciproducens. Appl Environ Microbiol 57:3013–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Werf MJ, Guettler MV, Jain MK, Zeikus JG. 1997. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z Arch Microbiol 167:332–342. [DOI] [PubMed] [Google Scholar]
- 10.Foster JW. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat Rev Microbiol 2:898–907. doi: 10.1038/nrmicro1021. [DOI] [PubMed] [Google Scholar]
- 11.Warnecke T, Gill RT. 2005. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Fact 4:25. doi: 10.1186/1475-2859-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsuji A, Okada S, Hols P, Satoh E. 2013. Metabolic engineering of Lactobacillus plantarum for succinic acid production through activation of the reductive branch of the tricarboxylic acid cycle. Enzyme Microb Technol 53:97–103. doi: 10.1016/j.enzmictec.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Lv F-X, Xing X-H. 2011. Bioengineering of the Enterobacter aerogenes strain for biohydrogen production. Bioresour Technol 102:8344–8349. doi: 10.1016/j.biortech.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Jung M-Y, Ng CY, Song H, Lee J, Oh M-K. 2012. Deletion of lactate dehydrogenase in Enterobacter aerogenes to enhance 2,3-butanediol production. Appl Microbiol Biotechnol 95:461–469. doi: 10.1007/s00253-012-3883-9. [DOI] [PubMed] [Google Scholar]
- 15.Nwachukwu R, Shahbazi A, Wang L, Ibrahim S, Worku M, Schimmel K. 2012. Bioconversion of glycerol to ethanol by a mutant Enterobacter aerogenes. AMB Express 2:20. doi: 10.1186/2191-0855-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Converti A, Perego P. 2002. Use of carbon and energy balances in the study of the anaerobic metabolism of Enterobacter aerogenes at variable starting glucose concentrations. Appl Microbiol Biotechnol 59:303–309. doi: 10.1007/s00253-002-1009-5. [DOI] [PubMed] [Google Scholar]
- 17.Shin SH, Kim S, Kim JY, Lee S, Um Y, Oh M-K, Kim Y-R, Lee J, Yang K-S. 2012. Complete genome sequence of Enterobacter aerogenes KCTC 2190. J Bacteriol 194:2373–2374. doi: 10.1128/JB.00028-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tajima Y, Kaida K, Hayakawa A, Fukui K, Nishio Y, Hashiguchi K, Fudou R, Matsui K, Usuda Y, Sode K. 2014. Study of the role of anaerobic metabolism in succinate production by Enterobacter aerogenes. Appl Microbiol Biotechnol 98:7803–7813. doi: 10.1007/s00253-014-5884-3. [DOI] [PubMed] [Google Scholar]
- 19.Jantama K, Zhang X, Moore JC, Shanmugam KT, Svoronos SA, Ingram LO. 2008. Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol Bioeng 101:881–893. doi: 10.1002/bit.22005. [DOI] [PubMed] [Google Scholar]
- 20.Inui M, Murakami S, Okino S, Kawaguchi H, Vertès AA, Yukawa H. 2004. Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7:182–196. doi: 10.1159/000079827. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Song H, Lee SY. 2006. Genome-based metabolic engineering of Mannheimia succiniciproducens for succinic acid production. Appl Environ Microbiol 72:1939–1948. doi: 10.1128/AEM.72.3.1939-1948.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SY, Hong SH, Moon SY. 2002. In silico metabolic pathway analysis and design: succinic acid production by metabolically engineered Escherichia coli as an example. Genome Inform 13:214–223. [PubMed] [Google Scholar]
- 23.Wendisch VF, Bott M, Eikmanns BJ. 2006. Metabolic engineering of Escherichia coli and Corynebacterium glutamicum for biotechnological production of organic acids and amino acids. Curr Opin Microbiol 9:268–274. doi: 10.1016/j.mib.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Thakker C, Martínez I, San K-Y, Bennett GN. 2012. Succinate production in Escherichia coli. Biotechnol J 7:213–224. doi: 10.1002/biot.201100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez AM, Bennett GN, San K-Y. 2005. Novel pathway engineering design of the anaerobic central metabolic pathway in Escherichia coli to increase succinate yield and productivity. Metab Eng 7:229–239. doi: 10.1016/j.ymben.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Kim P, Laivenieks M, Vieille C, Zeikus JG. 2004. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli. Appl Environ Microbiol 70:1238–1241. doi: 10.1128/AEM.70.2.1238-1241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okino S, Noburyu R, Suda M, Jojima T, Inui M, Yukawa H. 2008. An efficient succinic acid production process in a metabolically engineered Corynebacterium glutamicum strain. Appl Microbiol Biotechnol 81:459–464. doi: 10.1007/s00253-008-1668-y. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Zhu J, Bennett GN, San K-Y. 2011. Succinate production from different carbon sources under anaerobic conditions by metabolic engineered Escherichia coli strains. Metab Eng 13:328–335. doi: 10.1016/j.ymben.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Lin H, San K-Y, Bennett GN. 2005. Effect of Sorghum vulgare phosphoenolpyruvate carboxylase and Lactococcus lactis pyruvate carboxylase coexpression on succinate production in mutant strains of Escherichia coli. Appl Microbiol Biotechnol 67:515–523. doi: 10.1007/s00253-004-1789-x. [DOI] [PubMed] [Google Scholar]
- 30.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K–12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katashkina JI, Hara Y, Golubeva LI, Andreeva IG, Kuvaeva TM, Mashko SV. 2009. Use of the lambda Red-recombineering method for genetic engineering of Pantoea ananatis. BMC Mol Biol 10:34. doi: 10.1186/1471-2199-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peredelchuk MY, Bennett GN. 1997. A method for construction of E. coli strains with multiple DNA insertions in the chromosome. Gene 187:231–238. doi: 10.1016/S0378-1119(96)00760-3. [DOI] [PubMed] [Google Scholar]
- 33.Minaeva NI, Gak ER, Zimenkov DV, Skorokhodova AY, Biryukova IV, Mashko SV. 2008. Dual-in/out strategy for genes integration into bacterial chromosome: a novel approach to step-by-step construction of plasmid-less marker-less recombinant E. coli strains with predesigned genome structure. BMC Biotechnol 8:63. doi: 10.1186/1472-6750-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fukui K, Koseki C, Yamamoto Y, Nakamura J, Sasahara A, Yuji R, Hashiguchi K, Usuda Y, Matsui K, Kojima H, Abe K. 2011. Identification of succinate exporter in Corynebacterium glutamicum and its physiological roles under anaerobic conditions. J Biotechnol 154:25–34. doi: 10.1016/j.jbiotec.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Gokarn RR, Evans JD, Walker JR, Martin SA, Eiteman MA, Altman E. 2001. The physiological effects and metabolic alterations caused by the expression of Rhizobium etli pyruvate carboxylase in Escherichia coli. Appl Microbiol Biotechnol 56:188–195. doi: 10.1007/s002530100661. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez AM, Bennett GN, San K-Y. 2005. Efficient succinic acid production from glucose through overexpression of pyruvate carboxylase in an Escherichia coli alcohol dehydrogenase and lactate dehydrogenase mutant. Biotechnol Prog 21:358–365. doi: 10.1021/bp049676e. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez AM, Bennett GN, San K-Y. 2006. Batch culture characterization and metabolic flux analysis of succinate-producing Escherichia coli strains. Metab Eng 8:209–226. doi: 10.1016/j.ymben.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Liang L-Y, Liu R, Ma J, Chen K, Jiang M, Wei P. 2011. Increased production of succinic acid in Escherichia coli by overexpression of malate dehydrogenase. Biotechnol Lett 33:2439–2444. doi: 10.1007/s10529-011-0707-4. [DOI] [PubMed] [Google Scholar]
- 39.Tseng CP, Albrecht J, Gunsalus RP. 1996. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol 178:1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martínez I, Lee A, Bennett GN, San K-Y. 2011. Culture conditions' impact on succinate production by a high succinate producing Escherichia coli strain. Biotechnol Prog 27:1225–1231. doi: 10.1002/btpr.641. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO. 2009. Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci U S A 106:20180–20185. doi: 10.1073/pnas.0905396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh A, Cher Soh K, Hatzimanikatis V, Gill RT. 2011. Manipulating redox and ATP balancing for improved production of succinate in E. coli. Metab Eng 13:76–81. doi: 10.1016/j.ymben.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Tang J, Zhu X, Lu J, Liu P, Xu H, Tan Z, Zhang X. 2013. Recruiting alternative glucose utilization pathways for improving succinate production. Appl Microbiol Biotechnol 97:2513–2520. doi: 10.1007/s00253-012-4344-1. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Jantama K, Shanmugam KT, Ingram LO. 2009. Reengineering Escherichia coli for succinate production in mineral salts medium. Appl Environ Microbiol 75:7807–7813. doi: 10.1128/AEM.01758-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tajima Y, Keita F, Hashiguchi K. August 2012. Method for producing an organic acid. US patent 8,247,201 B2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.