Abstract
The United States has federal regulations in place to reduce the risk of seafood-related infection caused by the estuarine bacteria Vibrio vulnificus and Vibrio parahaemolyticus. However, data to support the development of regulations have been generated in a very few specific regions of the nation. More regionally specific data are needed to further understand the dynamics of human infection relating to shellfish-harvesting conditions in other areas. In this study, oysters and water were collected from four oyster harvest sites in North Carolina over an 11-month period. Samples were analyzed for the abundances of total Vibrio spp., V. vulnificus, and V. parahaemolyticus; environmental parameters, including salinity, water temperature, wind velocity, and precipitation, were also measured simultaneously. By utilizing these data, preliminary predictive management tools for estimating the abundance of V. vulnificus bacteria in shellfish were developed. This work highlights the need for further research to elucidate the full suite of factors that drive V. parahaemolyticus abundance.
INTRODUCTION
In the United States, it is estimated that as many as 84,000 people annually contract food-borne infections caused by Vibrio bacteria (1). These aquatic bacteria are found in coastal or estuarine environments as part of the natural flora but can become highly concentrated in filter-feeding sea life, including shellfish such as oysters (2, 3). Because oysters are often consumed raw or undercooked, vibrios concentrated within the oysters remain viable and infectious. Reported infections from food-borne Vibrio spp. are on the rise and are currently at the highest level since tracking began (4). While no fewer than 12 species of Vibrio are capable of infection, the 2 most common in the United States are Vibrio parahaemolyticus and Vibrio vulnificus, which cause the most infections and the most deaths, respectively (5–7). Symptoms associated with infections caused by these two species range from gastroenteritis to grievous wound infections or primary septicemia, with case fatality rates as high as 50% (2, 7–11).
Both of these important bacterial species have been reported to exhibit seasonality, with warmer water temperatures resulting in increased Vibrio occurrence and concentrations in oysters (12–15). As a consequence, more than 75% of the infections caused by Vibrio spp. in the United States are observed between May and October (14). While no maximum environmental temperature has been reported, the minimum water temperature needed for the isolation of culturable V. vulnificus from oysters differs among studies but is most often reported in the range of 12 to 17°C; however, lower temperatures have also been documented in individual studies (3, 12, 13, 15–20). Similarly, V. parahaemolyticus can grow in culture at a minimum temperature of approximately 10°C (21). The typical minimum water temperatures associated with oyster-related human disease reported for V. vulnificus and V. parahaemolyticus are ca. 20°C and 15°C, respectively (20–22). In addition to water temperature, the warmer air temperatures from June through September in the United States can also contribute to the increased rate of infections. Empirical data and predictive modeling have both shown that the growth of Vibrio spp. in shellfish after harvest poses considerable risk to consumers. This bacterial growth is a result of the internal warming that oysters undergo after being removed from the water. The inability of oysters to expel these growing bacteria also contributes to their increased numbers. Currently, federal regulations are in place that limit the time oysters can be exposed to warm air temperatures during harvest (23–26).
Salinity is also a factor in Vibrio abundance. V. vulnificus and V. parahaemolyticus concentrations in oysters appear to have a nonlinear relationship with water column salinity, although this is confounded by conflicting reports of positive, negative, and noncorrelating data (3, 13, 15, 16, 27–35). The optimal salinity ranges for V. vulnificus and V. parahaemolyticus in oysters have been reported to be 5 to 25‰ and 10 to 34‰, respectively (3, 20). Not surprisingly, given their close relationship, these salinity values overlap the optimal salinity ranges for oyster growth, survival, and recruitment. Salinity ranges of 10 to 28‰ have been found permissive for growth and reproduction, and salinity ranges of 18 to 22‰ have been found permissive for the settlement of oyster larvae (36, 37). Confounding these findings are the combined effects of salinity and temperature on the concentrations of Vibrio spp. in oysters. Most research finds that as water temperatures increase, so do the survivability and abundance of V. vulnificus at greater salinities. This indicates that the salinity and water temperature should be viewed in conjunction and the individual effect of each should be observed with caution (3, 12, 19).
V. vulnificus strains are not equal in potential infectivity. Typically, the strains of V. vulnificus that most often cause disease via ingestion are those containing the C allele of vcg (the virulence-correlated gene). Those with the E allele of vcg are typically less likely to cause seafood-related disease (38, 39). Strains with the C allele of the vcg gene are referred to as C-genotype strains, and those with the E allele are termed E-genotype strains. Understanding the relative abundances of these two types could play a role in determining the risk to an oyster consumer.
In this study, oysters and water were collected from four actively utilized oyster harvest sites in North Carolina over an 11-month period. Samples were analyzed for the abundances of total Vibrio spp., V. vulnificus, and V. parahaemolyticus; environmental parameters, including salinity, water temperature, wind velocity, and precipitation, were also measured simultaneously. By utilizing these data, preliminary predictive management tools for estimating the abundances of these Vibrio spp. in shellfish were developed.
MATERIALS AND METHODS
Sampling sites.
Samples were collected from four oyster harvest sites in eastern North Carolina, including North River, South River, Hoop Pole Creek, and Harlowe Creek (Fig. 1). These sites were selected because they represent areas that include high and low salinities, that experience either wide fluctuations in water column salinity or very small exchanges in salinity, and that are accessible (within 10 km) to the laboratory, allowing for rapid processing after oyster harvest and water collection.
FIG 1.
Map of eastern North Carolina. The oyster and water collection sites, South River (A), North River (B), Harlowe Creek (C), and Hoop Pole Creek (D), are indicated. Image from the North Carolina Department of Transportation (NCDOT).
Oyster sample collection and processing.
Oyster samples were collected from 4 February 2013 to 18 December 2013. There were 56 separate sampling events each for water and oyster samples. Ten market-sized oysters were collected from each sampling site on each of the days of sampling. Sites were typically sampled every 2 weeks, and alternate sites were sampled weekly. Shellfish were collected by dredge, rake, tongs, or hand, and were then placed in plastic bags, which were kept in coolers on ice during transport to the laboratory. In all cases, samples were transported and processed within 5 h of collection. Shellfish were cleaned of mud with a brush, rinsed with 70% ethanol, and dried with paper towels. Oysters were aseptically shucked with ethanol-sterilized instruments, and oyster meat was rinsed gently with sterile phosphate-buffered saline (PBS; Amresco, Solon, OH) to remove sediment. The 10 oysters from each site were separated into two groups of 5 oysters each. The meats from each group were pooled, drained of mantle fluid and hemolymph, and weighed, and an equal amount of PBS (wt/vol) was added to each batch of oyster tissues. The tissues were homogenized in a Waring (Stamford, CT) blender with three cycles of blending for 15 s, followed by 5 s of rest. Oyster homogenates were diluted 1:10 with PBS, and 100 μl of the original homogenate and 100 μl the diluted sample were each used for bacterial culture, as described below.
Water sample collection and processing.
Water samples for each site were collected simultaneously with oyster samples. Sterile clear plastic 1-liter Nalgene (Rochester, NY) bottles were rinsed three times with water immediately surrounding the oyster sample collection area; then they were filled, capped, and placed on ice. Salinity was measured with an HI 96822 digital refractometer (Hanna Instruments, Carrollton, TX). Water temperature was measured at the time of collection, and statistics for wind speed and cumulative 24-h precipitation around the sample area were collected from local weather stations. Water samples of 1 to 10 ml were vacuum filtered through a 47-mm-diameter, 0.45-μm-pore-size mixed cellulose ester filter (Pall, Port Washington, NY) and were placed on selective media as described below.
Media and growth conditions.
CHROMagar Vibrio medium (CHROMagar, Paris, France) was prepared as per the manufacturer's instructions and was used to select for presumptive V. vulnificus (dark blue colonies) and V. parahaemolyticus (dark purple colonies) isolates from water and oysters. Thiosulfate-citrate-bile salts-sucrose (TCBS) agar and heart infusion broth (HI) were prepared according to the manufacturer's instructions (Becton, Dickinson and Company [BD], Franklin Lakes, NJ). TCBS was used to estimate total Vibrio sp. abundance, with green and yellow colonies summed. Heart infusion broth was used to grow pure cultures of individual isolates, as detailed below. All media were incubated at 37°C for 24 h. After incubation, the colonies growing on these plates were counted, and the data were transformed to CFU per gram of oyster or CFU per milliliter of water. This presumed number was then multiplied by the percentage of isolates that were molecularly confirmed to be V. vulnificus or V. parahaemolyticus (via the method described below) in order to obtain an assumed value at each point.
Molecular confirmation of isolates.
After incubation and enumeration, 10 presumptive V. vulnificus colonies and 10 presumptive V. parahaemolyticus colonies from both water and oyster samples were isolated from each site at each sampling point. These were grown in pure culture in HI broth, boiled for 10 min, and centrifuged at 10,000 × g for 10 min, and the pellet was discarded. The remaining supernatants were stored at −20°C until they were used as templates for PCR confirmation. Molecular identification of V. parahaemolyticus was confirmed by targeting the flaE gene, using primers flaE F and flaE R as described by Tarr et al. (40). V. vulnificus was confirmed based on the presence of the hemolysin/cytolysin gene vvhA and was further genotyped using a multiplex PCR based on the identification of one of two alleles of the virulence-correlated gene, vcgC or vcgE, by using primers and protocols previously published by Warner and Oliver (41), with slight modifications. The master mix comprised 1× GoTaq buffer, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTP), 0.4 μM vvhA, vcgC, and vcgE primers, and 1.25 U of GoTaq DNA polymerase. Molecular-grade water (9.25 μl/reaction) and dimethyl sulfoxide (DMSO; 1 μl/reaction) were added. All PCRs were performed in a Techne TC-5000 thermal cycler (Bibby Scientific US, Burlington, NJ).
Statistics.
Mean values were compared using one-way analysis of variance (ANOVA) with a Holm-Sidak posttest for multiple comparisons. Multiple linear regression analysis included all recorded variables, with backward and forward stepwise regression performed using F-tests to ascertain the variables that best explained the output variable. The significant variables were then used to create multiple linear regression equations. Segmented regression analysis used the Levenberg-Marquardt algorithm with either two or three points, and iterations were performed until convergence was achieved and a chi-square tolerance of 1E−9 was reached. A two-tailed test was used to calculate the Spearman rank correlation coefficients. Statistical significance for all tests was measured at an α value of 0.05.
RESULTS
Environmental conditions measured.
The minimum, maximum, and mean values for salinity, water temperature, and wind velocity at the time of sample collection, the total-Vibrio, V. vulnificus, and V. parahaemolyticus abundances in both water and oyster samples, and the total precipitation 24 h prior to sample collection at each site are displayed in Table S1 in the supplemental material. Hoop Pole Creek had the highest maximum and mean salinities (P < 0.01), while South River had the lowest minimum and average salinities (P < 0.001). The Hoop Pole Creek and South River sites had the narrowest salinity ranges, while Harlowe Creek had the largest salinity range (see Table S1 and Fig. S1 in the supplemental material).
Vibrio spp. in North Carolina water and oysters.
Culturable Vibrio spp. were detected in all water samples, including samples in all temperature and salinity ranges (Fig. 2A; see also Table S1 and Fig. S1 in the supplemental material). Log total-Vibrio concentrations in the water column exhibited a strong, significant linear relationship with water temperature (Table 1), but not with salinity. Culturable Vibrio spp. were recovered from all but two oyster samplings (Fig. 3A), and there was a significant but weak linear relationship between log total-Vibrio concentrations in oyster meats and water temperature (Table 1). Water temperature was the only factor that exhibited a significant correlation with total Vibrio spp. in both oysters (n = 56; r = 0.64; P < 0.0001) and water (n = 56; r = 0.72; P < 0.0001). There was no significant difference in mean total-Vibrio concentrations in water or oyster samples among the sampling sites, although Hoop Pole Creek exhibited the highest variability of total-Vibrio concentrations (see Table S1 in the supplemental material).
FIG 2.
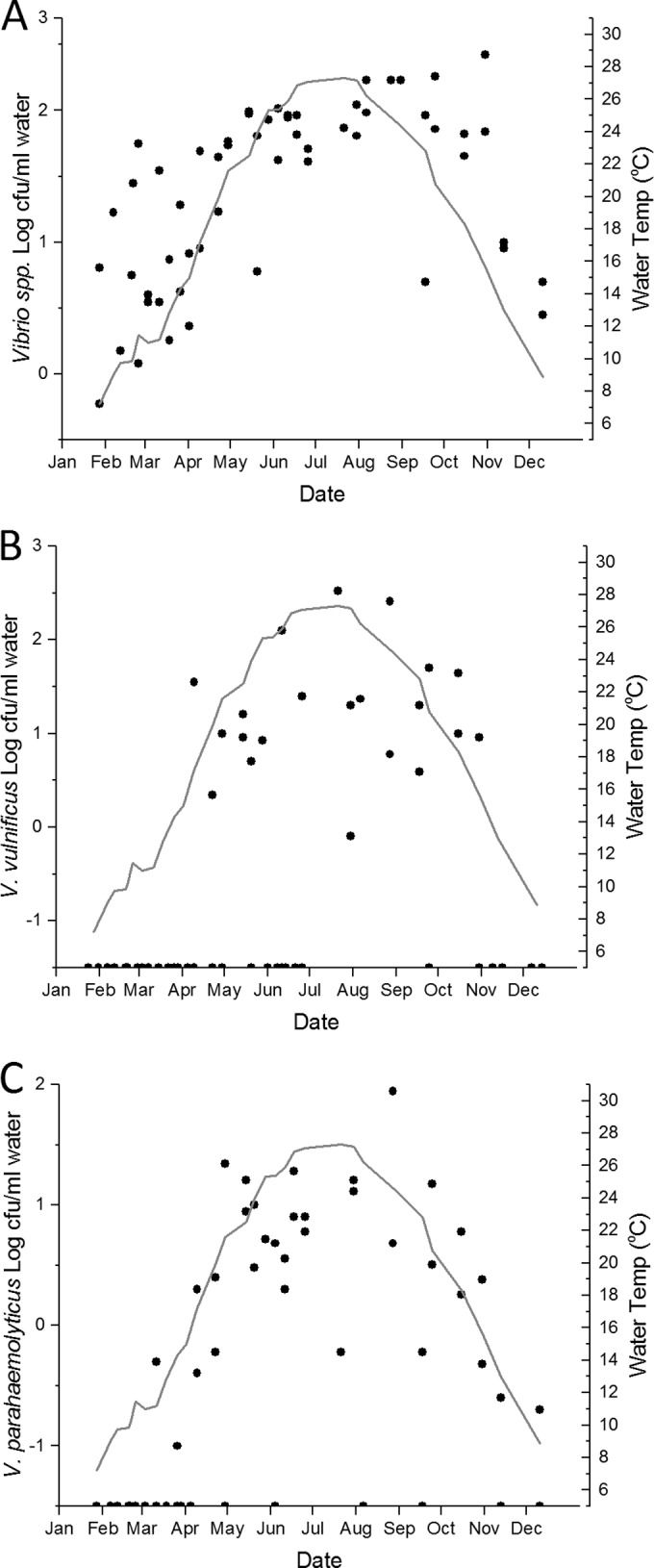
Log10 CFU of total Vibrio spp. (A), V. vulnificus (B), or V. parahaemolyticus (C) per milliliter of water sample by collection date (black dots). Results below the limit of detection were assigned the value of −1.5 log CFU/ml. The gray line represents the monthly moving average of the water temperature at the time of sample collection.
TABLE 1.
Individual statistics from each iteration of significanta linear regression of Vibrio abundance and water temperature organized by species
| Vibrio abundance | Result of linear regression analysis with water temp |
||
|---|---|---|---|
| Intercept (SE) | Slope (SE) | Adjusted R2 | |
| In water samples (log CFU/ml) | |||
| Total Vibrio spp. | 0.07735 (0.17455) | 0.07051 (0.00881) | 0.53413 |
| V. vulnificus | −2.51143 (0.46534) | 0.11205 (0.02341) | 0.29652 |
| V. parahaemolyticus | −2.47574 (0.34208) | 0.11772 (0.01689) | 0.48765 |
| In oyster samples (log CFU/g) | |||
| Total Vibrio spp. | 1.72746 (0.3125) | 0.08538 (0.01577) | 0.33971 |
| V. vulnificus | −0.44585 (0.4805) | 0.08297 (0.02394) | 0.17195 |
| V. parahaemolyticus | −0.95556 (0.34248) | 0.1221 (0.01729) | 0.47062 |
At a P value of <0.05.
FIG 3.
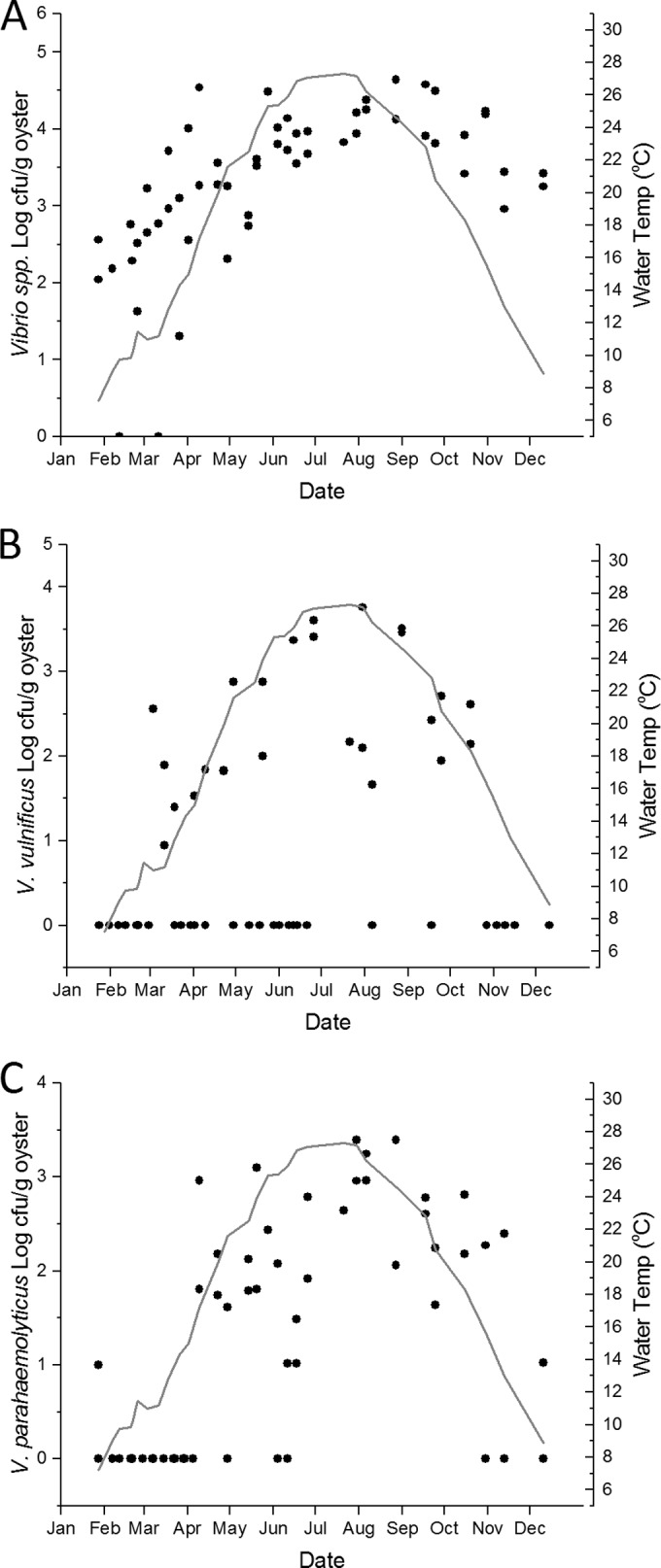
Log10 CFU of total Vibrio spp. (A), V. vulnificus (B), or V. parahaemolyticus (C) per gram of oyster sample by collection date (black dots). Results below the limit of detection were assigned the value of 0 log CFU/g. The gray line represents the monthly moving average of the water temperature at the time of sample collection.
Concentrations of culturable V. vulnificus bacteria in both water and oyster samples (n, 53 for each sample type) correlated positively with water temperature (for water samples, r was 0.53 and P was <0.0001; for oyster samples, r was 0.41 and P was 0.002) and negatively with salinity (for water samples, r was −0.37 and P was 0.007; for oyster samples, r was −0.47 and P was <0.001). A seasonal trend was observed with both water and oyster samples, but even when the waters were very warm (>22°C), numerous samples were below the limit of detection (Table 1; Fig. 2B and 3B). Multiple linear regression analysis revealed that the combined factors of salinity and temperature are best for predicting the abundance of V. vulnificus in water and oyster samples (Table 2).
TABLE 2.
Statistics from significanta multiple linear regression analysis of abundance of V. vulnificus bacteria from either oyster or water samples with water temperature and salinity
| V vulnificus abundance | Intercept (SE) | Slope from multiple linear regression analysis with: |
Adjusted R2 | |
|---|---|---|---|---|
| Salinity (SE) | Water temp (SE) | |||
| In water samples (log CFU/ml) | −0.38346 (0.5486) | −0.08876 (0.018) | 0.12896 (0.01969) | 0.51726 |
| In oyster samples (log CFU/g) | 0.20309 (0.71867) | −0.02648 (0.02188) | 0.08758 (0.02414) | 0.17928 |
At a P value of <0.05.
Heat maps of the combined effects of water temperature and salinity on V. vulnificus in North Carolina water and oysters serve as a quick visual aid for assessing V. vulnificus concentrations and are intended for future use by water quality managers (Fig. 4A and B). Segmented regression of temperature and bacterial abundance revealed that the critical temperature for V. vulnificus cells to be culturable in the estuarine waters of central eastern North Carolina is ca. 16°C. If water temperature is <16.1°C, log [V. vulnificus] = −1.5 + (−4.03367E−9 × water temperature); if water temperature is >16.1°C and <16.8°C, log [V. vulnificus] = −1.5 + [2.20882 × (water temperature − 16.1)]; and if water temperature is >16.8°C, log [V. vulnificus] = 0.0462 + [0.02754 × (water temperature − 16.8)]; R2 = 0.31.
FIG 4.
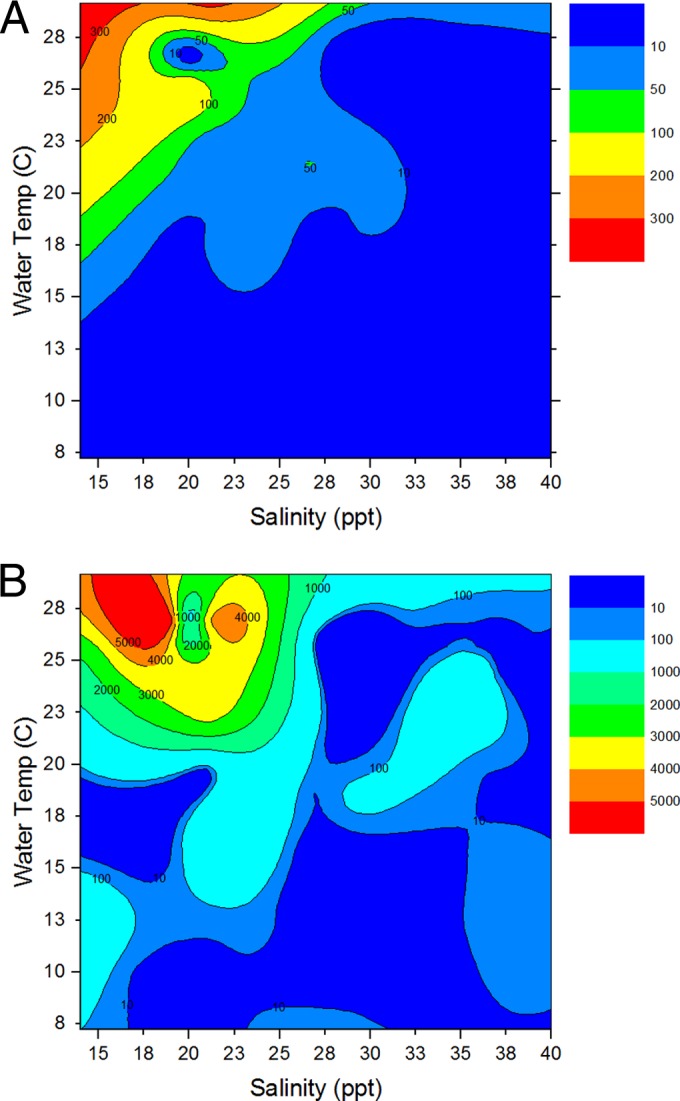
Heat maps of V. vulnificus abundances in water (A) or oyster (B) samples by salinity and water temperature during the collection period. Numbers on heat maps and in keys represent bacterial counts in CFU/ml for water and CFU/g for oysters.
Hoop Pole Creek had significantly lower V. vulnificus levels in water samples than South River, a finding that mirrors the differing salinity levels at the two sites (Fig. 5; see also Fig. S1 in the supplemental material) (P < 0.001). Nevertheless, there were no significant differences in mean V. vulnificus concentrations in oysters among the sites.
FIG 5.
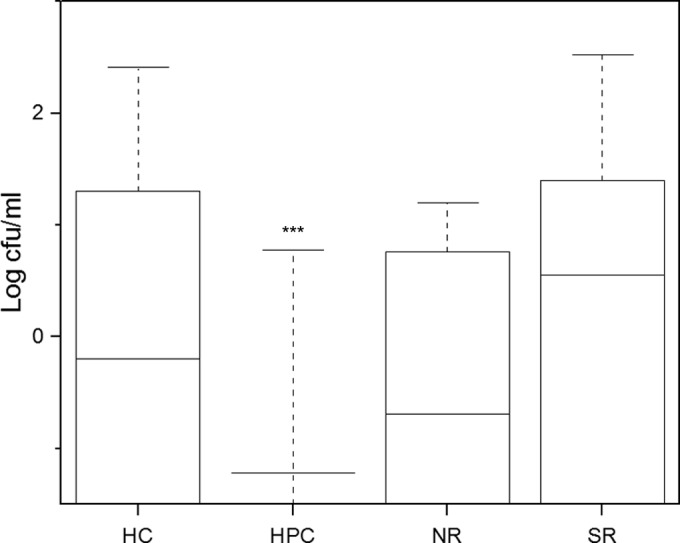
Average yearly concentrations of V. vulnificus recovered in water samples at different collection sites. HC, Harlowe Creek; HPC, Hoop Pole Creek; NR, North River; SR, South River. Boxes represent 25th to 75th percentiles; whiskers represent the maximum; and solid horizontal lines within boxes represent the means. HPC had numerous points below the limit of detection; therefore, the box containing the 25th to 75th percentiles is a single line at −1.5 log. Asterisks represent means significantly different from the others (***, P < 0.001).
Culturable V. parahaemolyticus abundances in water and oysters (n, 51 for each sample type) correlated only with water temperature among the parameters measured (for water samples, r was 0.69 and P was <0.0001; for oyster samples, r was 0.68 and P was <0.0001). Although V. parahaemolyticus abundance was tightly coupled with temperature, there were cases in both water and oysters where V. parahaemolyticus was undetectable, despite relatively warm water temperatures (Fig. 2C and 3C). There were strong, significant linear relationships between water temperature and V. parahaemolyticus abundances in both water and oysters (Table 1). The critical temperature for V. parahaemolyticus abundance in water was determined by segmented regression to be ca. 16°C, similar to that for V. vulnificus in water. If water temperature is <16.1°C, then log [V. parahaemolyticus] = −1.78149 + (0.04163 × water temperature); if water temperature is >16.1°C but <16.8°C, then log [V. parahaemolyticus] = −1.1112 + [1.71055 × (water temperature − 16.1)]; and if water temperature is >16.8°C, then log [V. parahaemolyticus] = −0.3374 + [0.04782 × (water temperature − 16.8)]; R2 = 0.52. There were no significant differences in the mean V. parahaemolyticus concentrations in water or oyster samples among the sites (data not shown).
Genotyping of recovered V. vulnificus bacteria.
V. vulnificus can be subdivided into allelic variants based on the virulence-correlated gene (vcg). The two genotypes, termed E-type and C-type, correlate with environmental or clinical isolation, respectively, and can be of use in determining potential virulence via ingestion (38, 39, 42, 43). There were five instances of recovery of PCR-confirmed C-genotype bacteria from water samples, and five instances from oyster samples, during the entire sampling period, from a total of 616 individual presumptive isolates sampled during 56 sampling events. The dates, sites, and sampling conditions for these occurrences are listed in Table 3. The average salinity of sites containing PCR-confirmed C-type isolates was 28‰, while the mean water temperature was 19°C. Only on one occasion (7 August 2014), at one site (Hoop Pole Creek), were confirmed C-type V. vulnificus bacteria isolated from both the oyster meat and water column portions of the samples. In total, only 5% of confirmed V. vulnificus isolates belonged to the C-genotype. When the isolates were separated by source, 9% of confirmed water column isolates and 5% of confirmed oyster isolates belonged to the C-genotype.
TABLE 3.
Dates, salinities, and water temperatures of samples from which C-type Vibrio vulnificus strains were isolated
| Source of C-type V. vulnificus | Date collected (mo/day/yr) | Sitea | Salinity (‰) | Water temp (°C) | % C-type strainsb |
|---|---|---|---|---|---|
| Oysters | 7/29/13 | SR | 21 | 29 | 20 |
| 8/7/14 | HPC | 28 | 27 | 50 | |
| 8/7/14 | HC | 20 | 27 | 25 | |
| 9/4/14 | HC | 15 | 25 | 10 | |
| 10/2/14 | HC | 27 | 21 | 100 | |
| Water | 4/17/14 | HC | 21 | 20 | 25 |
| 6/5/14 | NR | 36 | 24 | 20 | |
| 8/7/14 | HPC | 28 | 27 | 100 | |
| 8/14/14 | SR | 19 | 27 | 33 | |
| 9/4/14 | HC | 15 | 26 | 25 |
SR, South River; HPC, Hoop Pole Creek; HC, Harlowe Creek; NR, North River.
Percentage of all confirmed V. vulnificus strains in each sample that were identified as C-type.
DISCUSSION
Four North Carolina oyster-harvesting sites were chosen that provided high and low, broad and narrow salinity ranges, in order to maximize the range of collection conditions encountered during the study. There were no observable relationships between log total-Vibrio, V. parahaemolyticus, or V. vulnificus concentrations and precipitation or wind speed at the collection sites. All bacterial species tested exhibited significant seasonality, as has been observed previously across many members of this genus. We detected Vibrio spp. in all water samples and all but two of the oyster meat samples over the course of the study. Total-Vibrio abundance in water was related to water temperature, but in contrast to previous studies on total-Vibrio abundance in North Carolina waters, no relationship with salinity was observed (44–46). Furthermore, there was no difference in the mean abundance of total-Vibrio spp. observed in either water or oysters at any of the sampling sites, despite the differences in tidal influence on environmental conditions. This lack of difference is likely due to the ability of bacteria in the Vibrio genus to flourish at various temperatures and salinities ranging from freshwater to full-strength marine water, and while the numbers of individual species might differ at each location, the total number of vibrios remains relatively unchanged.
V. vulnificus in both oyster and water samples exhibited significant positive correlations with water temperature and negative correlations with salinity. We found that in North Carolina coastal water, 16°C was the minimum temperature at which culturable V. vulnificus bacteria were commonly isolated, a finding similar to a report by Pfeffer et al. (47), although our study did not find a maximum temperature at which V. vulnificus could be isolated. Despite the correlation with water temperature, there were numerous oyster and water samples collected in very warm waters in which no detectable V. vulnificus were observed. A reason for such lack of detection of this species at these times is apparent when the effect of salinity is also incorporated into the analysis. The sampling site with the highest average salinity, Hoop Pole Creek, was also the site with the lowest average number of recoverable V. vulnificus bacteria from the water. Interestingly, there was no statistical difference in the average abundance of V. vulnificus in oysters among the sites, indicating that colonization of oyster matrices could have a protective effect against external environmental conditions, such as the high levels of salinity reported in this study. However, this appears to be true only up to a point, since extreme or prolonged periods of elevated salinity have been shown to alter the oyster microflora, including V. vulnificus (31, 48–50). It was found that the combination of salinity and water temperature provided the best-fitting linear regression models for V. vulnificus in both water and oysters, and these models were used to generate matrix tools that provide easy-to-interpret visual references about the potential concentrations of V. vulnificus in North Carolina oysters. The use of such tools could allow oyster harvesters and water quality managers to make rapid decisions as to whether the oysters collected from these sites should be served raw or as a shucked product meant to be cooked.
Each V. vulnificus isolate that was molecularly confirmed was subjected to vcg genotyping in order to determine the proportions, in each oyster or water sample, of the more-virulent C-genotype strains and the less-virulent E-genotype strains. Only five of the samples collected from water throughout the year, and five separate samples collected from oysters, contained PCR-confirmed C-genotype V. vulnificus cells. Furthermore, on only one sample date did both the oyster and water samples contain C-genotype cells. No correlation was found between the environmental parameters measured and the occurrence or abundance of C-genotype strains, but this is likely due to the low recovery of C-type strains.
The only environmental parameter that correlated with the concentration of V. parahaemolyticus bacteria, in both water and oyster samples, was water temperature. Previous studies have differed in showing salinity to be correlated or not correlated with salinity, and this study supports the latter finding (32, 34). The minimum temperature for V. parahaemolyticus to be detectable in North Carolina oyster-harvesting waters was 16°C, with no detectable difference in mean V. parahaemolyticus abundance in water or oysters at any of the sampling sites. Thus, in this study, salinity does not appear to play a significant role in driving the occurrence or concentration of V. parahaemolyticus. Yet, remarkably, although there was a tight coupling of V. parahaemolyticus abundance with water temperature, there still remained instances in both water and oyster samples in which there were no detectable V. parahaemolyticus bacteria when water temperatures were relatively warm. This suggests that there are yet unrevealed or possibly stochastic factors that contribute to the frequency and distribution of V. parahaemolyticus bacteria.
The maxima of the bacterial concentrations encountered in this study were compared to those in another, similarly performed study that observed Vibrio counts for a year in shellfish and oysters, with sites that included the Gulf Coast (51). The maximum V. vulnificus concentrations recovered from water and oysters in that study were, respectively, 332 CFU/ml and 25,000 CFU/g (51). In this study, the maximum V. vulnificus concentrations were found to be 191 CFU/ml in water and 5,740 CFU/g in oysters, or nearly one-half of the concentrations in water from the Gulf Coast study, and one-fifth of the concentrations in oyster meats. For V. parahaemolyticus, the same study found concentrations of 204 CFU/ml in water and 22,000 CFU/g in oyster tissues, while this study of North Carolina water samples had a maximum of 88 CFU/ml and oysters contained a maximum of 2,479 CFU/g (51). These lower concentrations of vibrios in water and oysters in North Carolina could partially explain the relatively low number of vibrio-related infections in North Carolina.
It should be noted that the U.S. Food and Drug Administration administers the Bacteriological Analytical Manual (BAM), wherein the agency's preferred media for isolating V. vulnificus are given as modified cellobiose-polymyxin-colistin (mCPC) and cellobiose-colistin (CC) (52–54). While the newer medium CPC+ is not preferred, the FDA found no difference in efficacy (55). In this study, the CHROMagar Vibrio medium was used. The use of the CHROMagar Vibrio medium for the isolation of V. vulnificus has been compared with that of CPC+ previously, and the former has been found to yield fewer false-positive results, making environmental analysis easier (56). For V. parahaemolyticus, the CHROMagar Vibrio medium has been shown to produce the best results of culture-based isolation methods (57).
The matrix tools presented here for V. vulnificus in water and oysters are potentially useful for North Carolina commercial or recreational shellfishermen, water quality managers, and consumers (especially those at risk for V. vulnificus infections), informing decisions about the consumption of raw or undercooked oysters harvested from particular sites on particular days. The aim has been to use environmental measurements that are easy to achieve, using simple, cost-effective tools such as thermometers and refractometers. These results indicate interesting patterns, particularly in the strength (or lack thereof) of the relationships between important potential Vibrio pathogens and salinity. Continued data collection over the next few years will result in the capture of a wider range of environmental conditions over which to examine relationships and will improve our understanding of the combined effects of salinity and temperature on vibrios. Further data collection will also permit the examination and continued comparison of the density maxima of the two pathogenic Vibrio spp. examined here and their Gulf Coast counterparts. The findings of this study highlight apparent regional differences and indicate the need for Vibrio-related shellfish-harvesting regulations to be tailored to the state or region in which the oysters are actually harvested, rather than using a “one-size-fits-all” approach.
Supplementary Material
ACKNOWLEDGMENTS
We thank everyone at the North Carolina Division of Marine Fisheries, including Shannon Jenkins, Paul Moore, Mike Millard, Timmy Moore, Phil Piner, and Steve Murphy, for assistance in collecting oysters and for scientific input. We thank Tiffany Williams for critical analysis and discussions. We thank Mary-Jo Weiss and Katie Dugan for their efforts in oyster collection and analysis. We thank Kyle Hernandez for assistance with statistical analysis.
This work was supported by funding from the College of Liberal Arts and Sciences at UNC Charlotte, the Office of the Vice Chancellor for Research at UNC Chapel Hill, the National Science Foundation's Research Experiences for Undergraduates Program (OCE-1156625), and the NSF/NIH Joint Program in Ecology and Evolution of Infectious Diseases (grant OCE-0812913), and by Agriculture and Food Research Initiative Competitive Grant 11352692 from the USDA National Institute of Food and Agriculture.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03206-14.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M-A, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.09-1101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver JD. 2006. Vibrio vulnificus, p 349–366. In Thompson FL, Austin B, Swings J (ed), The biology of vibrios. American Society for Microbiology, Washington, DC. [Google Scholar]
- 3.Froelich B, Oliver J. 2013. The interactions of Vibrio vulnificus and the oyster Crassostrea virginica. Microb Ecol 65:807–816. doi: 10.1007/s00248-012-0162-3. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2013. Trends in foodborne illness in the United States, 2013. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/features/dsfoodsafetyreport/. [Google Scholar]
- 5.Oliver JD. 2013. Vibrio vulnificus: death on the half shell. A personal journey with the pathogen and its ecology. Microb Ecol 65:793–799. doi: 10.1007/s00248-012-0140-9. [DOI] [PubMed] [Google Scholar]
- 6.Jones MK, Oliver JD. 2009. Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723–1733. doi: 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. 2013. Vibrio parahaemolyticus. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/vibrio/vibriop.html. [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2007. Summary of human Vibrio cases reported to CDC, 2007. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/nationalsurveillance/PDFs/CSTEVibrio2007.pdf. [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2013. Vibrio vulnificus. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/vibrio/vibriov.html. [Google Scholar]
- 10.Oliver JD. 2006. Vibrio vulnificus, p 253–276. In Belkin S, Colwell RR (ed), Oceans and health: pathogens in the marine environment. Springer, New York, NY. [Google Scholar]
- 11.Mead PS, Slusker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. 1999. Food-related illness and death in the United States. Emerg Infect Dis 5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaspar CW, Tamplin ML. 1993. Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol 59:2425–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motes ML, DePaola A, Cook DW, Veazey JE, Hunsucker JC, Garthright WE, Blodgett RJ, Chirtel SJ. 1998. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl Environ Microbiol 64:1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Givens CE, Bowers JC, DePaola A, Hollibaugh JT, Jones JL. 2014. Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus—potential roles for fish, oyster, sediment and water. Lett Appl Microbiol 58:503–510. doi: 10.1111/lam.12226. [DOI] [PubMed] [Google Scholar]
- 15.Wright AC, Hill RT, Johnson JA, Roghman MC, Colwell RR, Morris JG. 1996. Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol 62:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamplin M, Rodrick GE, Blake NJ, Cuba T. 1982. Isolation and characterization of Vibrio vulnificus from two Florida estuaries. Appl Environ Microbiol 44:1466–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tilton RC, Ryan RW. 1987. Clinical and ecological characteristics of Vibrio vulnificus in the Northeastern United States. Diagn Microbiol Infect Dis 6:109–117. doi: 10.1016/0732-8893(87)90094-0. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima H, Seki R. 2004. Ecology of Vibrio vulnificus and Vibrio parahaemolyticus in brackish environments of the Sada River in Shimane Prefecture, Japan. FEMS Microbiol Ecol 48:221–229. doi: 10.1016/j.femsec.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Randa MA, Polz MF, Lim E. 2004. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl Environ Microbiol 70:5469–5476. doi: 10.1128/AEM.70.9.5469-5476.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Urtaza J, Bowers JC, Trinanes J, DePaola A. 2010. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res Int 43:1780–1790. doi: 10.1016/j.foodres.2010.04.001. [DOI] [Google Scholar]
- 21.McLaughlin JB, DePaola A, Bopp CA, Martinek KA, Napolilli NP, Allison CG, Murray SL, Thompson EC, Bird MM, Middaugh JP. 2005. Outbreak of Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. N Engl J Med 353:1463–1470. doi: 10.1056/NEJMoa051594. [DOI] [PubMed] [Google Scholar]
- 22.Nishibuchi M, DePaola A. 2005. Vibrio species, p 251–272. In Fratamico PM, Bhunia AK, Smith JL (ed), Foodborne pathogens: microbiology and molecular biology. Horizon Scientific Press, Norfolk, United Kingdom. [Google Scholar]
- 23.DaSilva L, Parveen S, DePaola A, Bowers J, Brohawn K, Tamplin ML. 2012. Development and validation of a predictive model for the growth of Vibrio vulnificus in postharvest shellstock oysters. Appl Environ Microbiol 78:1675–1681. doi: 10.1128/AEM.07304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez-Piquer J, Bowman JP, Ross T, Tamplin ML. 2011. Predictive models for the effect of storage temperature on Vibrio parahaemolyticus viability and counts of total viable bacteria in Pacific oysters (Crassostrea gigas). Appl Environ Microbiol 77:8687–8695. doi: 10.1128/AEM.05568-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro RL, Altekruse S, Hutwagner L, Bishop R, Hammond R, Wilson S, Ray B, Thompson S, Tauxe V, Griffin PM. 1998. The role of Gulf Coast oysters harvested in warmer months in Vibrio vulnificus infections in the United States. J Infect Dis 178:752–759. doi: 10.1086/515367. [DOI] [PubMed] [Google Scholar]
- 26.Gooch JA, DePaola A, Bowers J, Marshall DL. 2002. Growth and survival of Vibrio parahaemolyticus in postharvest American oysters. J Food Prot 65:970–974. [DOI] [PubMed] [Google Scholar]
- 27.Lin M, Schwarz JR. 2003. Seasonal shifts in population structure of Vibrio vulnificus in an estuarine environment as revealed by partial 16S ribosomal DNA sequencing. FEMS Microbiol Ecol 45:23–27. doi: 10.1016/S0168-6496(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 28.Johnson CN, Flowers AR, Noriea NF, Zimmerman AM, Bowers JC, DePaola A, Grimes DJ. 2010. Relationships between environmental factors and pathogenic vibrios in the Northern Gulf of Mexico. Appl Environ Microbiol 76:7076–7084. doi: 10.1128/AEM.00697-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parvathi A, Kumar HS, Karunasagar I, Karunasagar I. 2004. Detection and enumeration of Vibrio vulnificus in oysters from two estuaries along the southwest coast of India, using molecular methods. Appl Environ Microbiol 70:6909–6913. doi: 10.1128/AEM.70.11.6909-6913.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ristori CA, Iaria ST, Gelli DS, Rivera ING. 2007. Pathogenic bacteria associated with oysters (Crassostrea brasiliana) and estuarine water along the south coast of Brazil. Int J Environ Health Res 17:259–269. doi: 10.1080/09603120701372169. [DOI] [PubMed] [Google Scholar]
- 31.Froelich BA, Williams TC, Noble RT, Oliver JD. 2012. Apparent loss of Vibrio vulnificus from North Carolina oysters coincides with a drought-induced increase in salinity. Appl Environ Microbiol 78:3885–3889. doi: 10.1128/AEM.07855-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DePaola A, Kaysner CA, Bowers J, Cook DW. 2000. Environmental investigations of Vibrio parahaemolyticus in oysters after outbreaks in Washington, Texas, and New York (1997 and 1998). Appl Environ Microbiol 66:4649–4654. doi: 10.1128/AEM.66.11.4649-4654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staley C, Chase E, Harwood VJ. 2013. Detection and differentiation of Vibrio vulnificus and V. sinaloensis in water and oysters of a Gulf of Mexico estuary. Environ Microbiol 15:623–633. doi: 10.1111/1462-2920.12045. [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman AM, DePaola A, Bowers JC, Krantz JA, Nordstrom JL, Johnson CN, Grimes DJ. 2007. Variability of total and pathogenic Vibrio parahaemolyticus densities in Northern Gulf of Mexico water and oysters. Appl Environ Microbiol 73:7589–7596. doi: 10.1128/AEM.01700-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffitt KJ, Grimes DJ. 2013. Abundance and distribution of Vibrio cholerae, V. parahaemolyticus, and V. vulnificus following a major freshwater intrusion into the Mississippi Sound. Microb Ecol 65:578–583. doi: 10.1007/s00248-013-0203-6. [DOI] [PubMed] [Google Scholar]
- 36.Wilson C, Scotto L, Scarpa J, Volety A, Laramore S, Haunert D. 2005. Survey of water quality, oyster reproduction and oyster health status in the St. Lucie Estuary. J Shellfish Res 24:157–165. doi: 10.2983/0730-8000(2005)24[157:SOWQOR]2.0.CO;2. [DOI] [Google Scholar]
- 37.Chatry M, Dugas R, Easley KA. 1983. Optimum salinity regime for oyster production on Louisiana's state seed grounds. Contrib Mar Sci 26:81–94. [Google Scholar]
- 38.Rosche TM, Yano Y, Oliver JD. 2005. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical and environmental isolation. Microbiol Immunol 49:381–389. doi: 10.1111/j.1348-0421.2005.tb03731.x. [DOI] [PubMed] [Google Scholar]
- 39.Rosche TM, Binder EA, Oliver JD. 2010. Vibrio vulnificus genome suggests two distinct ecotypes. Environ Microbiol Rep 2:128–132. doi: 10.1111/j.1758-2229.2009.00119.x. [DOI] [PubMed] [Google Scholar]
- 40.Tarr CL, Patel JS, Puhr ND, Sowers EG, Bopp CA, Strockbine NA. 2007. Identification of Vibrio isolates by a multiplex PCR assay and rpoB sequence determination. J Clin Microbiol 45:134–140. doi: 10.1128/JCM.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warner EB, Oliver JD. 2008. Multiplex PCR assay for detection and simultaneous differentiation of genotypes of Vibrio vulnificus biotype 1. Foodborne Pathog Dis 5:691–693. doi: 10.1089/fpd.2008.0120. [DOI] [PubMed] [Google Scholar]
- 42.Warner JM, Oliver JD. 1999. Randomly amplified polymorphic DNA analysis of clinical and environmental isolates of Vibrio vulnificus and other Vibrio species. Appl Environ Microbiol 65:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chatzidaki-Livanis M, Hubbard MA, Gordon K, Harwood VJ, Wright AC. 2006. Genetic distinctions among clinical and environmental strains of Vibrio vulnificus. Appl Environ Microbiol 72:6136–6141. doi: 10.1128/AEM.00341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Froelich B, Bowen J, Gonzalez R, Snedeker A, Noble R. 2013. Mechanistic and statistical models of total Vibrio abundance in the Neuse River Estuary. Water Res 47:5783–5793. doi: 10.1016/j.watres.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh JL, Fries JS, Noble RT. 2008. Dynamics and predictive modelling of Vibrio spp. in the Neuse River Estuary, North Carolina, USA. Environ Microbiol 10:57–64. doi: 10.1111/j.1462-2920.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- 46.Wetz JJ, Blackwood AD, Fries JS, Williams ZF, Noble RT. 2008. Trends in total Vibrio spp. and Vibrio vulnificus concentrations in the eutrophic Neuse River Estuary, North Carolina, during storm events. Aquat Microb Ecol 53:141–149. doi: 10.3354/ame01223. [DOI] [Google Scholar]
- 47.Pfeffer CS, Hite MF, Oliver JD. 2003. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl Environ Microbiol 69:3526–3531. doi: 10.1128/AEM.69.6.3526-3531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motes ML, DePaola A. 1996. Offshore suspension relaying to reduce levels of Vibrio vulnificus in oysters (Crassostrea virginica). Appl Environ Microbiol 62:3875–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Audemard C, Kator HI, Rhodes MW, Gallivan T, Erskine AJ, Leggett AT, Reece KS. 2011. High salinity relay as a postharvest processing strategy to reduce Vibrio vulnificus levels in Chesapeake Bay oysters (Crassostrea virginica). J Food Prot 74:1902–1907. doi: 10.4315/0362-028X.JFP-11-152. [DOI] [PubMed] [Google Scholar]
- 50.Walton WC, Nelson C, Hochman M, Schwarz J. 2013. Preliminary study of transplanting as a process for reducing levels of Vibrio vulnificus and Vibrio parahaemolyticus in shellstock oysters. J Food Prot 76:119–123. doi: 10.4315/0362-028X.JFP-12-315. [DOI] [PubMed] [Google Scholar]
- 51.Johnson CN, Bowers JC, Griffitt KJ, Molina V, Clostio RW, Pei S, Laws E, Paranjpye RN, Strom MS, Chen A, Hasan NA, Huq A, Noriea NF, Grimes DJ, Colwell RR. 2012. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the coastal and estuarine waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl Environ Microbiol 78:7249–7257. doi: 10.1128/AEM.01296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaysner CA, DePaola A Jr. May 2004. Vibrio, chapter 9 In FDA, Bacteriological analytical manual. US Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070830.htm. [Google Scholar]
- 53.Elliot EL, Kaysner CA, Jackson L, Tamplin ML. 1995. Vibrio cholerae, V. parahaemolyticus, V. vulnificus and other Vibrio spp., p 9.01–9.27. In FDA, Bacteriological analytical manual, 8th ed. AOAC International, Gaithersburg, MD. [Google Scholar]
- 54.Høi L, Dalsgaard I, Dalsgaard A. 1998. Improved isolation of Vibrio vulnificus from seawater and sediment with cellobiose-colistin agar. Appl Environ Microbiol 64:1721–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones JL, Lüdeke CHM, Bowers JC, DePaola A. 2013. Comparison of plating media for recovery of total and virulent genotypes of Vibrio vulnificus in U.S. market oysters. Int J Food Microbiol 167:322–327. doi: 10.1016/j.ijfoodmicro.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Froelich BA, Weiss MJ, Noble RT. 2014. The evaluation of four recent culture-based methods for the isolation and enumeration of Vibrio vulnificus bacteria from oyster meat. J Microbiol Methods 97:1–5. doi: 10.1016/j.mimet.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Blanco-Abad V, Ansede-Bermejo J, Rodriguez-Castro A, Martinez-Urtaza J. 2009. Evaluation of different procedures for the optimized detection of Vibrio parahaemolyticus in mussels and environmental samples. Int J Food Microbiol 129:229–236. doi: 10.1016/j.ijfoodmicro.2008.11.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.