Abstract
Norovirus is one of the most common causes of acute viral gastroenteritis. The virus is spread via the fecal-oral route, most commonly from infected food and water, but several outbreaks have originated from contamination of surfaces with infectious virus. In this study, a close surrogate of human norovirus causing gastrointestinal disease in mice, murine norovirus type 1 (MNV-1), retained infectivity for more than 2 weeks following contact with a range of surface materials, including Teflon (polytetrafluoroethylene [PTFE]), polyvinyl chloride (PVC), ceramic tiles, glass, silicone rubber, and stainless steel. Persistence was slightly prolonged on ceramic surfaces. A previous study in our laboratory observed that dry copper and copper alloy surfaces rapidly inactivated MNV-1 and destroyed the viral genome. In this new study, we have observed that a relatively small change in the percentage of copper, between 70 and 80% in copper nickels and 60 and 70% in brasses, had a significant influence on the ability of the alloy to inactivate norovirus. Nickel alone did not affect virus, but zinc did have some antiviral effect, which was synergistic with copper and resulted in an increased efficacy of brasses with lower percentages of copper. Electron microscopy of purified MNV-1 that had been exposed to copper and stainless steel surfaces suggested that a massive breakdown of the viral capsid had occurred on copper. In addition, MNV-1 that had been exposed to copper and treated with RNase demonstrated a reduction in viral gene copy number. This suggests that capsid integrity is compromised upon contact with copper, allowing copper ion access to the viral genome.
INTRODUCTION
Noroviruses are responsible for approximately half of all cases of gastroenteritis worldwide. Their low infectious dose, ability to persist in an infectious state in the environment, and resistance to many commonly used cleaning agents have led to many disease outbreaks that have proved very difficult to contain (1, 2). The virus is spread directly via the fecal-oral route but also from touching contaminated surfaces, which has recently been found to be more significant than originally thought in the spread of many diseases (3). Ineffective cleaning agents may leave on surfaces residual virus particles which can initiate an infection (4). Norovirus disease is usually self-limiting, with symptoms lasting a few days, but can be more serious in severely ill or immunocompromised individuals, especially if the causative agent is one of the emerging recombinant strains, including GII.g/GII.12, which appeared in 2008 and has enhanced virulence and severity of clinical symptoms. Asymptomatic carriage and extended virus shedding also increase the risk of transmission (5, 6). A recent study of a large waterborne outbreak in Nokia, Finland, also observed that norovirus exposure may result in long-term health effects, which can persist for 15 months after the initial infection (7). This may mean that the considerable public health costs incurred in initial outbreaks, estimated in the United States to be more than $2 billion per year, may be just the tip of the iceberg if the virus is responsible for long-term pathologies.
The use of antimicrobial surfaces in high-risk environments may help to prevent the spread of many infectious agents that are able to retain infectivity on surfaces. Copper and copper alloys have been shown to be effective at rapidly killing a range of bacterial, fungal, and viral pathogens in laboratory studies at a range of temperatures and under various humidity conditions (8–13). This has led to clinical trials incorporating copper surfaces in busy wards, where reductions in the bioburden and infection rate have now been observed in rooms equipped with just a few copper surfaces (14–17). The mechanism of action has also been shown to be complex and variable, involving the release from the surfaces of copper ions, which have a direct action and/or lead to the generation of secondary agents of toxicity, such as reactive oxygen species, which affect a variety of targets (18, 19). We have previously shown with a small range of copper alloys the rapid destruction of murine norovirus (MNV), a close surrogate for the human norovirus causing gastrointestinal disease, which is necessary because of the lack of a suitable infectivity assay for the human norovirus (20). This study observed that the rate of inactivation was affected by temperature and aqueous content and did not involve generation of reactive oxygen species. The current study has continued this work by investigating a larger range of copper nickel and brass alloys, as well as the effect of surface texture, and nickel and zinc controls. The persistence of a range of commonly used nonmetal surfaces was also investigated. The previous study observed that exposure to copper resulted in destruction of the viral positive-strand RNA genome. In this study, the effect on the viral capsid was investigated.
MATERIALS AND METHODS
MNV-1 and cell lines.
Acquisition, preparation, and maintenance of virus stocks and cell lines have been described previously (20). MNV type 1 (MNV-1) CW1 and the mouse monocyte macrophage line RAW 264.7 were kindly supplied by Herbert Virgin IV, Washington University.
Preparation of purified MNV-1.
A preparation of purified MNV was prepared by adapting the method of Wobus et al. (21). Briefly, RAW 264.7 cells were infected at a multiplicity of infection (MOI) of 2 and incubated at 37°C in 5% CO2 for 48 h, when significant cytopathic effect (CPE) was visible. Following 3 freeze-thaw cycles, the virus was precipitated out of the cell lysate with polyethylene glycol (PEG) (BioVision PEG virus precipitation kit) and further purified by cesium chloride isopycnic centrifugation. The virus band was aspirated and dialyzed overnight against phosphate-buffered saline (PBS). The virus was then concentrated by pelleting it through a sucrose cushion (30% [wt/vol] sucrose) at 90,000 × g for 2.5 h at 4°C in a Beckman Coulter L7 65 ultracentrifuge; 100 μl ice-cold PBS was added to the virus pellet, which was incubated on ice for 30 min. The virus was resuspended by gentle pipetting and stored at −80°C until required.
Preparation of sample surfaces.
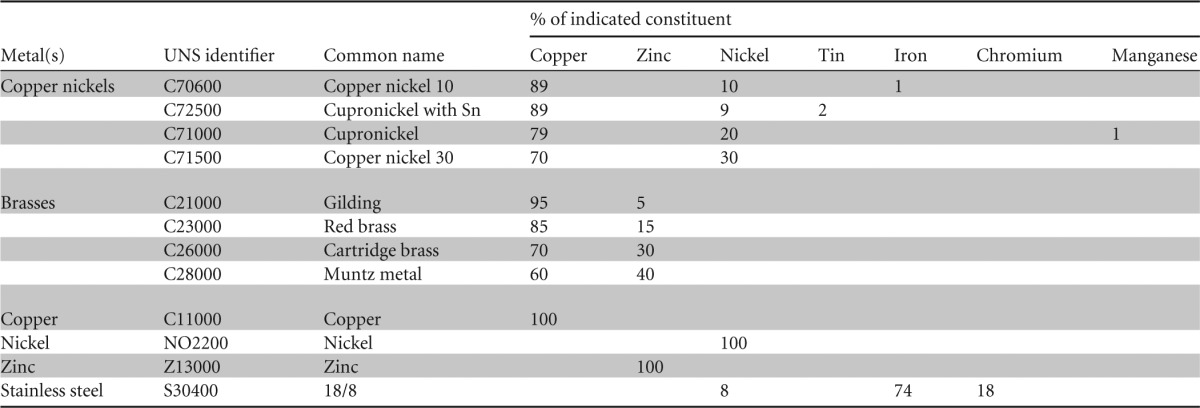
Metal coupons with a surface area of 1 cm2 and a thickness of 0.5 mm were degreased in acetone (to remove any lipid film that might delay the antimicrobial effect of copper [22]), stored in absolute ethanol, and flamed before use as described previously (20). The constituents of each metal tested are detailed in Table 1; they were supplied by the Copper Development Association.
TABLE 1.
Constituents of metals used in the study
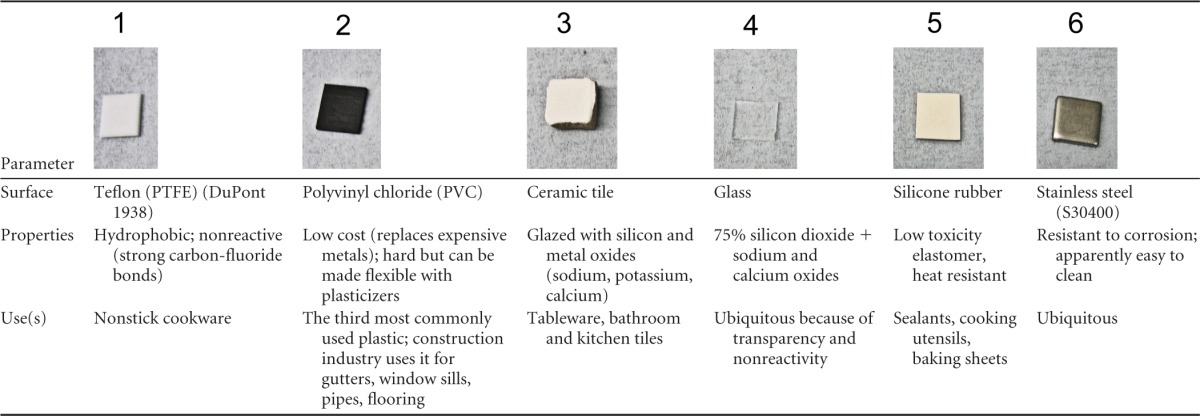
Nonmetal surfaces (Table 2) {Teflon (polyfluorotetraethylene [PTFE]), polyvinyl chloride (PVC), ceramic tiles, glass, silicone rubber} were also cut into 1-cm2 coupons and were sterilized by autoclaving. Metal controls for comparison were also autoclaved for method consistency for these experiments.
TABLE 2.
Range of commonly used surfaces to investigate the persistence of MNV-1 at room temperature
Increasing the surface roughness of metal coupons was investigated with some alloys. Coupons were abraded by rubbing them with coarse sandpaper for 5 min, rinsed well in doubly distilled water (DDW), and checked by episcopic differential interference contrast (EDIC) microscopy to verify that no grains remained on the coupons. The coupons were then prepared for surface testing as described above.
Plaque assay for infectious virus recovered from test surfaces.
Stock cell lysate preparations of MNV-1 were spread over the surfaces of the coupons at room temperature (approximately 5 × 105 PFU in 20 μl per coupon). This is 10 to 50 times more virus than was used in the previous study, where the virus was diluted in cell growth medium for comparison between dry-touch contamination and simulated-wet-fomite contamination (20). This new study has investigated simulated-wet-fomite surface contamination only. The virus was removed from the coupons at various times and assayed for infectivity in RAW 264.7 cells as described previously (20). The method resulted in a recovery rate of >95% of the inoculum.
Morphology of purified MNV-1 recovered from metal surfaces.
The purified virus was inoculated onto copper and stainless steel coupons (1 cm2) and incubated for 2 h at room temperature. The virus was removed from the coupons by gentle pipetting in sterile, nuclease-free distilled deionized water. Five-microliter preparations of virus exposed to metal surfaces and of untreated virus were dried on copper grids, stained with the negative stain phosphotungstic acid (PTA) or ammonium molybdate for 5 s, and observed using a transmission electron microscope (TEM).
Determination of capsid integrity by pretreatment with RNase.
MNV was inoculated onto 10 replicate coupons for copper or stainless steel as described for surface testing. Virus was removed immediately or after 2 h of contact by pipetting in as small a volume of PBS as possible (200 μl). Half of the sample was treated with 1 μl RNase plus 11 μl RNase buffer in a Promega RNase ONE RNase kit for 15 min at 37°C. Viral RNA preparations and cDNA were prepared, and real-time PCR amplification of a viral gene important in early stages of infection (the gene for VPg [viral protein, genome linked]) was performed on untreated and RNase-treated samples as described previously (20). Exposure to copper surfaces has been shown to result in massive degradation of the genome, suggesting that the results may apply with any viral gene (20).
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEM) and are from multiple independent experiments. Differences between duplicate samples were assessed using the Mann-Whitney rank t test. Group comparisons were analyzed using the Mann-Whitney U test where statistical significance was expressed as a P of <0.05. Statistical analyses and graphical representations were performed using Sigma Plot version 12.5 and GraphPad Prism 6.
RESULTS AND DISCUSSION
Infectious MNV-1 retains infectivity on nonmetallic surfaces for several weeks at room temperature.
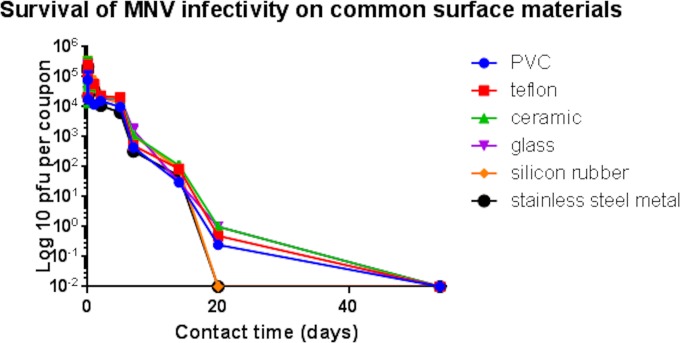
A 1-log reduction in infectivity was observed over the first 5 days of exposure to the nonmetallic surfaces: Teflon (PTFE), polyvinyl chloride (PVC), ceramic tiles, glass, and silicone rubber (Fig. 1). Between 5 and 14 days, there was a steady reduction in infectivity, but given that the infectious dose was very low, approximately 10 virions, there was a considerable risk of infection transmission from all surfaces tested at 2 weeks. In this test, the inoculating concentration was approximately 5 × 105 PFU per cm2 of test surface. This concentration is similar to that expected for surface contamination by vomitus, which can contain up to 107 virions per 30 ml. However, the feces of an infected individual may contain up to 1011 virions per g, and survival of infectivity on fecally contaminated surfaces may be considerably longer than reported here (23). It is interesting to observe that the highest levels of infectivity were retained on ceramic tiles, which may be significant, as this material is often employed in bathrooms and kitchens, where the majority of norovirus contaminations will occur.
FIG 1.
Persistence of infectious murine norovirus on common surface materials. Approximately 5 × 105 PFU of infectious virus was applied to 1-cm2 samples of test surfaces and incubated at room temperature. At various time points, virus was removed from the surfaces and assessed for infectious virus by plaque assay as described in the text. No significant reduction in infectivity of norovirus occurs on any surface over 2 h at room temperature. This was followed by a steady decline in the infectivity of norovirus. Infectious virus was present on all surfaces except stainless steel and silicon rubber at 20 days. However, because the infectious dose was very low (only 10 virus particles), this represents a considerable risk of infection spread. Slightly higher levels of infectious virus were recovered from ceramic surfaces commonly used in bathroom and kitchen tiles. Error bars represent ± standard deviations, and data are from multiple experiments.
Inactivation of MNV-1 on copper nickels.
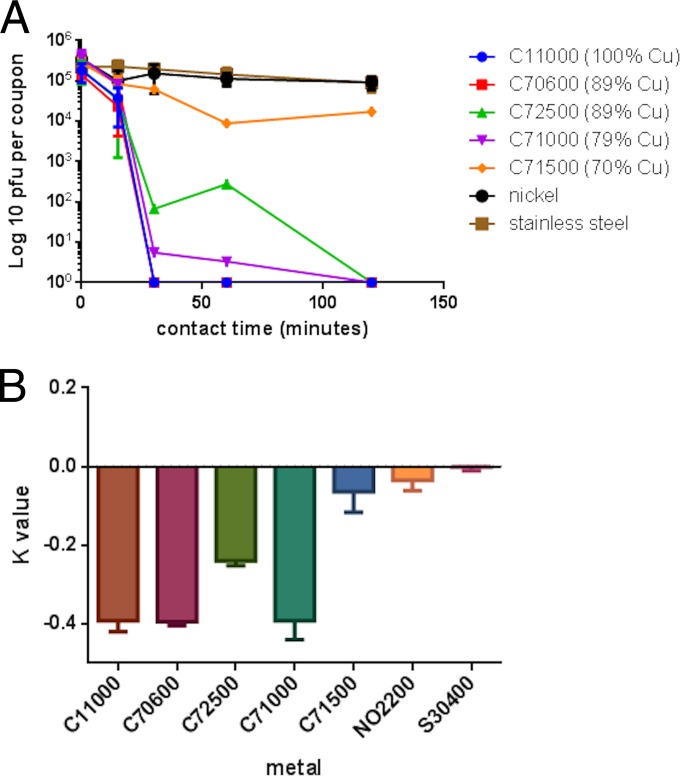
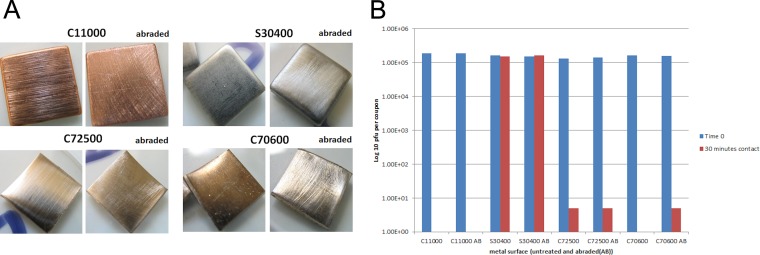
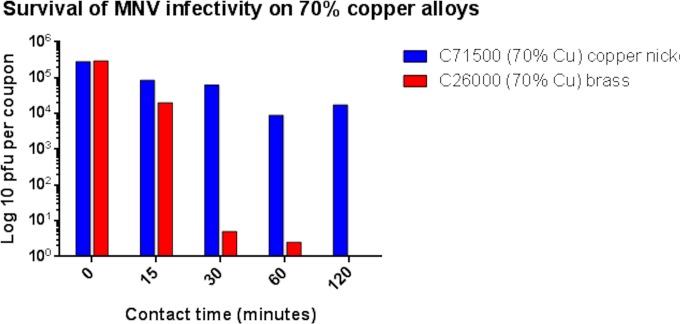
Our previous study suggested that copper nickel (number C70600 of the Unified Numbering System for Metals and Alloys [UNS]) was very efficacious at inactivating MNV-1, even compared to phosphor bronze, an alloy that contains 6% more copper. In this study, a further 3 alloys were tested (Fig. 2). MNV-1 was rapidly inactivated on alloys containing 79 to 89% copper, but efficacy was lost at 70%, suggesting that a small difference in copper content, 70 to 79%, can have a large effect on antiviral efficacy. The greatest difference in inactivation rate occurred over the first 30 min of contact (Fig. 2B), when C71000 had an inactivation rate very similar to those of pure copper and C70600. The exception to this was alloy C72500, which had reduced efficacy compared to C70600, despite having the same copper content. The appearances of these alloys are very different: C72500 is very shiny and polished compared to the dull alloy C70600. This may be due to a passivating oxide layer, which may contribute to efficacy (this alloy is employed in marine engineering because of excellent antifouling properties). Hans et al. (24) reported that a layer of cuprous oxide (Cu2O) was as effective at killing Enterococcus hirae as pure copper but found that copper II oxide, CuO, is inhibitory by forming a protective layer that reduces the rate of corrosion (passivation layer). We observed that if the surfaces of alloys C70600 and C72500 were abraded by rubbing with coarse sandpaper (Fig. 3A), the efficacy of the former to inactivate MNV-1 was reduced following 30 min of contact at room temperature (Fig. 3B). This suggests that the presence of an oxide layer on this alloy contributes to its antiviral efficacy. In contrast, abrading the surfaces of copper, stainless steel, and the alloy C72500 did not have any effect on their untreated antiviral efficacies. In the previous study (20), phosphor bronze was not as effective as alloys with lower percentages of copper, and this alloy contains 5% tin. Alloy C72500 contains 2% tin, and it is unclear whether the presence of tin has an inhibitory effect on copper ion release and therefore reduces the ability of copper to inactivate norovirus. The shiny polished surfaces of our samples also exhibited increased hydrophobicity when virus was inoculated onto the metal surfaces. Although the inoculating virus was 10 to 50 times higher than in the previous study, MNV-1 was inactivated in a shorter time on C70600. Perhaps the increased concentration of cellular debris, as well as virus, affected results. Stainless steel and nickel did not have any antiviral activity, suggesting that the inactivation of MNV-1 was due primarily to copper.
FIG 2.
Inactivation of MNV on copper nickels. (A) Survival of MNV infectivity on copper nickels. Approximately 5 × 105 PFU of infectious virus was applied to 1-cm2 samples of test surfaces and incubated at room temperature. At various time points, virus was removed from the surfaces and assessed for infectious virus by plaque assay as described in the text. MNV was rapidly inactivated on copper and C70600. The extent of inactivation on copper alloys was proportional to the percentage of copper, except with alloy C72500, which is not as effective as alloys with lower percentages of copper. Stainless steel and nickel do not have any antiviral activity. (B) Rate of MNV inactivation on copper nickels over the first 30 min of contact at room temperature The inactivation rate, K, was calculated as described previously (20). C11000 (100% copper), C70600 (89% copper), and C71000 (79% copper) all displayed similar, fast inactivations of MNV for the first 30 min of contact. Inactivation on C725000 was slower, even though its copper content is high (89%).
FIG 3.
Comparison between two copper nickels, C72500 and C70600) containing 89% copper. (A) Appearance of copper alloys, i.e., abraded and unabraded (copper and stainless steel controls). (B) Abrasion of the oxide layer on C70600 slightly reduces antiviral efficacy. Approximately 5 × 105 PFU of infectious virus was applied to 1-cm2 samples of abraded and unabraded metal coupons and incubated at room temperature. At various time points, virus was removed from the surfaces and assessed for infectious virus particles by plaque assay as described in the text. Abrading the surface of alloy C72500 did not make any difference in its efficacy in inactivating MNV. It is unclear whether the tin content of this alloy affects efficacy. Abrading the surface of alloy C70600 reduces antiviral efficacy; i.e., the alloy surface has an effect on MNV.
Inactivation on brasses and the influence of zinc.
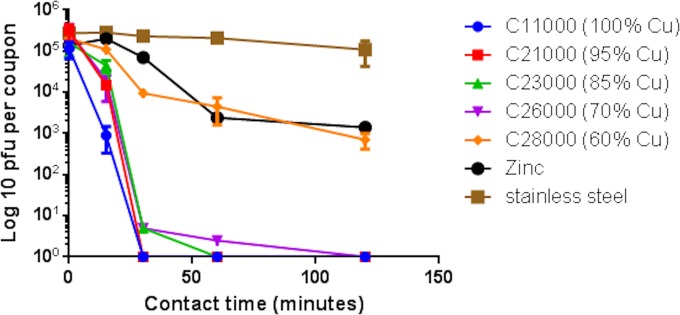
Our previous study suggested that the infectivity of MNV-1 was also reduced on cartridge brass, C26000. In our new study using a viral inoculum containing a higher concentration of virus and RAW264.7 cell debris, MNV inactivation was a little faster and totally inactivated at 2 h. The other alloys demonstrated MNV inactivation directly proportional to the percentage of copper (Fig. 4). However, over a 1-log10 reduction in MNV infectivity was observed on pure zinc. Comparison between copper-nickel and brass, both of which contained 70% copper, demonstrate the increased efficacy of brass (Fig. 5), which may be due to the synergistic actions of copper and zinc to inactivate MNV. Zinc was first reported to inhibit the replication of rhinoviruses in 1974 (25), and subsequently, zinc salts and lozenges have been used as therapies for the common cold and influenza. More recently, the antiviral effects of zinc ionophores, such as pyrithione, have been investigated. Increased uptake of Zn2+ through the ionophore results in inhibition of the replication of herpes simplex virus (HSV) (a large enveloped double-stranded DNA virus) by deregulating the ubiquitin-proteasome system (UPS) and activating NF-κB (nuclear factor kappa light-chain enhancer-activated B cells) (26). Here a small change in the percentage of the copper in the alloys tested (70 to 80%) had a large effect on efficacy in inactivating MNV. C28000 had the lowest copper content (60%) and an efficacy similar to that of pure nickel, despite the higher zinc content of 40%. All other copper alloys tested were very effective, totally inactivating 5 × 105 PFU per cm2 within 2 h at room temperature.
FIG 4.
Persistence of MNV infectivity on brasses. Approximately 5 × 105 PFU of infectious virus was applied to 1-cm2 samples of test surfaces and incubated at room temperature. At various time points, virus was removed from the surfaces and assessed for infectious virus by plaque assay as described in the text. The efficacy of these brasses in inactivating norovirus is directly proportional to the copper content; 100% Zn appeared to have some antiviral activity, similar to that of Muntz metal (C28000). The possible antiviral effect of zinc is discussed in the text.
FIG 5.
Comparison of the abilities of brass and copper nickel with the same percentage of copper (70%) to inactivate MNV. Brass is more effective at inactivating norovirus (the effect is more evident after 30 min of contact), presumably due to the synergistic action of copper and zinc.
A recent study has found that a zinc oxide passivation layer developed on brass surfaces in eccrine fingerprint sweat within an hour (27). It is yet to be determined whether this might affect the antiviral efficacy of brass.
Exposure to copper and copper alloy surfaces damages the norovirus capsid.
Observation by TEM of purified MNV-1 suggested that some damage to the capsid had occurred during the purification procedure (Fig. 6). The outer surface of the capsid was uneven and irregular (Fig. 6A). However, the purified fraction was found to be still infectious in the plaque assay, demonstrating the resilient nature of the capsid to protect the infectious viral RNA. Virus exposed to stainless steel was visible as individual particles (Fig. 6B) or had aggregated into large clumps of approximately 100 nm in diameter (Fig. 6C). This may offer some protection to virus particles within the aggregates and explain their persistence in an infectious state for weeks. The aggregation may be affected by the absence of cell debris and PBS diluent.
FIG 6.
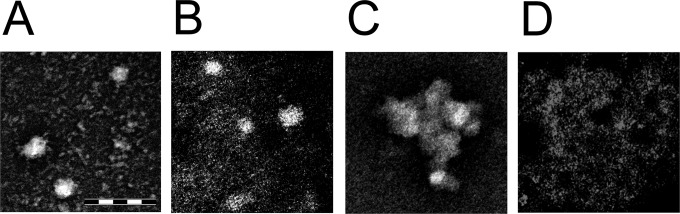
Contact with copper surfaces affects virus morphology. (A) Untreated virus. The uneven surface suggests that some damage has occurred to the outer capsid, resulting in a diameter slightly larger than 40 nm being reported for individual virions and disrupting the icosahedral symmetry. Plaque assay of this preparation suggests that the virus is still infectious, so the genome is still able to replicate. This demonstrates the protective nature of the capsid and its role in MNV persistence. (B and C) MNV recovered from stainless steel was observed as scattered individual particles (B) or large clumps (C) that had some damage to the outer capsid. Viral clumping may protect the inner virus particles from desiccation and explain the persistence on this material. (D) In contrast, exposure to copper resulted in viral fragments that were beyond the resolution of the TEM. Bar, 200 nm.
Purified MNV exposed to copper surfaces appeared to have disintegrated into fractions beyond the resolution of the TEM (Fig. 6D). Our previous study suggested that the release of cuprous and cupric ions was essential for antiviral activity, including the destruction of the viral genome. The TEM results suggest that extensive damage to the viral capsid must have occurred, presumably as a result of the direct or indirect action of copper ions. It is unclear whether capsid damage is a result of multiple, nonspecific attacks to the capsid surface or a dissociation of the capsomeres to expose the viral RNA.
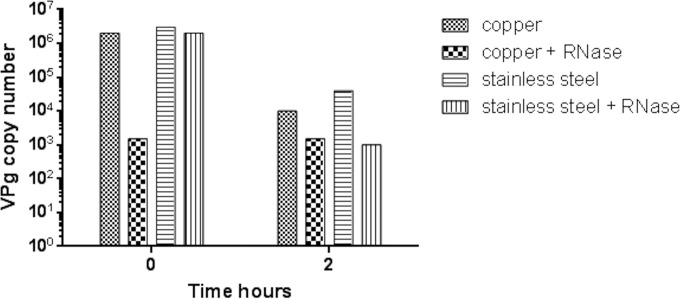
Comparisons between numbers of MNV virions removed from copper and stainless steel surfaces that had been treated with RNase support this premise (Fig. 7). Virus removed from stainless steel surfaces immediately after inoculation did not demonstrate any difference in copy number of a viral gene, the VPg gene, regardless of pretreatment with RNase. This suggests that the capsid is intact, with no exposed viral genome for RNase degradation. However, if virus is removed immediately from copper surfaces, the copy number is reduced in the RNase-treated sample. This suggests that damage to the capsid has already occurred in the time that it takes to process the sample (although infectivity is not reduced significantly at time zero). After 2 h of contact, the overall copy number was reduced on copper, but the reduced amplification of the VPg gene on both surfaces suggests that capsid damage occurred on copper and stainless steel but not so extensively on the latter. In our original work, we observed that if the virus is allowed to dry rapidly, infectivity is reduced much faster because the rapid drying process affects virus infectivity.
FIG 7.
Pretreatment of test samples with RNase to determine capsid integrity. If MNV is incomplete or the capsid is damaged, the viral nucleic acid may be exposed and susceptible to RNase treatment. Approximately 5 × 105 PFU of infectious virus was applied to 1-cm2 samples of test surfaces and incubated at room temperature. At various time points, virus was removed from the surfaces. Viral RNA was purified from the samples, and cDNA was prepared and assessed for viral genome by RT-quantitative PCR as described in the text. Copy number was derived from a standard curve for the standard VPg gene, supplied by PrimerDesign Ltd. Virus exposed to copper appears to have suffered damage to capsid immediately upon contact because the VPg gene copy number is reduced in RNase-treated samples, suggesting that the viral genome was exposed. This had not occurred on stainless steel at time zero, suggesting that intact capsid is impervious to RNase. After 30 min of contact, capsid damage is seen for both surfaces, which may be affected by the drying process as well as the antiviral effects of copper.
Potential application.
The highly infectious nature of norovirus makes it very difficult to stop the spread of infection from an outbreak, especially as no vaccine or treatment is available and the infection is often passed to the caregivers of infected individuals. Wheeler et al. (30) estimated that for every single case reported in the United Kingdom, this leads to 136 cases in the community. Morter et al. detected norovirus using real-time reverse transcription-quantitative PCR (RT-qPCR) in 31% of more than 30 surfaces tested in a hospital environment over a 4-month period, with 100% contamination on soap and alcohol dispensers, chairs, carpets, and Zimmer frames (28). Our results suggest that incorporation of copper alloy surfaces may help to prevent infection spread from contaminated surfaces. In many norovirus outbreaks reported, contaminated surfaces are responsible for a secondary outbreak that occurs primarily because of inadequate disinfection of vomitus and fecal norovirus contamination. For example, following an initial outbreak in a hospital, ward 2 carpet fitters contracted norovirus 12 days later when replacing the flooring in the outbreak area (29). It is very difficult to reduce the spread of norovirus infection from person to person in an initial outbreak in which segregation and quarantine of infected persons may be the only way to halt the spread of infection, although this is not always practical. There is a need for more-effective and safe disinfectants for decontaminating large areas of norovirus contamination. Incorporation of copper alloys may be very useful in preventing secondary transfer from ineffectively cleaned and highly contaminated surfaces in clinical facilities and closed environments, such as cruise ships, care homes, kitchens, bathrooms, and surfaces.
There is now a large body of evidence describing the importance of copper alloy touch surfaces as antibacterial surfaces; this evidence has been supported by hospital studies worldwide showing reduced bioburden on touch surfaces and decreased rates of infection in intensive care units. Recently, we demonstrated copper alloy antiviral activity against the enveloped and nonenveloped RNA viruses influenza virus and norovirus, respectively. We now report that the mechanism of copper inactivation for norovirus involves not only degradation of the RNA but also destruction of the capsid, which allows copper ions to enter the virus. These studies with RNA viruses suggest that copper alloy surfaces might exhibit antiviral activity against other important RNA viruses for which transmission via touch surfaces is important, including coronavirus (unpublished data) and Ebola virus.
ACKNOWLEDGMENTS
This research was supported by the Copper Development Association, New York, NY, and the International Copper Association, New York, NY.
We thank Herbert W. Virgin VI, Washington University, for providing MNV-1 and the RAW264.7 cell line. We also thank Anton Page, Biomedical Imaging Unit, University of Southampton, for his invaluable advice and assistance with transmission electron microscopy.
REFERENCES
- 1.Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, Rocks JJ, Kiel J, Montes JS, Moe CL, Eisenberg JN, Leon JS. 2012. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect 140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bert F, Scaioli G, Gualano MR, Passi S, Specchia ML, Cadeddu C, Viglianchino C, Siliquini R. 2014. Norovirus outbreaks on commercial cruise ships: a systematic review and new targets for the public health agenda. Food Environ Virol 6:67–74. doi: 10.1007/s12560-014-9145-5. [DOI] [PubMed] [Google Scholar]
- 3.Otter JA, Yezli S, Salkeld JA, French GL. 2013. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control 41:S6–S11. doi: 10.1016/j.ajic.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Patterson W, Haswell P, Fryers PT, Green J. 1997. Outbreak of small round structured virus gastroenteritis arose after kitchen assistant vomited. Commun Dis Rep CDR Rev 7:R101–R103. [PubMed] [Google Scholar]
- 5.Lai CC, Wang YH, Wu CY, Hung CH, Jiang DD, Wu FT. 2013. A norovirus outbreak in a nursing home: norovirus shedding time associated with age. J Clin Virol 56:96–101. doi: 10.1016/j.jcv.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Kirby AE, Shi J, Montes J, Lichtenstein M, Moe CL. 2014. Disease course and viral shedding in experimental Norwalk virus and Snow Mountain virus infection. J Med Virol 86:2055–2064. doi: 10.1002/jmv.23905. [DOI] [PubMed] [Google Scholar]
- 7.Laine J, Lumio J, Toikkanen S, Virtanen MJ, Uotila T, Korpela M, Kujansuu E, Kuusi M. 2014. The duration of gastrointestinal and joint symptoms after a large waterborne outbreak of gastroenteritis in Finland in 2. PLoS One 9:e85457. doi: 10.1371/journal.pone.0085457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilks SA, Michels H, Keevil CW. 2005. The survival of Escherichia coli O157 on a range of metal surfaces. Int J Food Microbiol 105:445–454. doi: 10.1016/j.ijfoodmicro.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 9.Noyce JO, Michels H, Keevil CW. 2006. Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. J Hosp Infect 63:289–297. doi: 10.1016/j.jhin.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Noyce JO, Michels H, Keevil CW. 2007. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol 73:2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver L, Michels HT, Keevil CW. 2008. Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. J Hosp Infect 68:145–151. doi: 10.1016/j.jhin.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 12.Weaver L, Michels HT, Keevil CW. 2010. Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminium. Lett Appl Microbiol 50:18–23. doi: 10.1111/j.1472-765X.2009.02753.x. [DOI] [PubMed] [Google Scholar]
- 13.Warnes SL, Green SM, Michels HT, Keevil CW. 2010. Biocidal efficacy of copper alloys against pathogenic enterococci involves degradation of genomic and plasmid DNAs. Appl Environ Microbiol 76:5390–5401. doi: 10.1128/AEM.03050-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey AL, Adams D, Karpanen TJ, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Shillam R, Christian P, Elliott TS. 2010. Role of copper in reducing hospital environment contamination. J Hosp Infect 74:72–77. doi: 10.1016/j.jhin.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Karpanen TJ, Casey AL, Lambert PA, Cookson BD, Nightingale P, Miruszenko L, Elliott TS. 2012. The antimicrobial efficacy of copper alloy furnishing in the clinical environment: a crossover study. Infect Control Hosp Epidemiol 33:3–9. doi: 10.1086/663644. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt MG, Attaway HH, Sharpe PA, John J Jr, Sepkowitz KA, Morgan A, Fairey SE, Singh S, Steed LL, Cantey JR, Freeman KD, Michels HT, Salgado CD. 2012. Sustained reduction of microbial burden on common hospital surfaces through introduction of copper. J Clin Microbiol 50:2217–2223. doi: 10.1128/JCM.01032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salgado CD, Sepkowitz KA, John JF, Cantey JR, Attaway HH, Freeman KD, Sharpe PA, Michels HT, Schmidt MG. 2013. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol 34:479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 18.Warnes SL, Keevil CW. 2011. Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Appl Environ Microbiol 77:6049–6059. doi: 10.1128/AEM.00597-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warnes SL, Caves V, Keevil CW. 2012. Mechanism of copper surface toxicity in Escherichia coli O157:H7 and Salmonella involves immediate membrane depolarization followed by lower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environ Microbiol 14:1730–1743. doi: 10.1111/j.1462-2920.2011.02677.x. [DOI] [PubMed] [Google Scholar]
- 20.Warnes SL, Keevil CW. 2013. Inactivation of norovirus on dry copper alloy surfaces. PLoS One 8:e75017. doi: 10.1371/journal.pone.0075017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. 2004. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol 2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noyce JO, Michels H, Keevil CW. 2006. Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing. Appl Environ Microbiol 72:4239–4244. doi: 10.1128/AEM.02532-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. 2008. Norwalk virus shedding after experimental human infection. Emerg Infect Dis 14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hans M, Erbe A, Mathews S, Chen Y, Solioz M, Mucklich F. 2013. Role of copper oxides in contact killing of bacteria. Langmuir 29:16160–16166. doi: 10.1021/la404091z. [DOI] [PubMed] [Google Scholar]
- 25.Korant BD, Kauer JC, Butterworth BE. 1974. Zinc ions inhibit replication of rhinoviruses. Nature 248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 26.Qiu M, Chen Y, Chu Y, Song S, Yang N, Gao J, Wu Z. 2013. Zinc ionophores pyrithione inhibits herpes simplex virus replication through interfering with proteasome function and NF-kappaB activation. Antiviral Res 100:44–53. doi: 10.1016/j.antiviral.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Bond JW, Lieu E. 2014. Electrochemical behaviour of brass in chloride solution concentrations found in eccrine fingerprint sweat. Appl Surf Sci 313:455–461. doi: 10.1016/j.apsusc.2014.06.005. [DOI] [Google Scholar]
- 28.Morter S, Bennet G, Fish J, Richards J, Allen DJ, Nawaz S, Iturriza-Gomara M, Brolly S, Gray J. 2011. Norovirus in the hospital setting: virus introduction and spread within the hospital environment. J Hosp Infect 77:106–112. doi: 10.1016/j.jhin.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Rzezutka A, Cook N. 2004. Survival of human enteric viruses in the environment and food. FEMS Microbiol Rev 28:441–453. doi: 10.1016/j.femsre.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Wheeler JG, Sethi D, Cowden JM, Wall PG, Rodrigues LC, Tompkins DS, Hudson MJ, Roderick PJ. 1999. Study of infectious intestinal disease in England: rates in the community, presenting to general practice, and reported to national surveillance. BMJ 318:1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]