Abstract
Pseudomonas plecoglossicida is a lethal pathogen of ayu (Plecoglossus altivelis) in Japan and is responsible for substantial economic costs to ayu culture. Previously, we demonstrated the efficacy of phage therapy against P. plecoglossicida infection using two lytic phages (PPpW-3 and PPpW-4) (S. C. Park, I. Shimamura, M. Fukunaga, K. Mori, and T. Nakai, Appl Environ Microbiol 66:1416–1422, 2000, http://dx.doi.org/10.1128/AEM.66.4.1416-1422.2000; S. C. Park and T. Nakai, Dis Aquat Org 53:33–39, 2003, http://dx.doi.org/10.3354/dao053033). In the present study, the complete genome sequences of these therapeutic P. plecoglossicida phages were determined and analyzed for deleterious factors as therapeutic agents. The genome of PPpW-3 (myovirus) consisted of 43,564 bp with a GC content of 61.1% and 66 predicted open reading frames (ORFs). Approximately half of the genes were similar to the genes of the Escherichia coli phage vB_EcoM_ECO1230-10 (myovirus). The genome of PPpW-4 (podovirus) consisted of 41,386 bp with a GC content of 56.8% and 50 predicted ORFs. More than 70% of the genes were similar to the genes of Pseudomonas fluorescens phage ϕIBB-PF7A and Pseudomonas putida phage ϕ15 (podoviruses). The whole-genome analysis revealed that no known virulence genes were present in PPpW-3 and PPpW-4. An integrase gene was found in PPpW-3, but other factors used for lysogeny were not confirmed. The PCR detection of phage genes in phage-resistant variants provided no evidence of lysogenic activity in PPpW-3 and PPpW-4. We conclude that these two lytic phages qualify as therapeutic agents.
INTRODUCTION
Bacteriophages (phages) have been studied for their therapeutic potential to control bacterial infections in animals and humans since their discovery in the mid-1910s. Studies on Escherichia coli infections in mice and farm animals by Smith et al. in the 1980s particularly opened the gate for phage therapy (1, 2, 3, 4). Thereafter, controlled studies with animal models indicated the potential of phage therapy and prompted renewed worldwide interest in this treatment method (reviewed in references 5, 6, and 7). In the aquaculture field, the first successful phage therapy was documented in an experimental Lactococcus garvieae infection of yellowtail Seriola quinqueradiata by intraperitoneal injection or oral administration of phages (8). Subsequently, the efficacy of phage therapy has been reported for several bacterial infections of fish and shellfish (9, 10, 11, 12, 13). However, inconsistent results have been reported for the use of this therapy against furunculosis of salmonids (14).
Bacterial hemorrhagic ascites emerged in cultured ayu (Plecoglossus altivelis) in Japan in the 1990s (15, 16). The disease is caused by the Gram-negative bacterium Pseudomonas plecoglossicida, which is highly homogeneous with respect to physiological, biochemical, and serological characteristics (17). P. plecoglossicida infection frequently results in high mortality at fish farms, because no licensed chemotherapeutic agents presently are available. To find an alternative method to control the disease, we isolated P. plecoglossicida lytic phages from the rearing water of ayu culture. Using two selected phages, designated PPpW-3 (myovirus) and PPpW-4 (podovirus), we demonstrated that phage treatment was effective for reducing mortality due to P. plecoglossicida infection when the ayu were fed a phage-impregnated diet in both laboratory and field settings (9, 10). However, some phages confer or raise the virulence of host bacteria (18). Therefore, for the safe application of phage therapy in aquaculture, we must demonstrate the absence of a deleterious factor(s) at the genetic level in the potential therapeutic phages (19). In the present study, we evaluated the suitability of two P. plecoglossicida phages as therapeutic agents by complete-genome sequence analysis.
MATERIALS AND METHODS
Phages and bacteria.
Two P. plecoglossicida phages, PPpW-3 (myovirus), which forms small plaques, and PPpW-4 (podovirus), which forms large plaques (9, 10), were used in this study. The P. plecoglossicida PTH-9802 strain (9) was used as the host bacterium and was subcultured in Trypto-soya broth (TSB; Nissui Pharmaceutical Co. Ltd., Tokyo, Japan) or on Trypto-soya agar (TSA; Nissui) at 25°C.
Purification of phages.
Phages were propagated by the agar overlay method (20) and then were purified as described previously (21). Phage-inoculated double-layer agar plates were incubated at 25°C. After 24 h of incubation, semisolid agar (TSB supplemented with 0.35% agar) containing 1,000 to 10,000 plaques per plate was recovered by washing the plates with 5 ml of PBS (0.1 M phosphate buffer, pH 7.2, 0.85% NaCl) and centrifuging at 5,000 × g for 10 min (4°C). The supernatant was filtered through a 0.45-μm membrane filter. The crude phage suspension (30 ml) was mixed with 10 ml of PEG solution (40% polyethylene glycol 6000, 2 M NaCl), followed by incubation on ice for 1 h. After centrifugation (25,000 × g, 10 min, 4°C), the sediment was resuspended in 1 ml of PBS. Solid CsCl was added to the concentrated phage suspension to bring the density to approximately 1.5 g/ml, and the suspension was centrifuged (150,000 × g, 18 h, 4°C) using a Hitachi CS100GS rotor. The phage fraction was dialyzed against PBS overnight and stored at 4°C until use.
Electron microscopy of purified phages.
Purified phages (1011 to 1012 PFU/ml) were negatively stained with 2% uranyl acetate on an elastic-carbon-coated copper grid (Okenshoji Co. Ltd., Tokyo, Japan). Grids were examined using a JEOL JEM-1200EX transmission electron microscope (TEM) at 80 kV.
Sequencing of phage DNA.
Phage genomic DNA was extracted from 100 μl of purified phage particles (1010 PFU/ml) using a standard phenol-chloroform extraction method (22). The concentration and purity of extracted phage genomic DNA in TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA) was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Products, Wilmington, DE). The Roche 454 GS-FLX Titanium sequencing platform (Roche Diagnostics, Branford, CT) was used to carry out the complete sequencing of phages. In total, 500 ng of each purified, enriched library was used for 454 FLX Titanium library preparations according to the manufacturer's protocols. DNA fragments (600 to 900 bp) from the library were amplified on beads by emulsion PCR (emPCR) using the GS FLX Titanium SV emPCR kit. For sequencing, the GS FLX Titanium sequencing kit XLR70 and GS FLX Titanium PicoTiterPlate kit 70x75 were used, and the DNA library with beads was sequenced in a 454 GS-FLX DNA sequencer. De novo assembly and contig alignment were performed using Newbler v. 2.5.3 (Roche Diagnostics, Branford, CT) and MAFFT6 (23), respectively. In advance, we optimized the number of sequence reads for de novo assembly and found that single contigs were produced most frequently using 5,000 reads for PPpW-3 and 1,000 reads for PPpW-4. For each strain, randomly picking up the reads 100 times, we performed de novo assemblies and aligned the single contigs obtained. Full-genome sequences of the phages were determined from the consensus sequence of the contig alignment.
ORF prediction and annotation.
The prediction of open reading frames (ORFs) was performed using Glimmer3 (24) and GeneMarkS (25). For tRNA gene identification, the tRNAscan-SE v.1.23 program was used (26). The function of each gene was predicted by comparison with known protein sequences in the GenBank database using BLASTp (27).
Examination of lysogenic activity of phages.
P. plecoglossicida strain PTH-9802 in the exponential growth phase (106 CFU/ml) was mixed with PPpW-3 or PPpW-4 at a multiplicity of infection (MOI) of approximately 10,000 (1010 PFU/ml) in PBS, and the mixture was incubated for 10 min to allow adsorption of phages. Five hundred μl of the mixture was added to 3 ml of dissolved semisolid agar and spread onto TSA plates. After incubation for 3 days at 25°C, single colonies were picked and subjected to subculture more than three times to remove infective phage particles. These mutants were suspended in TE buffer (approximately 107 CFU/ml) and boiled for 10 min to extract bacterial DNA. After centrifugation (12,000 × g, 10 min, 4°C), the supernatant was used as a template for PCR detection. The PCR primers used in this study are described in Table 1. All primer sets except PL-G2F and PL-G2R (28) were designed from sequence data of PPpW-3 and PPpW-4 using the Primer3 program (29). PCR amplifications were performed with SapphireAmp Fast PCR master mix (TaKaRa Bio, Otsu, Japan) according to the manual. The following temperature profile was used for amplification: preheating at 94°C for 1 min; 30 cycles of denaturation (98°C, 5 s), annealing (60°C, 5 s), and extension (72°C, 10 s); and a final extension at 72°C for 5 min. PCR products were electrophoresed with 3% agarose gels. Amplified DNA was stained with ethidium bromide and observed with a UV transilluminator.
TABLE 1.
PCR primers used in this study
| PCR detection target | Primer name | Primer sequence (5′ to 3′) | Product size (bp) | Reference |
|---|---|---|---|---|
| PPpW-3 ORF 5 (capsid) | W-3 ORF5F | AACCAGATGGCCTACGTGTC | 152 | This study |
| W-3 ORF5R | CCGTCAGAAATCTGGTTGGT | This study | ||
| PPpW-3 ORF 25 (integrase) | W-3 ORF25F | AACCTGAACGCAGTCGAAGT | 150 | This study |
| W-3 ORF25R | CCACAGCTTGGCCTTTATGT | This study | ||
| PPpW-3 ORF 34 (helicase) | W-3 ORF34F | GGGCGAATACTTTGACGAAA | 208 | This study |
| W-3 ORF34R | CTTGCGCCAGTGACTGATTA | This study | ||
| PPpW-4 ORF 25 (primase/helicase) | W-4 ORF25F | CGTCTGATGACCAAGCTGAA | 200 | This study |
| W-4 ORF25R | ATAGGGCATTGCTGGTTACG | This study | ||
| P. plecoglossicida (DNA gyrase) | PL-G2F | TGCTGAAGGACGAGCGTTCG | 520 | 28 |
| PL-G2R | ATCATCTTGCCGACAACAGC | 28 |
Nucleotide sequence accession numbers.
Complete genome sequences of the two P. plecoglossicida phage isolates were submitted to the DNA Data Bank of Japan (DDBJ) under accession numbers AB775548 (PPpW-3) and AB775549 (PPpW-4).
RESULTS AND DISCUSSION
TEM observation of PPpW-3 and PPpW-4.
Purified PPpW-3 phages were observed to have an isometric capsid of 59.4 ± 1.0 nm with a long rigid tail of 114.3 ± 1.4 × 20.4 ± 0.2 nm (n = 10) on TEM images (Fig. 1). In contrast, purified PPpW-4 phages had an isometric capsid of 54.5 ± 1.0 nm with a short tail of 11.2 ± 1.1 × 16.0 ± 0.9 nm (n = 10). From the morphological characteristics described in a previous report (9), PPpW-3 and PPpW-4 were identified as members of the families Myoviridae and Podoviridae, respectively. The size of PPpW-3 was similar to that of the P2-like viruses (head, 60 nm; tail, 135 by 18 nm) or the Mu-like viruses (head, 60 nm; tail, 120 by 18 nm) rather than the T4-like viruses (head, 111 by 78 nm; tail, 113 by 16 nm) of Myoviridae (30), while the size of PPpW-4 was almost the same as that of the T7-like viruses, which is the most common type of Podoviridae.
FIG 1.
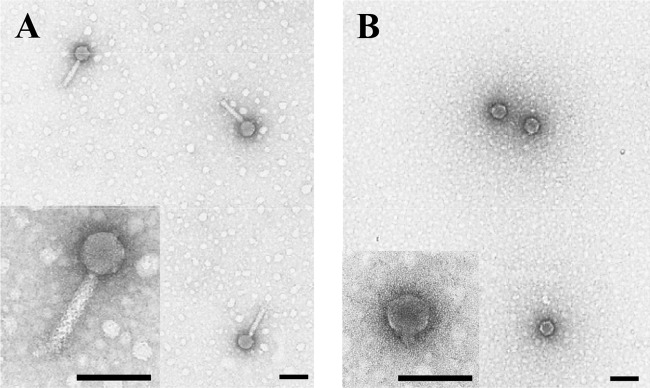
Electron micrograph of purified phage virion. (A) PPpW-3; (B) PPpW-4. Scale bars, 100 nm.
Overview of the PPpW-3 genome.
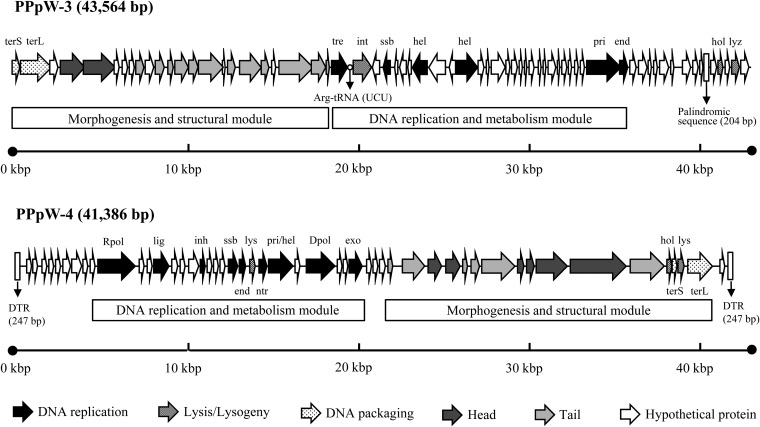
The genome of PPpW-3 consisted of linear double-stranded DNA of 43,564 bp, and the overall genomic guanine-plus-cytosine (GC) content was 61.1%. A total of 66 ORFs were predicted with one tRNA gene (Fig. 2). Based on the deduced amino acid sequence similarity (E value of <0.0001) using a BLASTp search of the GenBank database, 50 ORFs exhibited homology to known proteins of phage and bacteria (Table 2). Among these, 33 proteins were similar to Escherichia coli phage vB_EcoM_ECO1230-10. The particle size and content of morphogenesis proteins of this phage were almost the same as those of PPpW-3 (31). vB_EcoM_ECO1230-10 was isolated from the manure systems of dairy farms and caused the lysis of E. coli, which is one of the pathogens associated with puerperal metritis in dairy cows (31).
FIG 2.
Genome structures of PPpW-3 and PPpW-4. Gene abbreviations are the following: end, endonuclease; exo, exonuclease; hel, helicase; hol, holin; inh, RNA polymerase inhibitor; int, integrase; lig, DNA ligase; lys, lysis protein; lyz, lysozyme; ntr, nucleotidyl transferase; Rpol, RNA polymerase; Dpol, DNA polymerase; pri, primase; ssb, single-stranded DNA-binding protein; terS/L, terminase small/large subunit; tre, transcriptional regulator. The PPpW-3 genome contained a tRNA gene and long palindromic sequence (204 bp). The PPpW-4 genome had a 247-bp direct terminal repeat (DTR) at each of the genome termini.
TABLE 2.
Homologs of PPpW-3 genes
| ORF/strand | Length |
Homolog in GenBank |
|||
|---|---|---|---|---|---|
| bp | aa | Species (accession no.) | Putative function | E value (% identity) | |
| 1/+ | 519 | 172 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87936.1) | Terminase small subunit | 2e−40 (50) |
| 2/+ | 2,070 | 689 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87935.1) | Terminase large subunit | 0 (58) |
| 3/+ | 555 | 184 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87934.1) | 3e−71 (59) | |
| 4/+ | 1,617 | 538 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87933.1) | Portal protein | 0 (62) |
| 5/+ | 2,109 | 702 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87932.1) | Major capsid protein and peptidase U35 | 0 (67) |
| 6/+ | 306 | 101 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87931.1) | 2e−20 (52) | |
| 7/+ | 348 | 115 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87930.1) | 6e−36 (57) | |
| 8/+ | 342 | 113 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87929.1) | 2e−44 (66) | |
| 9/+ | 555 | 184 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87928.1) | Minor tail protein Z | 2e−71 (61) |
| 10/+ | 582 | 193 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87927.1) | 5e−67 (57) | |
| 11/+ | 609 | 202 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87926.1) | Baseplate assembly protein V | 3e−70 (53) |
| 12/+ | 348 | 115 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87925.1) | Baseplate assembly protein with lysozyme | 2e−50 (70) |
| 13/+ | 906 | 301 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87924.1) | Baseplate J-like protein | 3e−121 (60) |
| 14/+ | 621 | 206 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87923.1) | Tail protein I | 7e−52 (51) |
| 15/+ | 1,653 | 550 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87921.1) | Tail collar fiber protein | 2e−75 (42) |
| 17/+ | 513 | 170 | Bradyrhizobium sp. strain STM 3843 (ZP_09431603.1) | Tail collar domain-containing protein | 9e−32 (47) |
| 18/+ | 1,437 | 478 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87918.1) | Tail sheath protein | 0 (75) |
| 19/+ | 507 | 168 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87917.1) | Tail tube protein | 2e−74 (65) |
| 20/+ | 282 | 93 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87916.1) | 2e−36 (60) | |
| 21/+ | 2,280 | 759 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87915.1) | Tail protein | 0 (41) |
| 22/+ | 966 | 321 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87914.1) | Tail protein GpU | 3e−38 (49) |
| 23/+ | 210 | 69 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87913.1) | Tail protein X | 1e−21 (58) |
| 24/+ | 1,050 | 349 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87912.1) | Transcriptional regulator | 1e−159 (64) |
| 25/+ | 1,245 | 414 | Xanthobacter autotrophicus Py2 (YP_001415700.1) | Integrase family protein | 6e−50 (34) |
| 26/− | 480 | 159 | Enterobacteria phage Sf6 (NP_958202.1) | 6e−27 (43) | |
| 27/− | 489 | 162 | Pseudomonas putida F1 (YP_001269444.1) | Single-stranded DNA-binding protein | 2e−55 (61) |
| 28/− | 207 | 68 | Escherichia coli 536 (YP_672383.1) | 1e−08 (43) | |
| 29/− | 459 | 152 | Burkholderia phage BcepNY3 (YP_001294904.1) | 2e−34 (46) | |
| 30/− | 234 | 77 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87959.1) | 2e−27 (64) | |
| 31/− | 984 | 327 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87958.1) | Replicative DNA helicase | 2e−108 (51) |
| 32/− | 1,122 | 373 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87957.1) | 4e−126 (49) | |
| 33/− | 432 | 143 | Xanthomonas oryzae pv. oryzicola BLS256 (ZP_02243753.1) | 3e−15 (33) | |
| 34/+ | 1,410 | 469 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87956.1) | DNA helicase | 3e−173 (57) |
| 37/+ | 939 | 312 | Pseudomonas aeruginosa PACL_0465 (ACD39253.1) | 2e−111 (65) | |
| 39/+ | 390 | 129 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87953.1) | 4e−38 (52) | |
| 40/+ | 225 | 74 | Clostridium difficile QCD-63q42 (ZP_05332286.1) | 5e−11 (42) | |
| 41/+ | 156 | 51 | Burkholderia glumae BGR1 (YP_002911878.1) | 5e−11 (70) | |
| 45/+ | 507 | 168 | Burkholderia cenocepacia J2315 (YP_002233651.1) | 2e−06 (51) | |
| 49/+ | 192 | 63 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87949.1) | 1e−11 (56) | |
| 50/+ | 258 | 85 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87947.1) | 1e−11 (40) | |
| 51/+ | 2,274 | 757 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87946.1) | Primase | 0 (64) |
| 52/+ | 555 | 184 | Xanthomonas phage Xp10 (NP_858997.1) | HNH endonuclease | 4e−39 (46) |
| 53/+ | 414 | 137 | Methylosinus trichosporium OB3b (ZP_06890521.1) | 1e−70 (83) | |
| 54/+ | 576 | 191 | Pseudomonas phage AF (YP_007237221.1) | 3e−47 (79) | |
| 57/+ | 702 | 233 | Pseudomonas extremaustralis (WP_010566438.1) | 3e−09 (45) | |
| 62/+ | 435 | 144 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87941.1) | 2e−37 (50) | |
| 63/+ | 324 | 107 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87940.1) | Holin | 3e−15 (38) |
| 64/+ | 294 | 97 | Escherichia phage vB_EcoM_ECO1230-10 (ADE87939.1) | 3e−08 (36) | |
| 65/+ | 507 | 168 | Phage Gifsy-2 (NP_460003.1) | Lysozyme | 5e−42 (53) |
| 66/+ | 534 | 177 | Enterobacter asburiae LF7a (YP_004830970.1) | 7e−25 (41) | |
Similar to other small myoviruses, the genes on the left genome arm were conserved and encoded proteins involved in phage morphogenesis and structural modules (32). In the right genome arm, genes encoded DNA replication and metabolism modules, such as helicase (ORF31 and ORF34), primase (ORF51), and nuclease (ORF52). Additionally, there were many genes of unknown function (Fig. 2). The genes for holin (ORF63) and lysozyme (ORF65), which participate in cell lysis, were encoded close to the right-hand end of the PPpW-3 genome (Fig. 2). The role of holin is to permeabilize the cytoplasmic membrane and to facilitate lysozyme access to bacterial cell walls (33). Lysozyme degrades the cell wall by the hydrolysis of the beta-1,4-glycosidic bonds between N-acetylmuramic acid and N-acetyl-d-glucosamine residues in peptidoglycan (33).
Interestingly, the PPpW-3 genome had a long palindromic sequence (positions 41040 to 41243; 204 bp), and RNA transcripts might form stem-loop structures in the noncoding regions between ORF61 and ORF62. Small RNAs containing stem-loop structures, known as pRNA in Bacillus subtilis phage φ29, have an important function in packaging genomic DNA into procapsids (34), but the predicted secondary structure of these structures is completely different from that for pRNA (data not shown).
Overview of the PPpW-4 genome.
The genome of PPpW-4 consisted of linear double-stranded DNA of 41,386 bp, and the overall genomic guanine-plus-cytosine (GC) content was 56.8%. The genome had two 247-bp direct terminal repeats (DTR) in each genome termini, which is commonly observed in members of the T7-like viruses of Podoviridae. There were 50 predicted ORFs without a tRNA gene (Fig. 2). Using the BLASTp search, we found 45 ORFs that matched sequences in the GenBank database, which mainly originated from T7-like viruses (Table 3). In particular, 39 and 37 ORFs were similar to Pseudomonas fluorescens phage ϕIBB-PF7A (35) and Pseudomonas putida phage ϕ15 (36), respectively (Table 3). The phage ϕIBB-PF7A was isolated from dairy plant sewage and lysed dairy P. fluorescens isolates causing spoilage in milk (37). The phage ϕ15 is lytic and disrupts the biofilm of Pseudomonas putida, which is an opportunistic pathogen of humans (36).
TABLE 3.
Homologs of PPpW-4 genes
| ORF/Strand | Length |
Homolog on GenBanka |
|||
|---|---|---|---|---|---|
| bp | aa | Species (accession no.) | Putative function | E value (% identity) | |
| 2/+ | 246 | 81 | Escherichia phage rv5 (YP_002003688.1) | 2e−05 (38) | |
| 3/+ | 330 | 109 | ϕIBB-PF7A (YP_004306315.1), ϕ15 (YP_004286183.1) | 1e−13 (49), 4e−10 (41) | |
| 4/+ | 321 | 106 | ϕIBB-PF7A (YP_004306316.1) | 4e−40 (67) | |
| 6/+ | 543 | 180 | ϕIBB-PF7A (YP_004306317.1), ϕ15 (YP_004286185.1) | 8e−38 (48), 4e−36 (43) | |
| 7/+ | 711 | 236 | ϕIBB-PF7A (YP_004306318.1), ϕ15 (YP_004286186.1) | 2e−81 (55), 2e−5 (34) | |
| 8/+ | 276 | 91 | ϕIBB-PF7A (YP_004306319.1) | 9e−05 (41) | |
| 9/+ | 306 | 101 | ϕIBB-PF7A (YP_004306320.1) | 2e−06 (30) | |
| 10/+ | 2,673 | 890 | ϕIBB-PF7A (YP_004306321.1), ϕ15 (YP_004286187.1) | RNA polymerase | 0 (75), 0 (74) |
| 11/+ | 240 | 79 | ϕIBB-PF7A (YP_004306323.1) | 2e−24 (62) | |
| 12/+ | 264 | 87 | ϕ15 (YP_004286189.1) | 1e−34 (63) | |
| 13/+ | 1,086 | 361 | ϕIBB-PF7A (YP_004306325.1), ϕ15 (YP_004286190.1) | DNA ligase | 5e−153 (63), 8e−170 (68) |
| 14/+ | 435 | 144 | ϕIBB-PF7A (YP_004306326.1), ϕ15 (YP_004286191.1) | 3e−6 (28), 7e−09 (31) | |
| 16/+ | 717 | 238 | ϕIBB-PF7A (YP_004306327.1), ϕ15 (YP_004286193.1) | 4e−88 (58), 1e−75 (52) | |
| 17/+ | 171 | 56 | ϕIBB-PF7A (YP_004306328.1), ϕ15 (YP_004286194.1) | Bacterial RNA polymerase inhibitor | 2e−10 (58), 2e−16 (71) |
| 19/+ | 291 | 96 | ϕ15 (YP_004286195.1) | 3e−08 (59) | |
| 20/+ | 354 | 117 | ϕIBB-PF7A (YP_004306329.1), ϕ15 (YP_004286196.1) | 1e−23 (49), 1e−27 (48) | |
| 21/+ | 672 | 223 | ϕIBB-PF7A (YP_004306330.1), ϕ15 (YP_004286197.1) | Single-stranded DNA-binding protein | 1e−87 (62), 2e−117 (79) |
| 22/+ | 441 | 146 | ϕIBB-PF7A (YP_004306331.1), ϕ15 (YP_004286198.1) | Endonuclease I | 2e−69 (71), 3e−89 (83) |
| 23/+ | 468 | 155 | ϕIBB-PF7A (YP_004306332.1), ϕ15 (YP_004286199.1) | N-acetylmuramoyl–l-alanine amidase | 3e−89 (81), 9e−74 (70) |
| 24/+ | 582 | 193 | ϕIBB-PF7A (YP_004306333.1), ϕ15 (YP_004286201.1) | Nucleotidyl transferase | 5e−86 (70), 3e−67 (58) |
| 25/+ | 1,728 | 575 | ϕIBB-PF7A (YP_004306334.1), ϕ15 (YP_004286202.1) | DNA primase/helicase | 0 (68), 0 (73) |
| 26/+ | 192 | 63 | ϕIBB-PF7A (YP_004306335.1), ϕ15 (YP_004286203.1) | 1e−13 (53), 8e−14 (62) | |
| 27/+ | 2,130 | 709 | ϕIBB-PF7A (YP_004306337.1), ϕ15 (YP_004286204.1) | DNA polymerase | 0 (75), 0 (79) |
| 29/+ | 210 | 69 | ϕ15 (YP_004286206.1) | 3e−36 (84) | |
| 30/+ | 933 | 310 | ϕIBB-PF7A (YP_004306340.1), ϕ15 (YP_004286208.1) | Exonuclease | 1e−148 (67), 0 (82) |
| 31/+ | 249 | 82 | ϕIBB-PF7A (YP_004306341.1), ϕ15 (YP_004286209.1) | 5e−30 (64), 2e−29 (59) | |
| 32/+ | 267 | 88 | ϕIBB-PF7A (YP_004306342.1), ϕ15 (YP_004286210.1) | 5e−10 (46), 2e−21 (52) | |
| 33/+ | 423 | 140 | Pseudomonas phage gh-1 (NP_813770.1) | 9e−39 (52) | |
| 34/+ | 261 | 86 | ϕIBB-PF7A (YP_004306343.1), ϕ15 (YP_004286211.1) | Tail assembly protein | 4e−05 (55), 7e−09 (52) |
| 35/+ | 1,605 | 534 | ϕIBB-PF7A (YP_004306344.1), ϕ15 (YP_004286212.1) | Head-tail connector protein | 0 (81), 0 (81) |
| 36/+ | 972 | 323 | ϕIBB-PF7A (YP_004306345.1), ϕ15 (YP_004286213.1) | Capsid assembly protein | 2e−98 (53), 3e−111 (59) |
| 37/+ | 1,026 | 341 | ϕIBB-PF7A (YP_004306347.1), ϕ15 (YP_004286214.1) | Major capsid protein | 0 (87), 0 (88) |
| 38/+ | 249 | 82 | Pantoea sp. strain At-9b (YP_004115985.1) | Ig domain-containing protein | 1e−20 (63) |
| 39/+ | 588 | 195 | ϕIBB-PF7A (YP_004306348.1), ϕ15 (YP_004286216.1) | Tail tubular protein A | 3e−112 (81), 4e−88 (63) |
| 40/+ | 2,385 | 794 | ϕIBB-PF7A (YP_004306349.1), ϕ15 (YP_004286217.1) | Tail tubular protein B | 0 (66), 0 (63) |
| 41/+ | 438 | 145 | ϕIBB-PF7A (YP_004306350.1), ϕ15 (YP_004286218.1) | Internal virion protein A | 4e−51 (55), 2e−33 (47) |
| 42/+ | 540 | 179 | ϕIBB-PF7A (YP_004306351.1), ϕ15 (YP_004286219.1) | Internal virion protein B | 8e−73 (69), 2e−70 (61) |
| 43/+ | 2,211 | 736 | ϕIBB-PF7A (YP_004306352.1), ϕ15 (YP_004286220.1) | Internal virion protein C | 0 (65), 0 (61) |
| 44/+ | 3,999 | 1,332 | ϕIBB-PF7A (YP_004306353.1), ϕ15 (YP_004286221.1) | Internal virion protein D | 0 (73), 0 (73) |
| 45/+ | 2,445 | 814 | ϕIBB-PF7A (YP_004306354.1) | Tail fiber protein | 2e−67 (75) |
| 46/+ | 216 | 69 | ϕIBB-PF7A (YP_004306355.1), ϕ15 (YP_004286223.1) | Type II holin | 5e−10 (40), 4e−09 (40) |
| 47/+ | 258 | 85 | ϕIBB-PF7A (YP_004306356.1), ϕ15 (YP_004286224.1) | Terminase small subunit | 1e−25 (63), 5e−38 (75) |
| 48/+ | 438 | 145 | ϕIBB-PF7A (YP_004306357.1), ϕ15 (YP_004286225.1) | Endopeptidase | 1e−36 (50), 5e−36 (46) |
| 49/+ | 1,764 | 587 | ϕIBB-PF7A (YP_004306358.1), ϕ15 (YP_004286227.1) | Terminase large subunit | 0 (86), 0 (83) |
| 50/+ | 213 | 70 | ϕIBB-PF7A (YP_004306360.1), ϕ15 (YP_004286228.1) | 2e−15 (61), 7e−15 (60) | |
Various T7-like viruses have three conserved functional gene clusters, known as class I, II, and III (38). The class I genes are transcribed by the host RNA polymerase and work to convert host metabolism. In this region of PPpW-4, the phage promoter-specific RNA polymerase (ORF10), which transcribes the subsequent gene cluster, was identified. The DNA primase/helicase (ORF25) and DNA polymerase (ORF27) genes involved in DNA replication and metabolism were found in the class II gene cluster in the middle of the PPpW-4 genome (Fig. 2). Class III genes, which consist of morphogenesis and structural modules, were highly conserved with Pseudomonas phages ϕIBB-PF7A and ϕ15. Three lysis proteins were identified in PPpW-4: N-acetylmuramoyl-l-alanine amidase (ORF23), holin (ORF46), and endopeptidase (ORF48). These cell lysis proteins, except for holin, hydrolyze the cross-linkage of each N-acetylmuramic acid residue of peptidoglycan. Lysozyme found in PPpW-3 degrades the glycan strand (33). When we prepared the phage suspensions for further experiments, the viscosity of the phage lysates was different for PPpW-3 and PPpW-4. This phenomenon appears to be due to the different systems of lysis of host cells used by each phage.
Examination of the lysogenic activity of PPpW-3 and PPpW-4.
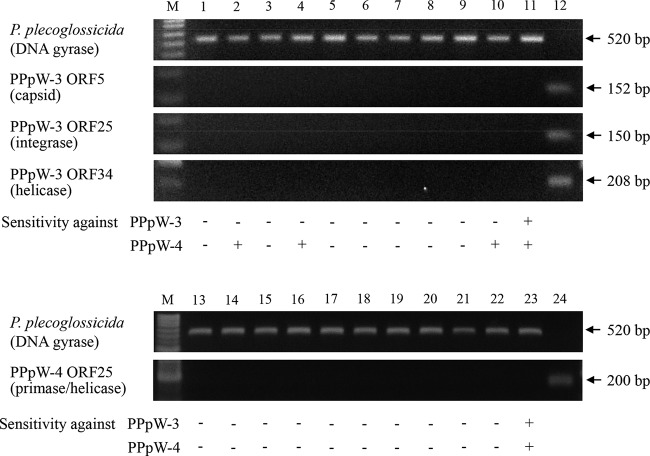
The lysogenic properties of phages often are related to phage conversion and may confer immunity against phage infection or enhance the virulence of host bacteria (18). In the present study, the lysogenic activity of PPpW-3 and PPpW-4 was examined by PCR detection of phage genes in phage-resistant bacteria. Approximately 0.01% of phage-inoculated bacteria became resistant to the inoculum, and 10 cloned variants from each phage strain were obtained. Several PPpW-3-resistant bacteria remained susceptible to PPpW-4, while all PPpW-4-resistant variants had no susceptibility against PPpW-3 (Fig. 3). In these variants, neither the major capsid gene (ORF5), the integrase gene (ORF25), the helicase gene (ORF34) of PPpW-3, nor the primase/helicase gene (ORF25) of PPpW-4 could be detected by PCR (Fig. 3). The integrase gene, which is strongly associated with lysogeny (39), was observed in the PPpW-3 genome as ORF25, but other lysogenic factors, such as excisionase, repressor, and antirepressor genes, were not identified (Table 2). Aeromonas salmonicida phage 56 and Idobacter sp. phage φPLPE are lytic phages that have only an antirepressor gene without any other factors for lysogeny (32). In these cases, the gene is thought to be derived from random horizontal transfer of lysogenic genes or residual lysogenic activity that has largely disappeared. On the other hand, the T7-like viruses are absolute lytic phages, and PPpW-4 also had no lysogenic genes that were similar to genes found in Pseudomonas phages ϕIBB-PF7A (35) and ϕ15 (36). Consequently, PPpW-3 and PPpW-4 appear to have no lysogenic activity in host bacteria.
FIG 3.
PCR detection of phage genes in the phage-resistant P. plecoglossicida variants. Lanes 1 to 10, phage-resistant variants produced by PPpW-3; lanes 13 to 22, phage-resistant variants produced by PPpW-4; lanes 11 and 23, PTH-9802 (parent strain); lane 12, PPpW-3; lane 24, PPpW-4; M, molecular size marker.
In regard to bacterial resistance against phage infection, our previous study demonstrated that phage-resistant P. plecoglossicida organisms, which are produced by PPpW-3 or PPpW-4 in vitro, lose pathogenicity against ayu (9), and phage treatment was successfully accomplished without isolation of phage-resistant bacteria (10). Recently, phage-resistant variants of Flavobacterium columnare, which is an opportunistic pathogen in salmonid fish, changed colony morphology and lost virulence (40). Therefore, the bacterial phage resistance mechanism appears to be closely associated with bacterial pathogenicity. A pathogenic E. coli strain (O18:K1:H7 ColV+) became resistant to phages during phage therapy in a mouse model due to the loss of K antigen, which is an essential factor for phage infection. However, the therapeutic trial was successful because K antigen also is associated with bacterial virulence (1). Other phage resistance mechanisms also have been reported to be involved in lysogeny-dependent resistance or lytic phage-inducible resistance, such as the CRISPR-Cas system (41). The present study shows that resistance to PPpW-3 and/or PPpW-4 infection is not due to lysogeny. Therefore, further studies into phage resistance mechanisms may shed light on virulence factors in P. plecoglossicida.
Evaluation of PPpW-3 and PPpW-4 as therapeutic agents.
Some phages are responsible for the virulence of host bacteria by aiding in the production of toxins, such as Shiga-like toxin by an E. coli O157:H7 strain (42) and cholera toxin by Vibrio cholerae (43). Other phages alter the bacterial properties, such as colonization, invasion, or susceptibility to antibiotics (18). In aquaculture, virulence of Vibrio harveyi, which causes vibriosis in shrimp, is induced by infection with specific phages, such as VHS1 (siphovirus [44, 45]) or VHML (myovirus [46, 47]). Complete genome sequence analysis of these phages revealed that VHML had a set of lysogenic genes and an ADP-ribosylating toxin gene (48), and VHS1 had another toxin gene associated with hemocyte agglutination (49). The complete genome sequencing of therapeutic phage is important to avoid lysogenic and virulence-inducible phages by confirming the absence of deleterious factors, such as a lyogenic gene or toxin gene. In the present study, no known lysogenic genes or toxin genes were identified in the whole genomes of PPpW-3 and PPpW-4, and we demonstrated that none of the analyzed phage-resistant variants contain PPpW-3 and PPpW-4 (Fig. 3). Furthermore, there was no possibility that these phages induced the virulence of P. plecoglossicida during phage treatment (9, 10). Although the analyzed phages had several genes of unknown function, based on the database analysis and our previous results from phage treatment, we concluded that these phages do not increase the pathogenesis of P. plecoglossicida.
The possibility of phage transduction is also a concern, because large amounts of phages are released to the rearing water of fish during the phage treatment. A therapeutic phage might transfer the virulence factor of the host bacteria to other nonpathogenic bacteria if the therapeutic phage has a broad host range that spans the bacterial species (50). In our previous study, the infectivity of PPpW-3 and PPpW-4 was examined against eight representative fish pathogenic bacteria (Aeromonas hydrophila, A. salmonicida, Edwardsiella tarda, Pseudomonas putida, Pseudomonas fluorescens, Pseudomonas anguilliseptica, Vibrio anguillarum, and V. ordalii), including the other pathogens of ayu. However, these phages did not show infectivity in the examined bacteria (9). This result suggests that the host range of PPpW-3 and PPpW-4 is restricted to P. plecoglossicida, and the risk of transduction by these therapeutic phages is low. Consequently, PPpW-3 and PPpW-4 are safe for therapeutic use.
The research steps for phage therapy in aquaculture have been proposed in terms of practical application (51). The genome analysis of therapeutic phages performed in this study is an essential step in ensuring the safety of this type of treatment. In addition, the complete genome sequence allows the characterization of the phages in detail, and we will be able to distinguish between the administered phage and naturally occurring phage during the treatment.
In the present study, we determined the complete genome sequences of PPpW-3 and PPpW-4, which are candidate strains for practical therapeutic applications, and we confirmed that these phages have no known deleterious factors as therapeutic agents. Now that the practice of phage therapy for P. plecoglossicida infection of ayu is imminent, the possibility of unexpected phage-mediated environmental perturbation, as noted by Meaden and Koskella (52), must be investigated in the field.
ACKNOWLEDGMENTS
We thank K. Koike, on the technical staff of Hiroshima University, for observing the phage by TEM.
This study was partly supported by a special grant from the Ministry of Agriculture, Forestry and Fisheries of Japan.
REFERENCES
- 1.Smith HW, Huggins MB. 1982. Successful treatment of experimental Escherichia coli infections in mice using phage: its general superiority over antibiotics. J Gen Microbiol 128:307–318. [DOI] [PubMed] [Google Scholar]
- 2.Smith HW, Huggins MB. 1983. Effectiveness of phages in treating experimental Escherichia coli diarrhoea in calves, piglets and lambs. J Gen Microbiol 129:2659–2675. [DOI] [PubMed] [Google Scholar]
- 3.Smith HW, Huggins MB, Shaw KM. 1987. The control of experimental Escherichia coli diarrhoea in calves by means of bacteriophages. J Gen Microbiol 133:1111–1126. [DOI] [PubMed] [Google Scholar]
- 4.Smith HW, Huggins MB, Shaw KM. 1987. Factors influencing the survival and multiplication of bacteriophages in calves and in their environment. J Gen Microbiol 133:1127–1135. [DOI] [PubMed] [Google Scholar]
- 5.Barrow PA, Soothill JS. 1997. Bacteriophage therapy and prophylaxis: rediscovery and renewed assessment of potential. Trends Microbiol 5:268–271. doi: 10.1016/S0966-842X(97)01054-8. [DOI] [PubMed] [Google Scholar]
- 6.Merril CR, Scholl D, Adhya SL. 2003. The prospect for bacteriophage therapy in Western medicine. Nat Rev Drug Discov 2:489–497. doi: 10.1038/nrd1111. [DOI] [PubMed] [Google Scholar]
- 7.Kutter E, Vos DD, Gvasalia G, Alavidze Z, Gogokhia L, Kuhl S, Abedon ST. 2010. Phage therapy in clinical practice: treatment of human infections. Curr Pharm Biotechnol 11:69–86. doi: 10.2174/138920110790725401. [DOI] [PubMed] [Google Scholar]
- 8.Nakai T, Sugimoto R, Park KH, Matsuoka S, Mori K, Nishioka T, Maruyama K. 1999. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis Aquat Org 37:33–41. doi: 10.3354/dao037033. [DOI] [PubMed] [Google Scholar]
- 9.Park SC, Shimamura I, Fukunaga M, Mori K, Nakai T. 2000. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol 66:1416–1422. doi: 10.1128/AEM.66.4.1416-1422.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SC, Nakai T. 2003. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis Aquat Org 53:33–39. doi: 10.3354/dao053033. [DOI] [PubMed] [Google Scholar]
- 11.Vinod MG, Shivu MM, Umesha KR, Rajeeva BC, Krohne G, Karunasagar I, Karunasagar I. 2006. Isolation of Vibrio harveyi bacteriophage with a potential for biocontrol of luminous vibriosis in hatchery environments. Aquaculture 255:117–124. doi: 10.1016/j.aquaculture.2005.12.003. [DOI] [Google Scholar]
- 12.Karunasagar I, Shivu MM, Girisha SK, Krohne G, Karunasagar I. 2007. Biocontrol of pathogens in shrimp hatcheries using bacteriophages. Aquaculture 268:288–292. doi: 10.1016/j.aquaculture.2007.04.049. [DOI] [Google Scholar]
- 13.Matsuoka S, Hashizume T, Kanzaki H, Iwamoto E, Park SC, Yoshida T, Nakai T. 2007. Phage therapy against β-hemolytic streptococcicosis of Japanese flounder Paralichthys olivaceus. Fish Pathol 42:181–189. doi: 10.3147/jsfp.42.181. [DOI] [Google Scholar]
- 14.Verner-Jeffreys DW, Algoet M, Pond MJ, Virdee HK, Bagwell NJ, Roberts EG. 2007. Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 270:475–484. doi: 10.1016/j.aquaculture.2007.05.023. [DOI] [Google Scholar]
- 15.Nakatsugawa T, Iida Y. 1996. Pseudomonas sp. isolated from diseased ayu, Plecoglossus altivelis. Fish Pathol 31:221–227. doi: 10.3147/jsfp.31.221. [DOI] [Google Scholar]
- 16.Wakabayashi H, Sawada K, Ninomiya K, Nishimori E. 1996. Bacterial hemorrhagic ascites of ayu caused by Pseudomonas sp. Fish Pathol 31:239–240. doi: 10.3147/jsfp.31.239. [DOI] [Google Scholar]
- 17.Nishimori E, Kita-Tsukamoto K, Wakabayashi H. 2000. Pseudomonas plecoglossicida sp. nov., the causative agent of bacterial hemorrhagic ascites of ayu, Plecoglossus altivelis. Int J Syst Evol Microbiol 50:83–89. doi: 10.1099/00207713-50-1-83. [DOI] [PubMed] [Google Scholar]
- 18.Wagner PL, Waldor MK. 2002. Bacteriophage control of bacterial virulence. Infect Immun 70:3985–3993. doi: 10.1128/IAI.70.8.3985-3993.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sulakvelidze A, Kutter E. 2005. Bacteriophage therapy in humans, p 381–436. In Kutter E, Sulakvelidze A (ed), Bacteriophages: biology and application. CRC Press, Boca Raton, FL. [Google Scholar]
- 20.Carlson K. 2005. Working with bacteriophages: common techniques and methodological approaches, p 437-494. In Kutter E, Sulakvelidze A (ed), Bacteriophages: biology and application. CRC Press, Boca Raton, FL. [Google Scholar]
- 21.Kawato Y, Nakai T. 2012. Infiltration of bacteriophages from intestinal tract to circulatory system in goldfish. Fish Pathol 47:1–6. doi: 10.3147/jsfp.47.1. [DOI] [Google Scholar]
- 22.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 23.Katoh K, Toh H. 2008. Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298. doi: 10.1093/bib/bbn013. [DOI] [PubMed] [Google Scholar]
- 24.Delcher AL, Bratke KA, Powers EC, Salzberg SL. 19 January 2007. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besemer J, Lomsadze A, Borodovsky M. 2001. GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res 29:2607–2618. doi: 10.1093/nar/29.12.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowe TM, Eddy SR. 1997. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25:955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi S, Yamamoto M, Suzuki K, Shimizu A, Aranishi F. 2007. Identification and detection of Pseudomonas plecoglossicida isolates with PCR primers targeting the gyrB region. J Fish Dis 30:391–397. doi: 10.1111/j.1365-2761.2007.00820.x. [DOI] [PubMed] [Google Scholar]
- 29.Rozen S, Skaletsky HJ. 2000. Primer3 on the WWW for general users and for biologist programmers, p 365–386. In Krawetz S, Misener S (ed), Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 30.Lavigne R, Ceyssens PJ. 2012. Family Myoviridae, p 46-62. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy classification and nomenclature of viruses ninth report of the international committee on taxonomy of viruses, Elsevier Academic Press, San Diego, CA. [Google Scholar]
- 31.Santos TMA, Bicalho RC. 2011. Complete genome sequence of vB_EcoM_ ECO1230-10: a coliphage with therapeutic potential for bovine metritis. Vet Microbiol 148:267–275. doi: 10.1016/j.vetmic.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Comeau AM, Tremblay D, Moineau S, Rattei T, Kushkina AI, Tovkach FI, Krisch HM, Ackermann HW. 2012. Phage morphology recapitulates phylogeny: the comparative genomics of a new group of myoviruses. PLoS One 7:e40102. doi: 10.1371/journal.pone.0040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young R. 1992. Bacteriophage lysis: mechanism and regulation. Microbiol Rev 56:430–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo PX, Erickson S, Anderson D. 1987. A small viral RNA is required for in vitro packaging of bacteriophage φ29 DNA. Science 236:690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- 35.Sillankorva S, Kluskens LD, Lingohr EJ, Kropinski AM, Neubauer P, Azeredo J. 2011. Complete genome sequence of the lytic Pseudomonas fluorescens phage ϕIBB-PF7A. Virol J 8:142. doi: 10.1186/1743-422X-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cornelissen A, Ceyssens PJ, T'Syen J, Praet HV, Noben JP, Shaburova OV, Krylov VN, Volckaert G, Lavigne R. 2011. The T7-related Pseudomonas putida phage ϕ15 displays virion-associated biofilm degradation properties. PLoS One 6:e18597. doi: 10.1371/journal.pone.0018597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sillankorva S, Neubauer P, Azeredo J. 2008. Isolation and characterization of a T7-like lytic phage for Pseudomonas fluorescens. BMC Biotechnol 8:80. doi: 10.1186/1472-6750-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn JJ, Studier FW. 1983. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol 166:477–535. doi: 10.1016/S0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- 39.Canchaya C, Proux C, Fournous G, Bruttin A, Brüssow H. 2003. Prophage genomics. Microbiol Mol Biol Rev 67:238–276. doi: 10.1128/MMBR.67.2.238-276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laanto E, Bamford JK, Laakso J, Sundberg LR. 2012. Phage-driven loss of virulence in a fish pathogenic bacterium. PLoS One 7:e53157. doi: 10.1371/journal.pone.0053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- 42.O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 43.Waldor MK, Mekalanos JJ. 1996. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 44.Ruangpan L, Danayadol Y, Direkbusarakom S, Siurairatna S, Flegel TW. 1999. Lethal toxicity of Vibrio harveyi to cultivated Penaeus monodon induced by a bacteriophage. Dis Aquat Org 35:195–201. doi: 10.3354/dao035195. [DOI] [Google Scholar]
- 45.Khemayan K, Pasharawipas T, Puiprom O, Sriurairatana S, Suthienkul O, Flegel TW. 2006. Unstable lysogeny and pseudolysogeny in Vibrio harveyi siphovirus-like phage 1. Appl Environ Microbiol 72:1355–1363. doi: 10.1128/AEM.72.2.1355-1363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oakey HJ, Owens L. 2000. A new bacteriophage, VHML, isolated from a toxin-producing strain of Vibrio harveyi in tropical Australia. J Appl Microbiol 89:702–709. doi: 10.1046/j.1365-2672.2000.01169.x. [DOI] [PubMed] [Google Scholar]
- 47.Munro J, Oakey J, Bromage E, Owens L. 2003. Experimental bacteriophage-mediated virulence in strains of Vibrio harveyi. Dis Aquat Org 54:187–194. doi: 10.3354/dao054187. [DOI] [PubMed] [Google Scholar]
- 48.Oakey HJ, Cullen BR, Owens L. 2002. The complete nucleotide sequence of the Vibrio harveyi bacteriophage, VHML. J Appl Microbiol 93:1089–2002. doi: 10.1046/j.1365-2672.2002.01776.x. [DOI] [PubMed] [Google Scholar]
- 49.Khemayan K, Prachumwat A, Sonthayanon B, Intaraprasong A, Sriurairatana S, Flegel TW. 2012. Complete genome sequence of virulence-enhancing siphophage VHS1 from Vibrio harveyi. Appl Environ Microbiol 78:2790–2796. doi: 10.1128/AEM.05929-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koskella B, Meaden S. 2013. Understanding bacteriophage specificity in natural microbial communities. Viruses 5:806–823. doi: 10.3390/v5030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakai T. 2010. Application of bacteriophages for control of infectious diseases in aquaculture, p 257–272. In Sabour PM, Griffiths MW (ed), Bacteriophages in the control of food and waterborne pathogens. ASM Press, Washington, DC. [Google Scholar]
- 52.Meaden S, Koskella B. 2013. Exploring the risks of phage application in the environment. Front Microbiol 4:358. doi: 10.3389/fmicb.2013.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]