Abstract
Immune escape mechanisms are prevalent in tumors, while their influence on the potency of antitumor immunotherapy has yet to be distinguished. We recently showed that increased numbers of intratumoral T cells rather than immune-escape-mechanisms significantly correlated with clinical outcome of advanced melanoma patients to subsequent autologous tumor cell vaccination. Our data emphasize the therapeutic relevance of tumor-infiltrating T cells for the clinical outcome.
Keywords: clinical outcome, immunotherapy, immune-escape mechanisms, melanoma, tumor-infiltrating T-cells
Review
Immunotherapy currently represents the most promising therapeutic approach for advanced melanoma patients, offeringpotentially life-saving treatments. Despite improved immunotherapeutic regimens for melanoma, significant numbers of patients with advanced disease still respond poorly, or relapse. Many immune regulating factors and cell types play a decisive role in the potency of antitumor immune responses. T-cell inhibitory factors, such as programmed cell death (ligand)-1 (PD-1/PD-L1), indoleamine 2,3-dioxygenase (IDO), and galectin-1 and -3, hamper T-cell function and/or promote T-cell apoptosis.1-3 On the other hand, immune suppressive cells, including regulatory T- ells (Tregs), myeloid-derived suppressor cells (MDSC) and mast cells, hinder the effector function of T cells.4 Tumor-derived tolerogenic cytokines can promote the immunosuppressive environment of tumors, whereas downregulation or loss of HLA and/or melanocyte differentiation antigens among tumor cells lead to decreased cancer cell recognition and attack by T cells. Since all these immune escape mechanisms in melanoma can impair the function of immune cells, it is important to understand the influence of T-cell inhibitory factors or immune-suppressive cells within the tumor environment and how these may influence an effective antitumor immune response and, consequently, clinical outcome.
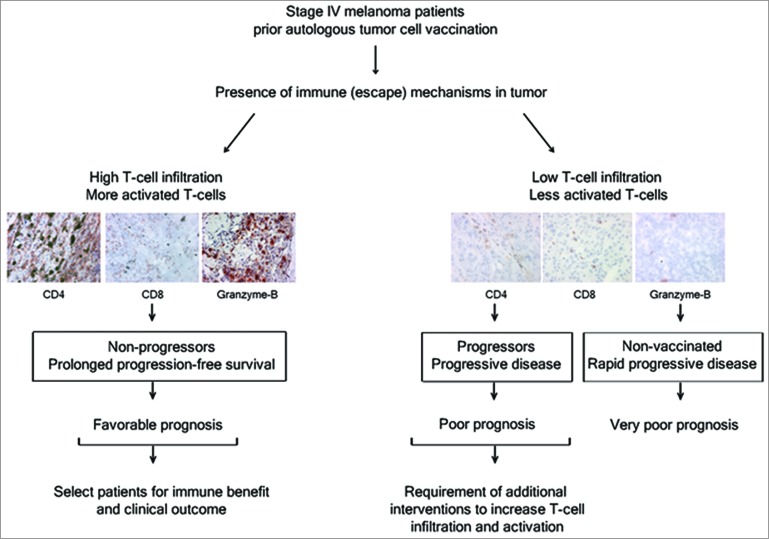
In a retrospective study of 43 Stage IV metastatic melanoma patients, we have investigated melanoma tissues of patients before receiving an autologous granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor cell vaccine.5 The presence of various T-cell related markers and immune escape markers of melanoma, including T-cell inhibitory factors, immune (suppressive) cells and tolerogenic cytokines were scored in one large analysis. The analyses incorporated the markers for immune (suppressive) cells consisting of CD4+ and CD8+ T-cells, CD11b+CD33+MDSCs, FoxP3+ Tregs, tryptase+ mast cells; markers associated with T-cell inhibition and activation (PD-1/PD-L1, IDO, galectin-1 and -3, granzyme-B); loss of melanocyte differentiation antigens (MART-1, gp100) or HLA Class-I, and tolerogenic cytokines in tumor cells (IL-1, IL-6, IL-10, TNFα and TGFβ). The results were related to patient clinical outcome, comprising clinical response, progression-free survival (PFS) and overall survival (OS). Based on their clinical responses, patients were assigned as 1) “non-progressors” when vaccinated patients had stable disease of non-assessable disease with prolonged PFS (median OS = 56 months); 2) “progressors” for vaccinated patients who experienced progressive disease (PD; median OS = 9.5 months; and 3) “non-vaccinated” for patients who were withdrawn before vaccination due to rapid disease progression (median OS = 3 months).
Significantly higher numbers of activated T cells were found in tumors at baseline of non-progressors as compared with progressors.5 Comparable low amounts of (activated) intratumoral T cells were detected in both progressors and the non-vaccinated group. Furthermore, a strong relationship between CD4, CD8 and granzyme-B with OS of vaccinated patients was found, suggesting that assessment of these markers can be clinically informative in predicting the immune benefit and the clinical outcome of melanoma patients receiving autologous tumor cell vaccine (Fig. 1).5 No correlation was found with OS and immune suppressive cells (Tregs, mast cells, MDSCs), T-cell inhibitory factors or loss of HLA class-I/melanocyte differentiation antigens in the patients in our study. Thus, advanced melanoma patients with sufficient numbers of (activated) T cells in the tumor at baseline may benefit from immunotherapy resulting in favorable clinical outcome.
Figure 1.
Immune markers correlate with clinical outcome in advanced melanoma patients following tumor cell vaccination. Analysis of immune-(escape) mechanisms in melanoma biopsies of patients showed that tumors at baseline with high numbers of activated CD4+ and CD8+ T cells manifest in prolonged progression-free survival (PFS) and/or overall survival (OS) in patients receiving autologous tumor cell vaccination. This indicates that a more prominent role for T-cell infiltration and activation in the tumor tissue for clinical outcome than immune-escape mechanisms. Therefore, analysis of tumor tissue characteristics before immunotherapy can be useful to optimally select patients, who will have increased chances of a favorable clinical outcome from the immunotherapy or to offer patients with low T cell presence in the tumor tissue additional inventions to increase T-cell tumor infiltration (e.g., by combining immune checkpoint blockade with vaccination or adoptive T-cell transfer).
Our results are in line with previous studies showing that the presence of tumor-infiltrating T cells is associated with a favorable outcome in melanoma patients, who underwent surgery, standard therapy or investigational immunotherapy, as measured in (primary) cutaneous tumor or sentinel lymph nodes.6,7 Most immunotherapeutic strategies aim to activate T cells or to interfere with immune checkpoints, such as cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) and the PD-1/PD-L1 axis, thereby regulating T-cell immune responses. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes (TILs) has already shown great potential to treat metastatic melanoma, which support the idea that increased levels of intratumoral T cells are beneficial for the outcome of advanced stage cancer patients.8 Current immunotherapies with anti-CTLA-4 and anti-PD-1 blocking antibodies are quite promising because they block inhibitory signals thereby reactivating T cells, and subsequently, antitumor T-cell immune responses.9,10 The success of immune checkpoint blockade may, however, depend on the presence of some level of pre-existent antitumor T-cell responses that become more efficient in attacking the tumor cells by releasing them from inhibitory signals. Since not all patients respond to immune checkpoint blockade, combination therapy with tumor cell or antigen vaccination may be required to induce antitumor T-cell responses that are potentiated by immune checkpoint blockade.
How can we translate our results to the field? Our study showed that tumor tissue with high numbers of activated CD4+ and CD8+ T cells manifest a prolonged PFS and/or OS in patients receiving autologous tumor cell vaccination. This indicates that analysis of tumor tissue characteristics before immunotherapy can be useful to optimally select patients, who will have increased chances of a favorable clinical outcome from the immunotherapy. Patients with low T cell presence in the tumor tissue may thus require additional inventions to increase T-cell infiltration in the tumor, e.g., by combining immune checkpoint blockade with vaccination or adoptive T-cell transfer. Combined with increasing evidence that (re)activation and attraction of T cells is an effective therapeutic strategy, further studies are required to better elucidate whether T-cell infiltration is of prognostic value and, if so, which patients’ organ(s) (e.g., tumor tissue or lymph nodes) can be used to predict clinical outcome and select the optimal immunotherapeutic strategy for individualized medicine.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
EPMT is supported by ZonMw-NGI-PreSeed Grant 93613005.
References
- 1. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537-44; PMID:; http://dx.doi.org/10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T cells mediated by galectin-1. Nature 1995; 378:736-9; PMID:; http://dx.doi.org/10.1038/378736a0 [DOI] [PubMed] [Google Scholar]
- 3. Zubieta M R, Furman D, Barrio M, Bravo AI, Domenichini E, Mordoh J. Galectin-3 expression correlates with apoptosis of tumor-associated lymphocytes in human melanoma biopsies. Am J Pathol 2006; 168:1666-75; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gabrilovich D I, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tjin EP, Krebbers G, Meijlink KJ, van de Kasteele W, Rosenberg EH, Sanders J, Nederlof PM, van de Wiel BA, Haanen JB, Melief CJ, et al. Immune-escape markers in relation to clinical outcome of advanced melanoma patients following immunotherapy. Cancer Immunol Res 2014; 2:538-46; PMID:; http://dx.doi.org/10.1158/2326-6066.CIR-13-0097 [DOI] [PubMed] [Google Scholar]
- 6. Ladanyi A, Somlai B, Gilde K, Fejos Z, Gaudi I, Timar J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res 2004; 10:521-30; PMID: [DOI] [PubMed] [Google Scholar]
- 7. Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol 2007; 25:869-75; http://dx.doi.org/10.1200/JCO.2006.08.9755 [DOI] [PubMed] [Google Scholar]
- 8. Besser MJ, Shapira-Frommer R, Itzhaki O, Treves AJ, Zippel DB, Levy D, Kubi A, Shoshani N, Zikich D, Ohayon Y, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013; 19:4792-800; PMID:; http://dx.doi.org/10.1158/1078-0432.CCR-13-0380 [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443-54. [DOI] [PMC free article] [PubMed] [Google Scholar]