Abstract
An increasing number of studies is focusing on the role of myeloid-derived suppressor cells (MDSCs) in the suppression of antitumor immune responses. Although the main site of action for MDSCs is most likely the tumor microenvironment, the study of these cells has been largely restricted to MDSCs derived from peripheral lymphoid organs. Only in a minority of studies MDSCs isolated from the tumor microenvironment have been characterized. This review will give an overview of the data available on the phenotypical and functional differences between tumor-derived MDSCs and MDSCs isolated from the spleen of tumor-bearing mice or from the peripheral blood of cancer patients.
Keywords: immunosuppression, myeloid-derived suppressor cells, tumor immunology, tumor microenvironment, tumor models
Abbreviations: ATRA, all-trans retinoic acid; Bv8, Bombina variagata peptide 8; CTLA-4, cytotoxic T-lymphocyte antigen-4; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; IL, interleukin; IL-4Rα, interleukin-4 receptor alpha; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinases; M-CSF, macrophage-colony stimulating factor; MDSCs, myeloid-derived suppressor cells; NS cells, natural suppressor cells; PD-L1, programmed death-ligand 1; PHA, phytohemagglutinin; ROS, reactive oxygen species; siRNA, small interfering ribonucleic acid; TAMs, tumor-associated macrophages; Treg, regulatory T cells; VEGF, vascular endothelial growth factor.
Introduction
The link between inflammation and tumor progression is longstanding. Already in 1863 Virchow observed that neoplastic tissue is infiltrated by leukocytes,1 giving rise to the hypothesis that cancer arises at sites of chronic inflammation. Under normal physiological conditions, inflammation is a self-limiting process, but dysfunctions in one of the inflammatory pathways can lead to pathogenesis and eventually to tumorigenesis.2 Despite the fact that our immune system is able to recognize and eliminate tumor cells, many tumors can escape immune control by various mechanisms. The presence of several subsets of suppressive immune cells, including regulatory T cells (Treg), tumor-associated macrophages (TAMs), and MDSCs, contribute to the immunosuppressive microenvironment3 and help tumors escape immune control. Already in the late 1970s different research groups described cells of myeloid origin that had the capacity to inhibit T-cell responses. These cells were termed natural suppressor (NS) cells,4,5 but because of technical and experimental limitations it was very difficult to fully characterize the phenotype and exact function of these cells. It was only in the late 1990s that two groups independently rediscovered these cells6,7 and since then the interest in immunosuppressive cells of myeloid origin has steadily increased. From then on, these cells were called “immature myeloid cells.” Since this term reflects only the origin of the cells and does not emphasize the most important characteristic of these cells, namely their ability to suppress immune responses, a consensus was reached to call these cells “myeloid-derived suppressor cells.”8,9 The increased interest in these cells is reflected by the fact that in 2013 over 300 research articles were published on this topic. Moreover, many research groups are developing strategies to specifically target these MDSCs in order to improve antitumor immune responses, further emphasizing the importance of these cells in the field of tumor immunology. These targeted strategies include blocking the differentiation and accumulation of MDSCs at the tumor site, blocking their expansion and interfering with their function. These strategies have been extensively reviewed in ref.10, and will, therefore, not be further addressed in this review. More and more evidence shows that MDSCs display a high phenotypic plasticity and can, under the influence of cytokines such as interleukin 12 (IL-12) and interferon-gamma (IFN-γ), even acquire characteristics of antigen-presenting cells.6,11 In contrast, all-trans retinoic acid (ATRA) was shown to trigger the differentiation of Gr-1+ cells into mature F4/80+ macrophages which were more potent immune suppressors on a per-cell basis. These data highlight the potent immunosuppressive functions of macrophages and support the development of therapeutic strategies to enhance antitumor immunity by targeting inhibitory myeloid cells as a collective group.12 Moreover, in analogy to the M1/M2 polarization in macrophages, MDSCs also show an M1/M2 classification in the tumor microenvironment. Different studies have shown that MDSCs present within the tumor microenvironment exhibit M2 characteristics, which accelerates tumor growth which is mediated by enhanced arginase activity, an increased secretion of immunosuppressive cytokines and the induction of angiogenesis.13,14 However, Umemura et al. showed that tumor-infiltrating MDSCs are pleiotropic-inflamed macrophages that can simultaneously display both M1- and M2-characteristics.15 In contrast, it has been shown that M2-type MDSCs can be skewed toward M1-type cells by lipopolysaccharide (LPS) through the p38 mitogen-activated protein kinases (MAPK) pathway,16 further underlining the plasticity of these cells. Thus, it is clear that MDSCs display a high degree of plasticity and that their exact fate will be determined by various factors inherent to the tumor type.
Murine MDSCs
Phenotype
MDSCs represent a heterogeneous population of myeloid cells that share some phenotypic characteristics with monocytes, macrophages, dendritic cells, and granulocytes,17 but can be distinguished from these cells by their potent immunosuppressive activities. The differentiation and accumulation of these MDSCs in tumor-bearing hosts is driven by different factors, such as IL-6,18 vascular endothelial growth factor (VEGF),19,20 granulocyte-macrophage colony-stimulating factor (GM-CSF),21 IL-1β,22 prostaglandin E2 (PGE2),23 S100A8/A9 proteins24 and hypoxia.25 These cells can express different surface markers, depending on the tumor type, disease stage, factors released by the tumors and their anatomical location. In mice, the markers CD11b and Gr-1 define this immunosuppressive cell population. Antibodies that specifically recognize Gr-1 bind to two separate antigens, Ly6G and Ly6C.26 The use of epitope specific antibodies together with morphological analysis has led to the identification of two functionally distinct subtypes of MDSCs: CD11b+Ly6G+Ly6Clow MDSCs, which are morphologically similar to polymorphonuclear granulocytes, whereas CD11b+Ly6G−Ly6Chigh MDSCs have a monocytic morphology.27 Both subsets can suppress T-cell proliferation, although they use different mechanisms to exert their function and various reports indicate that these two populations might have distinct functions in infectious diseases, autoimmune diseases, graft-versus-host disease, and cancer.28-31
Youn et al. were the first to perform a broad phenotypical and functional analysis of the two subtypes of MDSCs in 10 different models of lung cancer, breast cancer, colon cancer, melanoma, and sarcoma developed in three different strains of mice. Unfortunately, they only focused on spleen-derived MDSCs and they did not extend their findings to MDSCs present within the tumor microenvironment. In all tumor models studied, the number of MDSCs was significantly increased compared to that in naïve mice. However, the extent of MDSC expansion varied between different tumor models. Moreover, in most of the tumor models studied, it were predominantly the CD11b+Ly6G+Ly6Clow granulocytic MDSCs that expanded, except for the EL4 tumor model where both subsets were expanded equally.27,31 In a recent study, the same group showed that in tumor-bearing mice, monocytic MDSCs differentiate toward granulocytic MDSCs through the epigenetic silencing of the gene encoding retinoblastoma, a transcriptional regulator that controls cellular proliferation and differentiation. Thus, although it was previously assumed that monocytic and granulocytic MDSCs develop along a different pathway, this study suggests that, at least in cancer, MDSC development is altered, resulting in the conversion of monocytic MDSCs toward granulocytic MDSCs.32 However, more research will be required to fully elucidate the mechanisms and the tumor-derived factors that determine the fate of MDSCs and dictate the relationship between the different subsets of MDSCs. A number of different surface molecules implicated in the suppressive function of MDSCs have been described. The Interleukin-4 receptor α (IL-4Rα) has been reported to be essential for the development and function of MDSCs.33,34 However, these studies used in vitro generated MDSCs, so the relevance for primary MDSCs, either derived from the spleen or from the tumor, remains unclear. In contrast, other studies, using MDSCs isolated from the spleen of tumor-bearing animals and from IL-4Rα knock-out mice, demonstrated that the IL-4Rα is not essential for the accumulation and function of MDSCs.23,27,35 Unfortunately, none of these studies looked at the expression and function of the IL-4Rα on MDSCs derived from within the tumor microenvironment. Members of the B7-family were shown to be directly involved in the suppression of immune responses.36,37 CD80 (B7-1), expressed on antigen-presenting cells, has the ability to bind both to a stimulatory receptor, CD28, and to an inhibitory receptor CD152 (also known as CTLA-4, cytotoxic T-lymphocyte antigen-4) on T cells. Moreover, CD80 can also bind to programmed death-ligand 1 (PD-L1), which results in the delivery of inhibitory signals to T cells.38-40 Depending on these interactions an immune response will be evoked or dampened. The complexity of these ligand-receptor interactions hampers the study of the role of these molecules in the suppressive function of MDSCs and many conflicting results have been published. In their search for additional markers for MDSCs, Youn et al. did not observe higher levels of CD80, PD-L1, and PD-L2 on MDSCs isolated from the spleen of tumor-bearing animals compared to the expression levels found on immature myeloid cells isolated from the spleen of naïve animals. However, they did not look at the expression of these markers on MDSCs isolated from the tumor microenvironment.27 In a recent study Noman et al. showed that MDSCs at the tumor site had a higher expression of PD-L1 compared to MDSCs isolated from the spleen. PD-L1 is upregulated on these MDSCs under the influence of hypoxia and blockade of PD-L1 under hypoxic conditions abrogated the MDSC-mediated T-cell suppression by modulating MDSC cytokine production.41 In different tumor models it has been shown that CD80 expression is upregulated on tumor-derived MDSCs.42-45 Moreover in some tumor models CD80 expression was also elevated on spleen-derived MDSCs.24,42 These conflicting observations could be explained by the use of different tumor models (subcutaneously implanted tumors vs. tumors grown as ascites), the disease stage at which CD80 expression was evaluated and the subset of MDSCs under investigation. Conflicting results have been published about the role of this upregulated CD80 expression in the suppressive function of the MDSCs, which will be discussed further. Because of the myeloid origin of MDSCs, CD115 (macrophage-colony stimulating factor (M-CSF) receptor) and F4/80 are two additional markers used to further characterize MDSCs. It has been claimed that Gr-1 and CD115 may be better markers to define MDSCs compared to Gr-1 and CD11b.46 However, Youn et al. only found higher levels of CD115 on MDSCs in 2 out of 10 tested tumor models.27 Moreover, it has been shown in three different tumor models, including the EL4 thymoma, the B16.F10 melanoma, and the CC10 spontaneous lung tumor model, that only a minority of MDSCs express CD115 or F4/80. However, the expression of both molecules is slightly higher on tumor-associated MDSCs compared to spleen-derived MDSCs, although these differences were not statistically significant.25 One should be cautious when interpreting these data. Since protocols for isolating MDSCs vary extensively, it cannot be ruled out that these F4/80+ cells are actually contaminating TAMs rather than MDSCs. TAMs are a cellular population that can be histologically confused with MDSCs, but that are defined as mature and fully differentiated macrophages.47 However, distinguishing TAMs from MDSCs can be technically challenging. Gene expression profiling of CD11b+Gr-1+ cells from the spleens and tumors of 4T1 tumor-bearing mice revealed that genes involved in extracellular matrix remodeling, immunomodulation and hypoxia regulation were markedly upregulated in tumor-derived cells compared to their non-infiltrating counterparts.48 However, the functional importance of these upregulated genes in the function of tumor-derived MDSCs has so far not been determined.
Function
Only in a minority of studies MDSCs isolated from the tumor microenvironment have been fully characterized. In most of these studies, tumors are grown as ascites in order to facilitate the isolation of tumor-derived MDSCs. In order to directly compare the suppressive function of MDSCs from the tumor site to MDSCs from the spleen Corzo et al. developed a model where EL4 tumors were grown as ascites.25 They evaluated the effect of spleen-derived and tumor-derived MDSCs on the IFN-γ production and T-cell proliferation in both an antigen-specific and non-specific system. Both spleen- and tumor-derived MDSCs were able to suppress antigen-specific T-cell responses, although the level of suppression was significantly higher in tumor-derived MDSCs. However, the major differences were observed in a non-antigen specific setting, where spleen-derived MDSCs did not suppress T-cell responses while MDSCs isolated from the tumor exerted a profound suppressive effect on these T cells.25 This is in contrast with the data obtained by Haverkamp et al. who showed, in a model of acute and chronic prostate inflammation, that MDSCs derived from the spleen were not functional whereas cells isolated from the inflammatory site were able to inhibit T-cell proliferation and expressed higher levels of arginase and inducible nitric oxide synthase (iNOS).43 The difference between these 2 studies is the duration of the proliferation assay: 72 h in the experiments performed by Corzo et al. vs. only 12 h in the experiments of Haverkamp et al. The latter wanted to minimize the exposure of the MDSCs to IFNγ because it has been shown that IFNγ, produced during a standard proliferation assay of 3 d, converts precursor splenic CD11b+Gr-1+ cells (without suppressive activity) into fully functional MDSCs.43,49 However, we have shown in a non-antigen specific system, that spleen-derived MDSCs have the ability to suppress T-cell proliferation and cytokine secretion, although to a lower extent than the tumor-derived MDSCs. Moreover, we have shown that there is no production of IFN-γ when spleen-derived CD11b+Ly6G+Ly6Cint MDSCs were cocultured with splenocytes, indicating that at least for the granulocytic subset of MDSCs other mechanisms are responsible for the suppression of T-cell proliferation by spleen-derived MDSCs.45 Conflicting results concerning the suppressive activity of spleen- and tumor-derived MDSCs could be explained by differences in the activation status of the responder cells, differences in the duration of the suppression assay, differences between antigen-specific and antigen non-specific T-cell responses, differences in the subsets of MDSCs under investigation and the use of different tumor models.24,25,27,43,49
Different factors and mechanisms are involved in the suppressive activity of MDSCs, including arginase 1 activity, iNOS, the induction of Treg, downregulation of the T-cell receptor, etc. These mechanisms have been reviewed in detail elsewhere and will not be the subject of this review.50-52 Here, we will focus on the differences in the mechanisms used by spleen- or tumor-derived MDSCs to exert their function (Fig. 1). Several groups have shown an increased activity for both arginase and iNOS and a higher production of nitrite (NO2−) in tumor-derived MDSCs compared to their non-infiltrating counterparts. In contrast, higher amounts of reactive oxygen species (ROS) were detected in spleen-derived MDSCs.25,45,53 These data indicate that MDSCs isolated from either the spleen or the tumor microenvironment use different mechanisms to exert their function, which can explain the differences in suppressive strength between spleen- and tumor-derived MDSCs. However, further research is needed to fully elucidate these differences and their importance for the suppressive function of MDSCs.
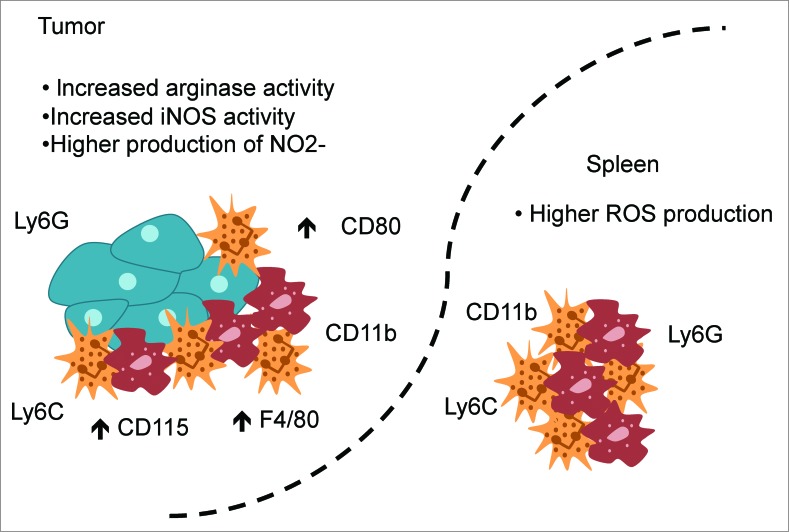
Figure 1.
MDSCs present in the tumor use different mechanisms to exert their suppressive function compared to their peripheral counterparts. Both spleen- and tumor-derived MDSCs express the markers CD11b, Ly6G, and Ly6C but MDSCs derived from the tumor express higher levels of CD115, F4/80 and CD80. An increased arginase activity, an increased inducible nitric oxide synthase (iNOS) activity, and a higher production of nitrite (NO2−) were observed in tumor-derived MDSCs, while spleen-derived MDSCs produced higher amounts of reactive oxygen species (ROS).
The importance of the CD80-CD28/CTLA-4 pathway in the suppressive function of MDSCs is not completely clear. As mentioned previously, CD80 expression is upregulated on tumor-derived MDSCs,42-45 as well as on spleen-derived MDSCs in some tumor models.24,42 The use of CD80-specific neutralizing antibodies or small interfering ribonucleic acid (siRNA) against CD80 was shown to partially inhibit the suppressive function of MDSCs, indicating that CD80 does play a role in the suppressive function of MDSCs but that other factors are also involved.42,45 Moreover, when mouse ovarian surface epithelial cell line (MOSEC) ID8 cells were injected subcutaneously into CD80−/− mice a slower tumor growth associated with a decreased suppressive potential of ovarian carcinoma-associated MDSCs was observed, compared to CD80+/+ mice.42 In contrast, Tomihara et al. showed that MDSCs derived from ascites of ovarian tumor-bearing mice are rather immunostimulatory and augment the proliferation of cytotoxic T cells via signaling through CD80.54 In order to fully understand these conflicting results, a consensus about the phenotype of MDSCs and the type of assays that have to be performed to evaluate their suppressive function has to be reached.
Human MDSCs
Phenotype
In humans, CD34+ cells that accumulate under the influence of GM-CSF and have the capacity to inhibit the secretion of IL-2 by activated lymphocytes, and, thus, possess MDSC-like properties, were described for the first time in patients with head and neck cancer.55 Human MDSCs have so far been inadequately characterized because of the lack of uniform markers (such as Gr-1 in mice), and a unified phenotype. The lack of defined and homogeneous markers for MDSCs in humans hampers the study of the biological function and clinical impact of these MDSCs in cancer patients. In accordance with the data obtained in murine models, attempts have been made to divide human MDSCs in two subtypes: a more granulocytic and a more monocytic type of cell. It has been shown that both subsets express the common myeloid markers CD11b and CD33 but lack the expression of mature myeloid markers. Moreover, the monocytic cells express CD14 while the granulocytic cells express CD15. Many laboratories are trying to further characterize different subsets of MDSCs in different types of cancer using different sets and combinations of markers. A comprehensive overview of the different phenotypes of MDSCs in different types of cancer can be found in ref56. These findings are summarized in Table 1. Whether this broad variety in described phenotypes can be attributed to different mechanisms of induction/expansion of MDSCs in different types of cancer or whether this is simply due to the variation in markers used by different research groups, is not clear yet. The variation in expression of different cell surface markers on MDSCs derived from patients with different types of cancer indicates the existence of subpopulations of MDSCs, as this is seen in different mouse models as well. This is not surprising since MDSCs accumulate under the influence of tumor-derived factors. Different types of tumors can secrete distinct sets of inflammatory molecules, which will lead to the accumulation of MDSCs with a particular and perhaps even unique phenotype. However, given the difficulties to reach the tumor and to obtain enough tumor material for research, almost all of the information on MDSCs in cancer patients has been obtained after research on peripheral blood samples. Whether this information truly correlates with the phenotype and function of MDSCs present within the tumor microenvironment, and, thus, under direct influence of factors secreted by these tumors, remains unclear. Significant controversy exists about the correlation between MDSCs and tumor stage and progression. A study conducted on peripheral blood samples from 106 cancer patients with newly diagnosed and histologically confirmed solid malignancies, revealed that there is a significantly higher percentage of circulating MDSCs in cancer patients relative to healthy volunteers.7 In several other studies, including breast cancer, gastric cancer, melanoma, pancreatic and esophageal cancer, it has been shown that the frequencies of circulating MDSCs correlate with disease stage and metastasis and that the increase in the number of these MDSCs is an independent prognostic factor and could predict response to therapy.57-59 Moreover, patients with stage IV solid tumors had the highest percentage of MDSCs. When they divided these stage IV patients into those with limited and those with extensive (defined as a diffuse involvement of one organ system or three or more distinct organ sites involved) metastatic tumor burden, the latter had both higher mean percentages and higher absolute numbers of circulating MDSCs. These data indicate that in different types of solid tumors, there is a correlation between MDSCs and both clinical cancer stage and tumor burden.60 A study conducted in patients with stage IV melanoma performed at the University of Colorado Cancer Center showed that immunosuppressive cells, including Treg, CD14+ MDSCs and CD14− MDSCs, are specifically increased in metastatic melanoma patients and are found in association with each other. Moreover, a high frequency of CD14− MDSCs was shown to predict poorer survival of the patients and faster disease progression.61 However, another study in patients with stage IV metastatic melanoma showed that the frequency of MDSCs was found to be rather low in melanoma patients and overlapped with the frequency detected in healthy donors.62 In contrast, a study performed in Germany at the University Medical Center in Mainz showed that the accumulation of CD11b+CD33+CD14+HLA-DRlow MDSCs was an early event already detectable in stage I/II melanoma. Progression of disease (stage III) and high tumor burden in metastatic disease (stage IV) did not result in a further increase of MDSC frequencies nor in changes of phenotypic markers.63 These contradictory results can be explained by different factors: differences in isolation methods used, different purification steps performed on the peripheral blood samples to isolate the MDSCs which may alter the natural frequencies of MDSCs, differences in cancer type, stage of disease and previous therapy of the patients. In order to compare data obtained in different laboratories it will be important to find a consensus about the markers used to define the phenotype of MDSCs and to perform studies on large groups of cancer patients.
Table 1.
Phenotype of MDSCs described in different cancer types
| Cancer type | SSC | CD3 | CD1a | CD11c | CD11b | CD13 | CD14 | CD15 | CD16 | CD19 | CD20 | CD24 | CD33 | CD34 | CD40 | CD56 | CD57 | CD62L | CD66b | CD68 | CD80 | CD83 | CD86 | CD205 | DC-Sign | HLA-DR | VEGR1 | IL4-Rα | CD115 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mRCC | ND | − | Int | ND | Int | Int | − | ND | − | − | − | ND | + | ND | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 83 |
| ND | ND | ND | + | + | + | − | + | − | ND | ND | + | + | ND | ND | ND | ND | − | + | ND | ND | ND | ND | ND | ND | ND | + | ND | ND | 84 | |
| ND | ND | ND | ND | + | ND | − | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | − | − | ND | ND | ND | ND | ND | ND | 64 | |
| ND | ND | ND | ND | ND | ND | − | + | ND | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 85 | |
| ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 85 | |
| ND | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | ND | 86 | |
| ND | − | ND | ND | ND | ND | − | ND | ND | − | ND | ND | + | ND | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 87 | |
| Melanoma | ND | ND | + | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | + | + | ND | + | low | ND | + | ND | 66 |
| ND | − | ND | ND | + | ND | + | ND | ND | − | − | ND | ND | ND | ND | − | − | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 69 | |
| + | − | ND | ND | + | ND | − | int | ND | − | − | ND | ND | ND | ND | − | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 69 | |
| ND | − | ND | ND | + | ND | − | − | ND | − | − | ND | ND | ND | ND | − | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 69 | |
| ND | ND | ND | ND | ND | ND | − | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | 88 | |
| Solid malignancies | ND | − | ND | ND | + | ND | − | ND | ND | − | ND | ND | + | ND | ND | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 60 |
| high | ND | ND | ND | ND | ND | ND | ND | ND | − or + | ND | ND | + | ND | ND | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 89 | |
| HNSCC | ND | − | ND | − | ND | ND | − | − | ND | − | ND | ND | + | − | − | ND | − | ND | ND | ND | ND | ND | − | ND | ND | − | ND | ND | + | 7 |
| ND | ND | ND | ND | + | ND | + | ND | ND | ND | ND | ND | + | + | ND | ND | ND | ND | ND | − | ND | ND | ND | ND | ND | low | ND | ND | ND | 67 | |
| Breast cancer | ND | − | ND | − | ND | ND | − | − | ND | − | ND | ND | + | − | − | ND | − | ND | ND | ND | ND | ND | − | ND | ND | − | ND | ND | + | 7 |
| HCC | ND | ND | − | + | + | ND | + | − | − | − | ND | ND | + | − | ND | ND | ND | ND | ND | ND | − | − | + | ND | ND | low | ND | ND | ND | 90 |
| Pancreatic and esophago-gastric cancer | ND | − | ND | ND | + | ND | − | ND | − | − | − | ND | + | ND | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | − | ND | ND | ND | 57 |
| Lung cancer | ND | − | ND | − | + | ND | ND | + | ND | ND | ND | ND | + | ND | ND | − | ND | ND | ND | − | − | ND | ND | − | ND | ND | ND | ND | ND | 91 |
| NSCLC | ND | ND | ND | ND | + | ND | − | + | ND | ND | ND | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | 92 |
| ND | − | ND | − | ND | ND | − | − | ND | − | ND | ND | + | − | − | ND | − | ND | ND | ND | ND | ND | − | ND | ND | − | ND | ND | + | 7 | |
| Colon cancer | ND | ND | ND | ND | ND | ND | − | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | 88 |
| Prostate cancer | ND | ND | ND | ND | + | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | low | ND | ND | ND | 68 |
Abbreviations: HCC, hepatocellular carcinoma; HNSCC, head and neck squamous cell carcinoma ; int, intermediate; mRCC, metastatic renal cell carcinoma; ND, not determined; NSCLC, non-small cell lung cancer.
Function
Although it has become clear in different murine tumor models that MDSCs derived from within the tumor microenvironment and those isolated from peripheral lymphoid organs have a distinct suppressive potential (see above), there are, to our knowledge, almost no studies comparing MDSCs from both sources head-to-head in cancer patients. The current understanding on the phenotype and especially the function of human MDSCs is almost completely derived from cells isolated from the peripheral blood of the patients and it is at this moment not clear yet whether this information is relevant for tumor-derived MDSCs as well. This could be partially explained by the difficulty to obtain tumor material from the clinic for research purposes, since this material is generally needed for diagnostic purposes. However, a few studies have made an attempt to investigate and compare the suppressive function of tumor-derived MDSCs and MDSCs isolated from the peripheral blood of cancer patients. A population of CD14−CD11b+CD33+ cells, previously shown to bear characteristics of MDSCs,64,65 were isolated from both the peripheral blood and tumor tissue obtained from three patients with head and neck cancer. In accordance with data obtained in tumor-bearing mice, MDSCs from within the tumor microenvironment produced significantly lower levels of ROS compared to blood-derived MDSCs while the iNOS levels were higher in tumor MDSCs than in blood MDSCs. Moreover, MDSCs derived from peripheral blood did not affect phytohemagglutinin (PHA)-induced T-cell proliferation, while MDSCs from the tumor microenvironment significantly suppressed T-cell proliferation.25 This is in contrast with different studies showing suppressive activity of MDSCs derived from the peripheral blood of cancer patients.66-68 However, in these studies a population of CD14+HLA-DR−/low cells was investigated (in contrast to the CD14−CD11b+CD33+ cell population) complicating direct comparisons of the suppressive function of these cells and again underlining the importance of consensus phenotypes to define MDSCs in cancer patients. In another study performed in patients with head and neck cancer, CD14+HLA-DRlow cells isolated from the tumor, the draining lymph node as well as the peripheral blood had the capacity to suppress antigen non-specific T-cell responses. However, also for this cell population it was clear that CD14+HLA-DRlow MDSCs from the tumor had a greater capacity to suppress autologous T cells compared to MDSCs derived from the peripheral blood.67 Possible explanations for the discrepancy observed in the suppressive function of MDSCs obtained from the peripheral blood of patients with head and neck cancer include differences in phenotype of the cells under investigation, in stimuli used to induce T-cell proliferation and in handling of the peripheral blood samples. In the study performed by Corzo et al. the peripheral blood samples were treated in the same way as the tumor tissue (i.e. by enzymatic digestion), which could account for the loss of the suppressive function of MDSCs isolated from the peripheral blood.25 In contrast to the two studies performed in patients with head and neck cancer, a study performed in metastatic melanoma patients showed that melanoma-infiltrating myeloid cells displayed an impaired suppressive capacity on antigen non-specific T-cell proliferation compared to cells with the same phenotype isolated from the peripheral blood of the same patient.69 However, these melanoma-infiltrating CD14+ cells expressed higher levels of HLA-DR compared to the cells isolated from the peripheral blood, and perhaps this more differentiated phenotype can explain the lack of suppressive function of these cells in melanoma tumors compared to peripheral blood or other tumor types. Moreover, it remains unclear whether the inability of these cells to suppress non-antigen specific T-cell proliferation, as opposed to antigen-specific T-cell responses, reflects the real functional status of these cells in the tumor microenvironment. Another possible explanation is that the accumulation of MDSCs is less evident in an immunogenic tumor, such as melanoma, capable of responding to immunotherapy.70-72 These data suggest that the contribution of MDSCs to the inhibition of T-cell proliferation in patients with metastatic melanoma may be less important than what was suggested by data obtained in murine tumor models. However, these are results from only a single study and more research will be needed in order to unravel the immunological relevance and the importance of MDSCs during tumor progression in cancer patients.
Trafficking of MDSCs
Infiltration of the tumor by inflammatory cells, including MDSCs, is an important factor in cancer progression. However, little is known about the mechanisms responsible for the mobilization and subsequent trafficking of MDSCs to the tumor site. Under normal conditions hematopoietic progenitors are predominantly found in the bone marrow, although low numbers can circulate in the blood. Mobilization is the first step in myeloid progenitor trafficking to inflammatory sites. The recruitment of MDSCs from the bone marrow to the peripheral blood can be mediated by Bv8 (Bombina variagata peptide 8, also known as prokineticin 1 and 2, a mitogen selective for endothelial cells) and endocrine-gland derived VEGF.73,74 Also GM-CSF, secreted by the tumor cells, can mobilize MDSCs from the bone marrow. From the peripheral blood, MDSCs can be recruited to the tumor site by a number of chemokines, and the migration of particular MDSC subsets is strongly determined by the tumor histology and the spectrum of chemokines produced by these tumors.75,76 The expression of several of these factors is increased by hypoxia, indicating that MDSCs might be preferentially recruited to sites of tumor hypoxia.77 Myeloid cells also express integrins, such as α4β1, the receptor for vascular cell adhesion molecule-1, which plays a role in the cellular trafficking to vascularized microenvironments.78 MDSC accumulation also results from a prolonged survival and decreased apoptosis. It has been shown in a 4T1 model that the spleen, but not the bone marrow is the primary site for MDSC proliferation and serves as a reservoir from which MDSCs rapidly enter the bloodstream.79
Concluding remarks
Data obtained in different murine tumor models showed the importance of MDSCs in the suppression of T-cell responses and the promotion of tumor growth. Different studies showed that MDSCs present within the tumor microenvironment possess a stronger suppressive capacity compared to their non-infiltrating counterparts. Given the important role for MDSCs in the suppression of T-cell responses different studies focus on the specific targeting of MDSCs in order to alleviate the suppressive tumor microenvironment (reviewed in refs.80,81) and to achieve complete tumor regression. However, in cancer patients the role of MDSCs and the relative contribution of these cells to tumor progression is less clear. The major hurdle here remains the lack of a unified phenotype and standardized assays to determine the suppressive function of these cells. Without this, a comprehensive analysis and comparison of data obtained in different laboratories and clinical centers is very difficult and can possible lead to an under- or overestimation of the importance of MDSCs in the suppression of immune responses in cancer patients. One way to overcome this problem could be the development of a system that allows for the large-scale production of MDSCs ex vivo. Although different research groups are working on this topic, the methods published so far are still heterogeneous, complicated and characterized by a low yield. Moreover, the cells obtained by these methods may not resemble closely enough the cells that accumulate in the tumor microenvironment.82 When the role of MDSCs in tumor progression and escape of immune control has become clear, strategies can be tested in order to reduce the number or inhibit the function of MDSCs, thereby creating a favorable tumor microenvironment for immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
SKM is funded by a PhD grant from the Agency for Innovation by Science and Technology in Flanders (IWT).
References
- 1.Balkwill F, Mantovani A. inflammation and cancer: back to Virchow? Lancet 2001; 357:539-45; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860-7; PMID:; http://dx.doi.org/ 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013; 2:e25961; PMID:; http://dx.doi.org/ 10.4161/onci.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roder JC, Duwe AK, Bell DA, Sinhal SK. Immunological senescence: the role of suppressor cells. Immunology 1978; 837-47; PMID: [PMC free article] [PubMed] [Google Scholar]
- 5.Maier T, Holda JH. Natural suppressor (NS) activity from murine neonatal spleen is responsive to IFN-gamma. J Immunol 1987; 138:4075-84; PMID: [PubMed] [Google Scholar]
- 6.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P, Dc W. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood 2000; 96:3838-46; PMID: [PMC free article] [PubMed] [Google Scholar]
- 7.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol 2001; 166:678-89; PMID:; http://dx.doi.org/ 10.4049/jimm-unol.166.1.678 [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Bronte V, Chen S-H, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res 2007; 67:425; author reply 426; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13:739-52; PMID:; http://dx.doi.org/ 10.1038/nrc3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells targets for therapy. OncoImmunology 2013; 2: 1-7; PMID:; http://dx.doi.org/ 10.4161/onci.24117; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011; 121:4746-57; PMID:; http://dx.doi.org/ 10.1172/JCI58814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton MJ, Bosiljcic M, Lepard NE, Halvorsen EC, Ho VW, Banáth JP, Krystal G, Bennewith KL. Macrophages are more potent immune suppressors ex vivo than immature myeloid-derived suppressor cells induced by metastatic murine mammary carcinomas. J Immunol 2014; 192:512-22; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1300096 [DOI] [PubMed] [Google Scholar]
- 13.Ma G, Pan P-Y, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen S-H. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity 2011; 34:385-95; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis 2012; 33:949-55; PMID:; http://dx.doi.org/ 10.1093/carcin/bgs123 [DOI] [PubMed] [Google Scholar]
- 15.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang G-F, Okada M, Balazs M, Adany R, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol 2008; 83:1136-44; PMID:; http://dx.doi.org/ 10.1189/jlb.0907611 [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Zhang R, Xia F, Zou T, Huang A, Xiong S, Zhang J. LPS converts Gr-1+CD115+ myeloid-derived suppressor cells from M2 to M1 via P38 MAPK. Exp Cell Res 2013; 319:1774-83; PMID:; http://dx.doi.org/ 10.1016/j.yexcr.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Youn J, Collazo M, Shalova IN, Biswas SK. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. 2012; 91:167-81; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S-J, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S-I, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 2004; 173:3844-54; PMID:; http://dx.doi.org/ 10.4049/jimmunol.173.6.3844 [DOI] [PubMed] [Google Scholar]
- 19.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998; 92:4150-66; PMID: [PubMed] [Google Scholar]
- 20.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood 2003; 102:2138-45; PMID:; http://dx.doi.org/ 10.1182/blood-2003-01-0190 [DOI] [PubMed] [Google Scholar]
- 21.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999; 162:5728-37; PMID: [PMC free article] [PubMed] [Google Scholar]
- 22.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1-secreting cells. J Immunol 2005; 175:8200-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.175.12.8200 [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res 2007; 67:4507-13; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-4174 [DOI] [PubMed] [Google Scholar]
- 24.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G, Hudson HF. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol 2008; 181:4666-75; PMID:; http://dx.doi.org/ 10.4049/jimmunol.181.7.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corzo C A, Condamine T, Lu L, Cotter MJ, Youn J-I, Cheng P, Cho H-I, Celis E, Quiceno DG, Padhya T, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207:2439-53; PMID:; http://dx.doi.org/ 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming TJ, Fleming M, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6–8C5 mAb to granulocyte-differentiation Antigen (Gr-1) Detects Members of the Ly-6 Family. J Immunol 1993; 151:2399-408; PMID: [PubMed] [Google Scholar]
- 27.Youn J-I, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:; http://dx.doi.org/ 10.4049/jimmu-nol.181.8.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dietlin TA, Hofman FM, Lund BT, Gilmore W, Stohlman SA, van der Veen RC. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol 2007; 81:1205-12; PMID:; http://dx.doi.org/ 10.1189/jlb.1006640 [DOI] [PubMed] [Google Scholar]
- 29.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK, Khoury SJ. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol 2007; 179:5228-37; PMID:; http://dx.doi.org/ 10.4049/jimmunol.179.8.5228 [DOI] [PubMed] [Google Scholar]
- 30.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 2010; 116:5738-47; PMID:; http://dx.doi.org/ 10.1182/blood-2010-06-287839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter J A. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008; 111:4233-44; PMID:; http://dx.doi.org/ 10.1182/blood-2007-07-099226 [DOI] [PubMed] [Google Scholar]
- 32.Youn J, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, Mccaffrey JC, Sarnaik A, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 2013; 14:211-20; PMID:; http://dx.doi.org/ 10.1038/ni.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apolloni E, Bronte V, Mazzoni A, Serafini P, Cabrelle A, Segal DM, Young HA, Zanovello P. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated T lymphocytes. J Immunol 2000; 165:6723-30; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.12.6723 [DOI] [PubMed] [Google Scholar]
- 34.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 2010; 32:790-802; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 35.Sinha P, Parker KH, Horn L, Ostrand-Rosenberg S. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-γ and IL-4Rα. Eur J Immunol 2012; 42:2052-9; PMID:; http://dx.doi.org/ 10.1002/eji.201142230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother 2007; 56:739-45; PMID:; http://dx.doi.org/ 10.1007/s00262-006-0272-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins M, Ling V, Carreno BM. The B7 family of immune-regulatory ligands. Genome Biol 2005; 6:223; PMID:; http://dx.doi.org/ 10.1186/gb-2005-6-6-223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seliger B, Marincola FM, Ferrone S, Abken H. The complex role of B7 molecules in tumor immunology. Trends Mol Med 2008; 14:550-9; PMID:; http://dx.doi.org/ 10.1016/j.molmed.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev 2011; 241:180-205; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2011.01011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res 2007; 13:5271-9; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1030 [DOI] [PubMed] [Google Scholar]
- 41.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:; http://dx.doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang R, Cai Z, Zhang Y, Yutzy WH, Roby KF, Roden RBS. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res 2006; 66:6807-15; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3755 [DOI] [PubMed] [Google Scholar]
- 43.Haverkamp JM, Crist SA, Elzey BD, Cimen C, Ratliff TL. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur J Immunol 2011; 41:749-59; PMID:; http://dx.doi.org/ 10.1002/eji.201041069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Yu Y, Yang S, Zeng B, Zhang Z, Jiao G, Zhang Y, Cai L, Yang R. Regulation of arginase I activity and expression by both PD-1 and CTLA-4 on the myeloid-derived suppressor cells. Cancer Immunol Immunother 2009; 58:687-97; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0591-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maenhout SK, Lint S Van, Emeagi PU, Thielemans K, Aerts JL. Enhanced suppressive capacity of tumor-infiltrating myeloid-derived suppressor cells compared with their peripheral counterparts. Int J cancer 2014; 1:1077-90; PMID: 23983191; http://dx.doi.org/ 10.1002/ijc.28449 [DOI] [PubMed] [Google Scholar]
- 46.Huang B, Pan P-Y, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen S-H. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66:1123-31; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1299 [DOI] [PubMed] [Google Scholar]
- 47.Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res 2010; 70:5728-39; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-4672 [DOI] [PubMed] [Google Scholar]
- 48.Elpek KG, Cremasco V, Shen H, Harvey CJ, Wucherpfennig KW, Goldstein DR, Monach PA, Turley SJ. The tumor microenvironment shapes lineage, transcriptional, and functional diversity of infiltrating myeloid cells. Cancer Immunol Res 2014; 2:655-67; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallina G, Dolcetti L, Serafini P, Santo C De, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest 2006; 116:2777-90; PMID:; http://dx.doi.org/ 10.1172/JCI28828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 2010; 59:1593-600; PMID:; http://dx.doi.org/ 10.1007/s00262-010-0855-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0802740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol 2012; 189:5602-11; PMID:; http://dx.doi.org/ 10.4049/jimmu-nol.1201018 [DOI] [PubMed] [Google Scholar]
- 54.Tomihara K, Guo M, Shin TT, Sun X, Ludwig SM, Brumlik MJ, Zhang B, Curiel TJ. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J Immunol 2010; 184:6151-60; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0903519 [DOI] [PubMed] [Google Scholar]
- 55.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res 1995; 1:95-103; PMID: [PubMed] [Google Scholar]
- 56.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319:47-65; PMID:; http://dx.doi.org/ 10.1111/nyas.12469; [DOI] [PubMed] [Google Scholar]
- 57.Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419-30; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014; 63:247-57; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding J, Zhu J, Wei H, Zhao K. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One 2013; 8:e57114; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0057114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58:49-59; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother 2013; 62:1711-22; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007; 25:2546-53; PMID:; http://dx.doi.org/ 10.1200/JCO.2006.08.5829 [DOI] [PubMed] [Google Scholar]
- 63.Rudolph BM, Loquai C, Gerwe A, Bacher N, Steinbrink K, Grabbe S, Tuettenberg A. Increased frequencies of CD11b(+) CD33(+) CD14(+) HLA-DR(low) myeloid-derived suppressor cells are an early event in melanoma patients. Exp Dermatol 2014; 23:202-4; PMID:; http://dx.doi.org/ 10.1111/exd.12336 [DOI] [PubMed] [Google Scholar]
- 64.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, Mcdermott D, Quiceno D, Youmans A, Neill AO, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients : a mechanism of tumor evasion arginase-producing myeloid suppressor cells in renal cell carcinoma patients : a mechanism of tumor evasion. Cancer Res 2005; 65:3044-8; PMID: [DOI] [PubMed] [Google Scholar]
- 65.Nagaraj S, Youn J-I, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res 2010; 16:1812-23; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 2010; 70:4335-45; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3767 [DOI] [PubMed] [Google Scholar]
- 67.Vasquez-dunddel D, Pan F, Zeng Q, Gorbounov M, Albesiano E, Fu J, Blosser RL, Tam AJ, Bruno T, Zhang H, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest 2013; 123:1580-9; PMID:; http://dx.doi.org/ 10.1172/JCI60083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vuk-pavlovi S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate 2010; 70:443-55; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res 2012; 18:5212-23; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 2012; 18:2039-47; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilgenhof S, Van Nuffel AMT, Benteyn D, Corthals J, Aerts C, Heirman C, Van Riet I, Bonehill A, Thielemans K, Neyns B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol 2013; 24:2686-93; PMID:; http://dx.doi.org/ 10.1093/annonc/mdt245 [DOI] [PubMed] [Google Scholar]
- 72.Wilgenhof S, Nuffel AMT Van, Corthals J, Heirman C, Tuyaerts S, Benteyn D, De Coninck A, Van Riet I, Verfaillie G, Vandeloo J, et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother 2011; 448-56; PMID:; http://dx.doi.org/ 10.1097/CJI.0b013e31821dcb31 [DOI] [PubMed] [Google Scholar]
- 73.LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA 2004; 101:16813-8; PMID:; http://dx.doi.org/ 10.1073/pnas.0407697101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shojaei F, Wu X, Zhong C, Yu L, Liang X-H, Yao J, Blanchard D, Bais C, Peale F V, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature 2007; 450:825-31; PMID:; http://dx.doi.org/ 10.1038/nature06348 [DOI] [PubMed] [Google Scholar]
- 75.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008; 8:618-31; PMID:; http://dx.doi.org/ 10.1038/nrc2444 [DOI] [PubMed] [Google Scholar]
- 76.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer Microenviron 2013; 6:169-77; PMID:; http://dx.doi.org/ 10.1007/s12307-012-0126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du R, Lu K V, Petritsch C, Liu P, Ganss R, Passegué E, Song H, Vandenberg S, Johnson RS, Werb Z, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008; 13:206-20; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2008.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res 2006; 66:2146-52; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-2704 [DOI] [PubMed] [Google Scholar]
- 79.Younos IH, Dafferner AJ, Gulen D, Britton HC, Talmadge JE. Tumor regulation of myeloid-derived suppressor cell proliferation and trafficking. Int Immunopharmacol 2012; 13:245-56; PMID:; http://dx.doi.org/ 10.1016/j.intimp.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 80.Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res 2014; 8-14; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Umansky V, Sevko A. Melanoma-induced immunosuppression and its neutralization. Semin Cancer Biol 2012; 22:319-26; PMID:; http://dx.doi.org/ 10.1016/j.semcancer.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 82.Escors D, Perez-janices N, Schwarze J, Dufait I, Goyvaerts C, Lanna A, Arce F, Blanco-luquin I, Kochan G, Guerrero-setas D, et al. Dendritic cells or myeloid-derived suppressor cells ? assessing T-cell responses in anticancer immunotherapy. OncoImmunology 2013:1-9; PMID:; http://dx.doi.org/ 10.4161/onci.26148; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, Yancey D, Dahm P, Vieweg J. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res 2008; 14:8270-8; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0165 [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009; 69:1553-60; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman P a, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15:2148-57; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1332 [DOI] [PubMed] [Google Scholar]
- 86.Kusmartsev S, Eruslanov E, Kübler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, et al. Oxidative stress regulates expression of VEGR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J Immunol 2008; 346-53; PMID:; http://dx.doi.org/ 10.4049/jimmunol.181.1.346 [DOI] [PubMed] [Google Scholar]
- 87.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Timothy J, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res 2007; 66:9299-307; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol 2009; 182:6562-8; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0803831 [DOI] [PubMed] [Google Scholar]
- 89.Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol 2011; 89:311-7; PMID:; http://dx.doi.org/ 10.1189/jlb.0310162 [DOI] [PubMed] [Google Scholar]
- 90.Hoechst B, Ormandy L a, Ballmaier M, Lehner F, Krüger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135:234-43; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 91.Srivastava MK, Bosch JJ, Thompson JA, Ksander B, Edelman M, Ostrand-Rosenberg S. Lung cancer patients’ CD4+ T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother 2008; 57:1493-504; PMID:; http://dx.doi.org/ 10.1007/s00262-008-0490-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C-Y, Wang Y-M, Wang C-L, Feng P-H, Ko H-W, Liu Y-H, Wu Y-C, Chu Y, Chung F-T, Kuo C-H, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 2010; 136:35-45; PMID:; http://dx.doi.org/ 10.1007/s00432-009-0634-0 [DOI] [PMC free article] [PubMed] [Google Scholar]