Abstract
Chimeric antigen receptor (CAR) modified T cells have shown early promise in hematological malignancies. However, in solid malignancies CAR T cells must overcome a distinct immunosuppressive microenvironment which may compromise their capacity to mediate antitumor activity.
Keywords: adoptive cell therapy, cancer, chimeric antigen receptor T cells, immune checkpoint molecules, tumor microenvironment
Abbreviations: CAR, chimeric antigen receptor; Lag3, lymphocyte-activation gene 3; PD1, programmed cell death 1; PDL1, programmed death ligand 1; TIM3, T cell immunoglobulin domain and mucin domain 3
CAR modified T cells have recently demonstrated impressive activity in the treatment of some patients with CD19+ hematologic malignancies.1 This finding has spurred strong interest in investigating a role for CAR T cells in the treatment of other advanced cancers, including solid malignancies. As a result, multiple clinical trials are currently underway investigating the utility of CAR T cells for the treatment of patients with advanced solid cancers.
CARs are modular polypeptides synthetically designed to transmit a cellular activation signal upon binding a target antigen expressed on the surface of an adjacent cell. T cells genetically modified to express a CAR that recognizes a tumor associated antigen are unaffected by tumor escape mechanisms involving the loss or downregulation of major histocompatibility molecules. However, it is possible that malignant cells may still escape elimination by CAR T cells by gaining immunosuppressive properties, by establishing an immunosuppressive microenvironment, or both. However, little is known about the role of these mechanisms in regulating the efficacy of CAR T cells in solid malignancies.
The use of CAR T cells for the treatment of solid malignancies has been less well explored than their use for the treatment of hematological malignancies. This is largely a result of the concern for on-target off-tumor toxicities due to the expression of target antigens on normal healthy cells. We recently reported on the use of T cells engineered to express a mesothelin-specific CAR in two patients with advanced cancer.2 Mesothelin, a glycosylphosphatidylinositol-linked glycoprotein, is expressed at low levels on the cell surface of normal peritoneal, pleural, and pericardial surfaces. However, mesothelin has also been demonstrated to be a target of an endogenous immune response in mesothelioma, ovarian carcinoma, and pancreatic carcinoma where it is frequently overexpressed.3 Our preliminary findings investigating adoptive therapy with autologous T cells transiently modified to express a mesothelin-specific CAR have shown safety and preliminary efficacy in two patients with advanced solid cancers. In a patient with chemotherapy-refractory pancreatic carcinoma, we observed evidence for CAR T cell infiltration of tumor tissue after intravenous infusion. In addition, our findings suggested a role for CAR T cells in inducing a vaccine effect with the development of novel antitumor antibody responses. However, disease control was transient with patients ultimately experiencing disease progression.
The tumor microenvironment is a critical determinant of antitumor immunity with the capacity to suppress the infiltration, activation, and effector activity of tumor-specific T cells. Leukocytes as well as other non-malignant cells, such as fibroblasts, present within the tumor microenvironment can suppress the antitumor activity of tumor-infiltrating T cells. However, because CAR T cells can be engineered with intrinsic co-stimulatory activity [e.g. incorporation of CD28 and/or 4-1BB (CD137) signaling domains], we initially hypothesized that they may be capable of resisting inhibitory elements present within the tumor microenvironment to which endogenous T cells are highly susceptible.
To understand the role of the tumor microenvironment in regulating the activity of tumor-infiltrating CAR T cells, we evaluated the capacity of mesothelin-specific CAR T cells to impact the growth of large, established tumors formed from a human mesothelioma cell line stably transduced with mesothelin.4 In contrast to prior studies with a different mesothelioma cell line in which major regressions and even cure were observed,5,6 tumor outgrowth was delayed but no regressions were seen. One explanation for this finding could be that the tumors underwent a process of “immunoediting” in which they downregulated or lost mesothelin expression allowing for the outgrowth of antigen-negative tumor variants. However, immunohistochemical studies showed intense and uniform expression of mesothelin on tumor cells despite significant CAR T cell infiltration. In addition, tumor-infiltrating T cells were found to retain CAR expression. As a result, we considered the possibility that tumors may evade immune elimination through their ability to orchestrate a microenvironment that induces a state of T cell hyporesponsiveness. To investigate this hypothesis, the functional capacity of CAR T cells was examined. This analysis revealed that tumor infiltrating CAR T cells rapidly lose their cytolytic and cytokine secretion capacity which is reversible by resting cells in vitro for 24 h. In contrast, CAR T cells that had infiltrated the spleen were significantly less hypofunctional, supporting the idea that the effect on CAR T cell function was specific to the tumor microenvironment.
Despite acquiring a fate of hypofunction, CAR T cells were found to accumulate progressively over time in the tumor microenvironment suggesting that they may retain their proliferative capacity – perhaps due to the 4-1BB cytoplasmic signaling domain of the CAR which can support T cell survival. However, we also found that CAR T cells acquired increased expression of several cell-surface inhibitory receptors including PD1, TIM3, Lag3, and 2B4. This expression, though, was reversible with a dramatic decrease observed after resting cells in vitro. Consistent with a role for these receptors in regulating CAR T cell hypofunction in vivo, blockade of the PD1/PDL1 interaction was also found to restore the cytolytic capacity of CAR T cells analyzed ex vivo.
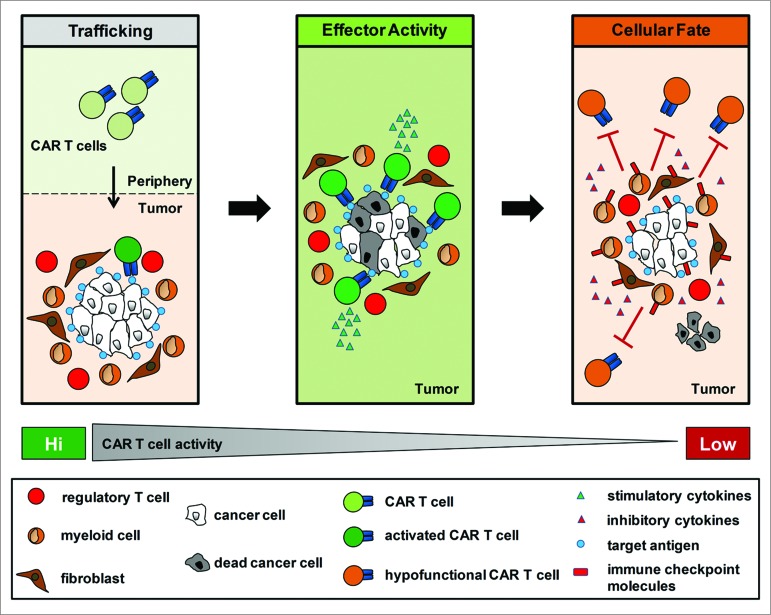
The inability of CAR T cells to eliminate tumor cells despite sufficient antigen expression argues for a critical role for the tumor microenvironment in regulating T cell immunosurveillance in cancer (Fig. 1). However, we believe it is important to note that CAR T cells were not irreversibly inactivated. This finding suggests that immune suppression in cancer is an active process that is local in nature and may be potentially reversible in vivo.
Figure 1.
The tumor microenvironment directs the cellular fate of chimeric antigen receptor modified T cells. The efficacy of autologous chimeric antigen receptor (CAR)-engineered T cells is dependent on their capacity to (1) traffick to tumor tissue,(2) mediate effector activity against tumor cells which express the target antigen on their cell surface, and (3) resist immunosuppressive signals within the tumor microenvironment which can induce a state of T cell hyporesponsiveness.
In summary, we believe that the tumor microenvironment will be a key determinant of the efficacy of CAR T cells in solid malignancies. We propose that combinatorial approaches involving strategies that redirect the tumor microenvironment from immunosuppressive to immunostimulatory, inhibit immune checkpoint signals in T cells, or both, will be necessary for realizing the full potential of CAR T cells.
Disclosure of potential conflicts of interest
G.L.B. declares receipt of research funding from Novartis. The authors have no additional financial interests.
Funding
Work was supported by grants from the National Institutes of Health to G.L.B. (NIH K08 CA138907) and to E.K.M. (NIH K08 CA 163941), from the Doris Duke Charitable Foundation (Grant# 2013107) to G.L.B., and from Precision Medical Research Associates (Grant# PMRA_2014-0612.1) to G.L.B.
References
- 1. Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013; 3:388-98; PMID:; http://dx.doi.org/ 10.1158/2159-8290.CD-12-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol Res 2014; 2:112-20; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014; 74:2907-12; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moon EK, Wang LC, Dolfi DV, Wilson CB, Ranganathan R, Sun J, Kapoor V, Scholler J, Pure E, Milone MC, et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin Cancer Res 2014; 20:4262-73; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, Varela-Rohena A, Haines KM, Heitjan DF, Albelda SM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009; 106:3360-5; PMID:; http://dx.doi.org/ 10.1073/pnas.0813101106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, Chew A, Carroll RG, Scholler J, Levine BL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res 2010; 70:9053-61; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2880 [DOI] [PMC free article] [PubMed] [Google Scholar]