Abstract
A Phase I trial of a p53-targeting modified vaccinia Ankara (p53MVA) vaccine in patients afflicted with refractory gastrointestinal cancers demonstrated enhanced T-cell recognition of p53 following vaccination. However, this effect was transient suggesting that p53MVA requires combination with immunomodulatory agents to deliver clinical benefit. Here, we outline our rationale for combining p53MVA with immunomodulatory chemotherapy in a forthcoming trial.
Keywords: clinical trial, gemcitabine, immunotherapy, MVA, ovarian cancer, p53
Abbreviations: MVA, modified vaccinia Ankara; PD-1, programmed death receptor1; PDL-1, programmed death receptor 1 ligand; DC, dendritic cells; MDSC, myeloid-derived suppressor cells; Tregs, regulatory T cells
The recognition that irradiation, and some chemotherapy agents, partly confer clinical benefit through immunological mechanisms, taken together with improved patient outcomes after checkpoint inhibition, has boosted the cancer immunotherapy field in recent years. However, while there is real cause for optimism, considerable obstacles to stimulating effective antitumor immunity remain.
The function of germline p53 protein is to maintain normal cell division. TP53 mutations are present in the majority of solid tumors, resulting in the accumulation of mutant p53 protein. In contrast, the concentration of normal p53 in healthy cells is low, making p53 an attractive cancer target with potentially wide therapeutic applications. We developed a MVA (modified vaccinia Ankara) vaccine expressing the wild-type p53 antigen (p53MVA), and performed the first-in-human trial in patients with advanced, treatment refractory gastrointestinal malignancies (trial reference no. NCT01191684).1 We found that immunological responses were robust after the first vaccination, but p53 T-cell responses did not show continued expansion with successive immunizations in the majority of patients. This transient p53 vaccination effect resembled that of another report of p53-based immunotherapy2 and may partly be due to peripheral tolerance to the self-antigen p53. Additionally, it's important to bear in mind that the immunosuppression prevalent in heavily pre-treated patients with advanced disease may be a barrier to inducing objective clinical responses.
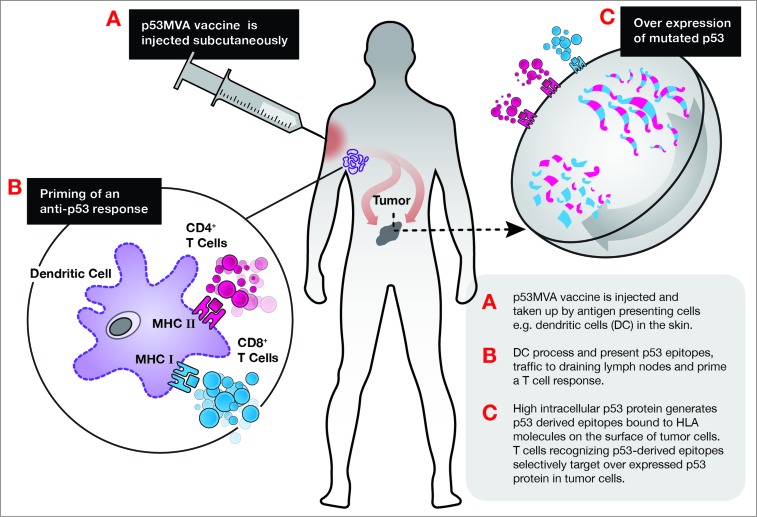
Upregulation of the immune checkpoint molecule programmed cell death 1 (PDCD1, better known as PD-1) on T cells commonly results from chronic antigen stimulation and negatively regulates T cells. The primary ligand, PD-L1, is often expressed on tumor cells, facilitating PD-1/PD-L1 suppression of antitumor immune responses. Administration of antibodies that block PD-1 or PD-L1 have elicited impressive therapeutic responses in patients with solid tumors.3 The finding that cancer patient peripheral blood dendritic cells (DCs) express elevated levels of PD-L14 is a concern for researchers developing viral vaccines, since these agents generally require uptake and presentation by DCs (see Fig. 1). Significantly higher frequencies of PD-1+ T cells were detected in our trial participants compared to healthy donors.1 Furthermore, lower frequencies of pre-vaccine PD-1+CD8+ lymphocytes correlated with higher p53-reactive CD8+ T cells. T-cell expansions in vitro also showed that the presence of anti-PD-1 antibody enhanced vaccine-induced responses, adding weight to the rationale for combining viral based vaccines with checkpoint inhibition antibodies.
Figure 1.
Proposed mechanism for priming of anti-p53 response by p53MVA vaccine.
The role of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in tumor immune evasion and cancer progression is well documented. These suppressive cell types impede effective antitumor immunity, with both Tregs and MDSCs being shown to affect vaccine-induced immune responses. One method of depleting these suppressive cell types is with chemotherapy agents, which can show useful preferential killing of suppressive cells relative to effector cells. The use of cancer vaccines in combination with chemotherapy has shown promise in cancer patients, such as in a report by Vermeij et al. demonstrating that responses to a p53 peptide vaccine were enhanced by low dose cyclophosphamide in ovarian cancer patients.5
Another chemotherapy agent that is being actively explored in this regard is gemcitabine. Like many second-line chemotherapy agents used to treat refractory, aggressive disease gemcitabine has a relatively low efficacy in terms of extending patient survival. However, it has recently become apparent that this agent may be useful when combined with immunotherapy. Although gemcitabine often induces neutropenia, it has been shown to induce positive immunomodulatory effects in pancreatic cancer patients, including decreased numbers of T regs6 and increased frequency of circulating monocytes and DCs.7 In addition, studies in pancreatic cancer indicate that gemcitabine may enhance responses to a variety of immunotherapies, including antigen-pulsed DCs8 and peptide vaccines.9 Many of these studies were single arm, pilot studies, so it is not possible to definitively conclude that gemcitabine plus vaccines are superior over either agent administered alone. However, induction of short-lived stable disease has been reported by some of these studies, justifying continued evaluation of this approach. A number of Phase II and some Phase III studies combining gemcitabine and various immunotherapies have recently been completed or are currently underway, it will be interesting to see what data emerge from these trials. In addition to its therapeutic application in pancreatic cancer, gemcitabine is frequently used to treat platinum-resistant ovarian cancer, late-stage disease with a very poor prognosis due to intrinsic and acquired chemotherapy resistance. Around 80% of patients initially respond to platinum-based chemotherapy (cisplatin/carboplatin) combined with paclitaxel, however, the vast majority later relapse with chemoresistant disease. Potentially accounting for this high rate of recurrence, immunosuppression within the ovarian tumor microenvironment is considerable. Suppressive cell types such as MDSCs and Tregs are known to accumulate during disease progression, and these immune inhibitory cells have been linked to poor prognosis.10
Since p53 mutation is associated with poor prognosis and platinum-based chemotherapy resistance, ovarian cancer is an ideal setting in which to evaluate p53-based immunotherapy. Our forthcoming clinical study will specifically evaluate the p53MVA vaccine in combination with the chemotherapy agent, gemcitabine. The combination of p53MVA-based cancer vaccines and gemcitabine is also being actively pursued as a therapy for pancreatic cancer. To our knowledge only one study, recently completed at the University of Leiden, has explored this fundamental approach in platinum-resistant ovarian cancer. In our study, patients will receive gemcitabine according to a modified standard-of-care regimen, with p53MVA being administered during treatment breaks. Our hope is that gemcitabine will synergize with the p53-targeting vaccine in this setting, as it has in some recent pancreatic cancer trials. If gemcitabine can be successfully utilized to at least partially inhibit the immunosuppressive action of MDSCs and Tregs, it has the potential to enhance the immunostimulatory action of p53MVA and deliver clinical benefit.
We apologize to authors whose work could not be cited in this commentary due to space constraints.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hardwick NR, Carroll M, Kaltcheva T, Qian D, Lim D, Leong L, Chu P, Kim J, Chao J, Fakih M, et al. p53MVA therapy in patients with refractory gastrointestinal malignancies elevates p53-specfic CD8+ T cell responses. Clin Cancer Res 2014; 20(17):1-12; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-3361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, Hepkema BG, Willemse PH, Molmans BH, Hollema H, et al. Immunization with a P53 synthetic long peptide vaccine induces P53-specific immune responses in ovarian cancer patients, a phase II trial. Int J Cancer 2009; 125:2104-13; PMID:; http://dx.doi.org/ 10.1002/ijc.24597 [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207-12; PMID:; http://dx.doi.org/ 10.1016/j.coi.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tjomsland V, Spangeus A, Sandstrom P, Borch K, Messmer D, Larsson M. Semi mature blood dendritic cells exist in patients with ductal pancreatic adenocarcinoma owing to inflammatory factors released from the tumor. PLoS One 2010; 5:e13441; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0013441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vermeij R, Leffers N, Hoogeboom BN, Hamming IL, Wolf R, Reyners AK, et al. Potentiation of a p53-SLP vaccine by cyclophosphamide in ovarian cancer: a single-arm phase II study. Int J Cancer 2012; 131:E670-80 [DOI] [PubMed] [Google Scholar]
- 6.Homma Y, Taniguchi K, Nakazawa M, Matsuyama R, Mori R, Takeda K, Ichikawa Y, Tanaka K, Endo I. Changes in the immune cell population and cell proliferation in peripheral blood after gemcitabine-based chemotherapy for pancreatic cancer. Clin Transl Oncol 2014; 16:330-5; PMID:; http://dx.doi.org/ 10.1007/s12094-013-1079-0 [DOI] [PubMed] [Google Scholar]
- 7.Soeda A, Morita-Hoshi Y, Makiyama H, Morizane C, Ueno H, Ikeda M, Okusaka T, Yamagata S, Takahashi N, Hyodo I, et al. Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c +dendritic cells in patients with advanced pancreatic cancer. Jpn J Clin Oncol 2009; 39:797-806; PMID:; http://dx.doi.org/ 10.1093/jjco/hyp112 [DOI] [PubMed] [Google Scholar]
- 8.Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M, Katano Y, Ohmiya N, Niwa Y, Yamamoto K, et al. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas 2009; 38:e69-74; PMID:; http://dx.doi.org/ 10.1097/MPA.0b013e318197a9e3 [DOI] [PubMed] [Google Scholar]
- 9.Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother 2011; 34:92-9; PMID:; http://dx.doi.org/ 10.1097/CJI.0b013e3181fb65b9 [DOI] [PubMed] [Google Scholar]
- 10.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942-9; PMID:; http://dx.doi.org/ 10.1038/nm1093 [DOI] [PubMed] [Google Scholar]