Abstract
This commentary provides the authors’ perspective on the article “Routes of delivery for CpG and anti-CD137 for the treatment of orthotopic kidney tumors in mice”, published in PLoS ONE. It also discusses the caveats of using subcutaneous tumors to model the treatment of human cancers versus orthotopic mouse models that more closely mimic human disease.
Keywords: cancer, immunotherapy, mouse model, orthotopic, preclinical
Immunotherapy is the delivery of agents which either directly stimulate and enhance the body's own immune system, or which block the suppressive and inhibitory factors acting against the immune system, thus enabling it to function more effectively. An effective immune response mediated by activated T cells is often key to successful immunotherapy. However, T cells must overcome immune regulation and the suppressive environments generated by cancers in order to become fully immune competent. An example of a stimulatory immunotherapy is CpG oligodeoxynucleotide (CpG ODN), a synthetic DNA molecule that mimics bacterial DNA sequences and binds to Toll-like receptor 9 (TLR9) on immune cell subsets, including monocytes, neutrophils, and natural killer (NK) cells. This binding elicits triggering of various pathways leading to production of proinflammatory cytokines that directly activate immune cells. Various CpGs are currently undergoing evaluation in clinical trials, but do not appear to be sufficient to eliminate cancers on their own. Thus, combinations of other therapies are being tested along with CpG-based treatments. In a previous study by our group,1 we combined CpG1826 with anti-CD137 monoclonal antibody (αCD137), an agonist to CD137 expressed on activated T cells, monocytes, natural killer (NK) cells and dendritic cells, enhancing T-cell receptor signaling. The combination of both immunotherapies resulted in over 80% survival of mice bearing established subcutaneous (SC) MC38 (derived from colon carcinoma) tumors, and over 60% survival of mice bearing established SC Renca (derived from renal carcinoma) tumors. The CpG was delivered intratumorally (IT) every 2–3 d for 4 doses, and the αCD137 was delivered intraperitoneally (IP) every 3–4 d for 3 doses. The combination therapy had an effect on contralateral MC38 SC tumors injected 2 d after the primary SC tumor, with 50% of mice surviving in this SC model. Mechanistically, CD8+ T cells, NK cells and interferons were required for the observed therapeutic effect.
There are now several published articles which attest that preclinical testing of anticancer therapies does not have a good record of predicting success once moved to the clinic.2 One of the problems attested to is the use of SC models, which are considered a poor surrogate model for metastatic human cancers. This is largely because the tumors are in an organ (the skin) distinct from that from which the neoplasms were derived and, therefore, are undergoing evaluation in a different microenvironment than their original context. Furthermore, SC tumors are seldom metastatic. Numerous articles have called for more clinically relevant mouse tumor models to be used in preclinical testing of anticancer agents.2,3 Neither the SC MC38 or SC Renca models we used for testing CpG with αCD137 are metastatic, and neither were they injected into the organ from which they were derived. With this in mind, we decided to choose one of these tumor lines (Renca) and injected it orthotopically into the kidney outer cortex where it metastasizes to the lungs, liver, diaphragm and abdominal cavity, and thus more closely models human renal carcinoma.
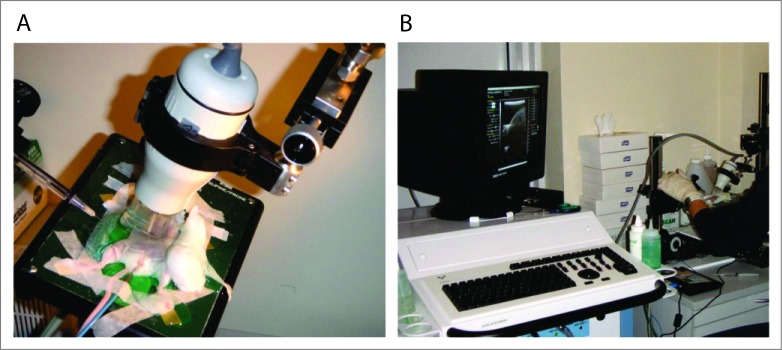
This work was published recently in PLoS ONE.4 In brief, an operation was performed to inject the Renca tumor cells into the renal outer cortex of one kidney per mouse. Twelve days later, αCD137 was injected IP every 3–4 d for 6 doses, but to deliver the CpG IT (as before in the SC tumor setting) over several days without surgically exposing the kidney each time, required ultrasound-guided injection of the CpG directly into the established tumor (see Fig. 1 showing the ultrasound imaging set up and equipment). It is possible with this imaging to see the CpG solution being injected into the tumor (it appears as a reduction in density spreading through the tumor) and this was readily and reproducibly observed. This novel technique has not been used before to treat renal tumors IT, and is minimally invasive, fast and suitable for multiple injections. By day 19, primary tumor growth in the kidney was inhibited significantly relative to non-treated controls. Nevertheless, all mice (except one in 25 mice) died of large renal tumors and/or metastatic burden, the majority with lung metastases. However, there was significantly enhanced survival compared with the non-treated group. We also tested CpG delivered intravenously for 3 injections combined with αCD137, and survival was significantly enhanced compared with IT delivered CpG plus αCD137, but with no long-term survivors. In this case, enhanced survival was most likely due to the reduction of metastatic burden mediated by systemic CpG. Thus, survival results using combination therapy and intratumoral CpG delivery to orthotopic tumors certainly did not match those of mice bearing SC tumors.
Figure 1.
Ultrasound imaging equipment setup for injecting CpG into orthotopic renal tumors in mice. The sedated mouse is shown strapped to a warm stage ready for ultrasound-guided needle injection of CpG oligodeoxynucleotide into the tumor implanted orthotopically in the kidney (A). Operator watches screen to visualize the tumor on the kidney and accurately inject CpG directly into the tumor (B).
We have found similar results for other immunotherapies. In 2 of our studies5,6 we tested a combination of 3 agonist antibodies (anti-DR5, anti-CD137 and anti-CD40) termed Tri-mAb and found significantly enhanced survival in SC models of MC38, Renca, CT26, and RM-1, with survival ranging from ∼60–90% in these models. However, treatment was not nearly as effective when tumors were injected orthotopically, with <40% survival of mice bearing CT26 intracecum-injected tumors, <10% survival of mice with Renca intrakidney-injected tumors, and nearly 3 times the tumor burden by day 12 in mice with RM-1 intraprostate-injected tumors. We found this was due to involvement of alternatively activated macrophages causing immunosuppression, which was tissue-specific. By neutralizing the macrophage-associated molecules CC motif chemokine ligand 2 (CCL2) or IL-13, the therapy was significantly improved in the orthotopic Renca kidney cancer model. Undoubtedly, the effect of immunosuppressive immune cells and molecules present within the tumor microenvironment has important consequences for immunotherapy,7,8 and combination therapies to overcome these effects will naturally be required. Most importantly however, our findings argue that these combined therapies should be tested in clinically relevant models. These models should comprise implantation of tumor cell lines in the organ from which they were derived and which form metastases in the appropriate organs similar to endogenous human disease. In addition, spontaneously arising tumor models and using older mice may represent more clinically relevant situations to test new immunotherapies.9,10
Funding Statement
This work was supported by NH&MRC (1013667).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Westwood JA, Haynes NM, Sharkey J, McLaughlin N, Pegram HJ, Schwendener RA, Smyth MJ, Darcy PK, Kershaw MH. Toll-like receptor triggering and T-cell costimulation induce potent antitumor immunity in mice. Clin Cancer Res 2009; 15:7624-33; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-2201. [DOI] [PubMed] [Google Scholar]
- 2.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov 2013; 12:217-28; PMID: 23449307;http://dx.doi.org/10.1038/nrd3870 [DOI] [PubMed] [Google Scholar]
- 3.Ruggeri BA, Camp F, Miknyoczki S. Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol 2014; 87:150-61; PMID: 23817077; http://dx.doi.org/10.1016/j.bcp.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 4.Westwood JA, Potdevin Hunnam TC, Pegram HJ, Hicks RJ, Darcy PK, Kershaw MH. Routes of delivery for CpG and anti-CD137 for the treatment of orthotopic kidney tumors in mice. PLOS One 2014; 9:e95847; PMID: 24788789; http://dx.doi.org/10.1371/journal.pone.0095847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westwood JA, Darcy PK, Guru PM, Sharkey J, Pegram HJ, Amos SM, Smyth MJ, Kershaw MH. Three agonist antibodies in combination with high-dose IL-2 eradicate orthotopic kidney cancer in mice. J Translational Med 2010; 8:42; PMID: 20426873; http://dx.doi.org/10.1186/1479-5876-8-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devaud C, Westwood JA, John LB, Flynn JK, Paquet-Fifield S, Duong CP, Yong CS, Pegram HJ, Stacker SA, Achen MG. Tissues in different anatomical sites can sculpt and vary the tumor microenvironment to affect responses to therapy. Mol Ther 2014; 22:18-27; PMID: 24048441; http://dx.doi.org/10.1038/mt.2013.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. OncoImmunology 2013; 2:e25962; PMID: 24327938; http://dx.doi.org/10.4161/onci.25962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaud C, John LB, Westwood JA, Darcy PK, Kershaw MH. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. OncoImmunology 2013; 2:e25961; PMID: 24083084; http://dx.doi.org/10.4161/onci.25961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strachan DC, Ruffell B, Oei Y, Bissell MJ, Coussens LM, Pryer N, Daniel D. CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. OncoImmunology 2013; 2:e26968; PMID: 24498562; http://dx.doi.org/10.4161/onci.26968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchlaka MN, Murphy WJ. Impact of aging in cancer immunotherapy: The importance of using accurate preclinical models. OncoImmunology 2013; 2:e27186; PMID: 24498569; http://dx.doi.org/10.4161/onci.27186 [DOI] [PMC free article] [PubMed] [Google Scholar]