Abstract
Interleukin-4 (IL4), a Th2 cytokine, signals through IL4 receptors (IL4Rs) to regulate increased lymphocyte proliferation and survival. We have recently discovered that IL4 also promotes these phenotypes in mammary cancer cells expressing IL4Rs to enhance their metastatic ability. Targeting IL4/IL4R signaling on cancer cells themselves may limit metastatic disease.
Keywords: Akt, breast cancer, Erk, liver metastasis, mTOR, proliferation, survival
Interleukin-4 (IL4) is one of the most studied Th2 cytokines of the immune system where it is mainly produced by activated T cells, mast cells, basophils, and eosinophils. On lymphocytes, IL4 activates the type I IL4 receptor (IL4R), consisting of the IL4Rα and common gamma (γc) chains, to promote differentiation, survival, and proliferation for clonal expansion (Fig. 1A).1 While most normal epithelial cells do not express IL4Rs, epithelial cancer cells including breast cancer cells upregulate the type II IL4R consisting of the IL4Rα and IL13 receptor α 1 (IL13Rα1) chains (Fig. 1B).2 Significantly, IL4 ligand is upregulated in the microenvironment of several epithelial tumor types including breast cancer.3 So, why have cancer cells apparently hijacked the immune IL4/IL4R signaling axis?
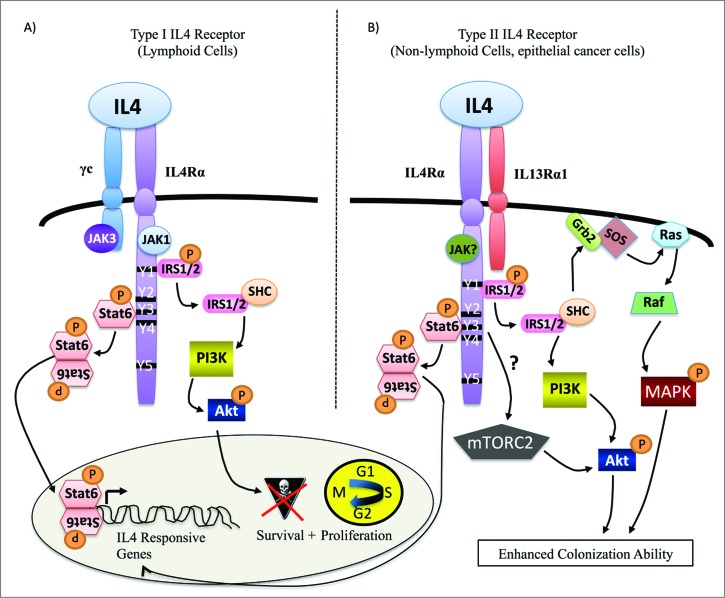
Figure 1.
IL4/IL4R-activated signaling pathways that promote growth phenotypes. (A) In lymphoid cells, IL4 induces Jak activation and heterodimerization of IL4Rα and γC chains to form the type I IL4 receptor. Effects of receptor activation include transcription of target genes, cell cycle progression, and survival. (B) Little is known about type II IL4R signaling in epithelial cancer cells in response to IL4 beyond the activation of Stat6 for transcription of pro-growth genes. We have shown that IL4-activated Akt, Erk, and mTOR mediate IL4/IL4R-induced colonization ability in mammary cancer cells.
Similar to immune functions, Il4/IL4R signaling may promote the survival and proliferation of cancer cells. In fact, IL4/IL4R signaling has been shown to increase survival via the upregulation of anti-apoptotic proteins (PED, cFLIP, Bcl-xL and Bcl-2) to promote human breast and colon tumor growth in nude mice.2 However, these studies did not examine the contribution of specific IL4/IL4R-induced anti-apoptotic pathways in models with intact immune systems, or consider how enhanced proliferation could contribute to tumor growth. Notably, IL4/IL4R signaling enhances the proliferation of several cancer types including breast cancer in vitro, and prostate cancer cells in vivo.4
IL4/IL4R signaling regulates pro-tumorigenic growth phenotypes in epithelial cancer cells, but can it promote metastasis? Overall, metastasis accounts for 85–90% of all cancer-related deaths. In the case of breast cancer, the 5-year survival rates plummet from 99% to 24% for patients that develop distant metastases.5 Our goal was to determine whether IL4/IL4R signaling could promote the survival and proliferation of mammary cancer cells for enhanced metastatic colonization and outgrowth in the lung and liver. We answered this question by performing shRNA-mediated knockdown (KD) of IL4Rα, in two murine mammary cancer cell lines, R221a and 4T1, and testing metastatic ability in immune competent murine models.
Experimental metastasis assays with control and IL4Rα KD mammary cancer cells revealed that IL4Rα is a strong promoter of metastatic mammary tumor growth in the lung and liver. IL4Rα-induced metastatic growth ability was attributed to increased survival and colonization ability and increased proliferation and outgrowth ability. Using IL4 knockout mice, we also showed that IL4 ligand drives enhanced lung metastatic tumor growth.6 However, our finding that IL4 knockout mice injected with IL4Rα KD cells had a greater reduction in tumor burden than either IL4 knockout or IL4Rα KD alone could implicate a role for IL13 in vivo. IL4 is the prototypical ligand for the IL4 receptor. However, IL13, the only other cytokine known to bind and activate type II IL4Rs, may also promote tumor growth, a concept we have reviewed.7
How does IL4 regulate the proliferation and survival of cancer cells? While both the type I and type II IL4Rs are bound and activated upon IL4 binding the IL4Rα chain, downstream signaling cascades controlling growth may differ. In lymphoid cells, Il4 induces proliferation and survival through two main pathways downstream of the type I IL4R; 1) the insulin receptor substrate protein (IRS)/PI3K/Akt pathway, and 2) the Jak/signal transducer and activator of transcription factor (Stat6) pathway (Fig. 1A).1 However, type II IL4R signaling in response to IL4 in epithelial cancer cells remains largely unresolved, with the exception of Stat6 activation.
IL4-activated Stat6 has been shown to mediate enhanced proliferation, and resistance to apoptosis in vitro.2,8 Yet, analysis of human breast cancer tissue revealed that total Stat6 is actually decreased in invasive breast ductal carcinomas compared to ductal carcinomas in situ.9 One caveat to this study is that only Stat6 protein expression was examined and not functional activation or nuclear localization. While it is possible that invasive breast tumors could have increased Stat6 function, we sought to elucidate other potentially novel IL4/IL4R-activated pathways that mediate metastatic tumor growth.
It is known that IRS1/2 activation by IL4/IL4R can result in the activation of downstream MAPK/Erk and/or PI3K/Akt in some non-hematopoietic cells.1 Both Erk and Akt have been implicated in inducing proliferation and survival in a variety of cancers. We found that both were phosphorylated in response to IL4 in addition to Stat6 in mammary cancer cells in vitro. Interestingly, Akt was strongly phosphorylated at serine 473 in both the R221a and 4T1 cell lines, implicating a possible role for upstream mTORC2 activation. mTOR was phosphorylated at serine 2481 in response to IL4, indicating an active mTORC2 complex. In vitro, pharmacologic blockade of each of the effector proteins (Akt, Erk, or mTOR) abrogated IL4-induced colony formation, indicating that all three pathways mediate IL4-enhanced colonization ability. In addition, we also saw a reduction in pAkt serine 473 and pErk levels in IL4Rα KD lung metastases compared to control, confirming the relevance of IL4/IL4Rα-activated pAkt and pErk in vivo.6
In summation, we have identified IL4/IL4R signaling as a potent driver of mammary cancer metastasis. Approximately 80% of cancers are epithelial in origin.5 Given that IL4/IL4R signaling regulates the survival and proliferation of many other epithelial cancer types, our findings could have a large impact on limiting metastatic disease. However, we must take into consideration that systemic inhibition of IL4/IL4R may not be feasible given the normal functions of IL4 in the immune system. Fortunately, modified IL4 ligands termed “superkines” that selectively target and inhibit the type II IL4R are in development.10 Further experimentation is needed to determine if IL4 superkines can thwart metastatic tumor growth.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 1999; 17:701-38; PMID:; http://dx.doi.org/10.1146/annurev.immunol.17.1.701 [DOI] [PubMed] [Google Scholar]
- 2. Todaro M, Lombardo Y, Francipane MG, Alea MP, Cammareri P, Iovino F, Di Stefano AB, Di Bernardo C, Agrusa A, Condorelli G, et al. Apoptosis resistance in epithelial tumors is mediated by tumor-cell-derived interleukin-4. Cell Death Differ 2008; 15(4):762-72; PMID:; http://dx.doi.org/10.1038/sj.cdd.4402305 [DOI] [PubMed] [Google Scholar]
- 3. Camp BJ, Dyhrman ST, Memoli VA, Mott LA, Barth RJ. In situ cytokine production by breast cancer tumor-infiltrating lymphocytes. Ann Surg Oncol 1996; 3(2):176-84; PMID:; http://dx.doi.org/10.1007/BF02305798 [DOI] [PubMed] [Google Scholar]
- 4. Roca H, Craig MJ, Ying C, Varsos ZS, Czarnieski P, Alva AS, Hernandez J, Fuller D, Daignault S, Healy PN, et al. IL-4 induces proliferation in prostate cancer PC3 cells under nutrient-depletion stress through the activation of the JNK-pathway and survivin upregulation. J Cell Biochem 2011; 113(5):1569-80; PMID:; http://dx.doi.org/10.1002/jcb.24025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Cancer Society Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. p11 [Google Scholar]
- 6. Venmar KT, Carter KJ, Hwang DG, Dozier EA, Fingleton B. IL-4 receptor ILR4α regulates metastatic colonization by mammary tumors through multiple signaling pathways. Cancer Res 2014; 74:4329-40; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-14-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hallett MA, Venmar KT, Fingleton B. Cytokine stimulation of epithelial cancer cells: the similar and divergent functions of IL-4 and IL-13. Cancer Res 2012; 72(24):6338-43; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-12-3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang WJ, Li BH, Yang XZ, Li PD, Yuan Q, Liu XH, Xu SB, Zhang Y, Yuan J, Gerhard GS, et al. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine 2008; 42(1):39-47; PMID:; http://dx.doi.org/10.1016/j.cyto.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 9. Porter HA, Perry A, Kingsley C, Tran NL, Keegan AD. IRS1 is highly expressed in localized breast tumors and regulates the sensitivity of breast cancer cells to chemotherapy, while IRS2 is highly expressed in invasive breast tumors. Cancer Lett 2013; 338(2):239-48; PMID:; http://dx.doi.org/10.1016/j.canlet.2013.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Junttila IS, Creusot RJ, Moraga I, Bates DL, Wong MT, Alonso MN, Suhoski MM, Lupardus P, Meier-Schellersheim M, Engleman EG, et al. Redirecting cell-type specific cytokine responses with engineered interleukin-4 superkines. Nat Chem Biol 2012; 8(12):990-8; PMID:; http://dx.doi.org/10.1038/nchembio.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]