Abstract
Natural killer (NK) cells eradicate galectin-deficient malignant gliomas without the necessity for T cell cooperation. This phenomenon was discovered as a consequence of reducing glioma-derived galectin-1. We propose that stimulation of endogenous antitumor NK cell activity may be achieved by reducing potent tumor-derived NK cell inhibitors, such as galectin-1, and that such agents be tested in the clinic to treatbrain tumors.
Keywords: malignant glioma, galectin-1, natural killer cells, innate antitumor immunity
If you come to a fork in the road, take it.
Yogi Berra
Even when patients that suffer from high grade malignant glioma are treated with the current state-of-the-art surgery, radiotherapy and chemotherapy, median survival following tumor resection remains less than 2 years.1 Today's statistics compare poorly with the ∼6 month survival reported in the late 30s. Slow progress in extending patients’ lives highlights the urgent need for new and effective treatments for malignant brain tumors. The unpredictable power of the adaptive immune system to eradicate human tumors, such as has been observed in the treatment of some patients with advanced melanomas, has encouraged the development of immunotherapies for the treatment of other cancers, including brain malignancies. Currently, www.clinicaltrials.gov, the website that lists all recently completed and ongoing clinical trials, lists ∼75 trials involving vaccination and/or immunotherapy for the treatment of brain tumors.
Given the limited long-term survival of glioma patients, any treatment that provides robust and reproducible extension in patient survival will likely be rapidly adopted worldwide. The fact that after 20years only a handful of immunotherapy/vaccination trials are being tested indicates that, so far, a clear winner has yet to become available.2,3 In view of the efforts invested in immunotherapy and the limited responses obtained, it is worth inquiring whether some important component of the immune system is not being sufficiently activated with immunotherapy for brain tumors. In 1989 Charles Janeway, Jr. called attention to the fact that immune responses to specific infectious antigens were not detected without the co-administration of poorly characterized adjuvants.4 This opened the floodgates to the cellular and molecular characterization of early immune system function. Though adjuvant-like molecules are included in immunotherapy trials, a lack of consideration of the antitumor activity of innate immune cells could limit the expected success of immunotherapies. In 2009, our Group discovered that an endogenous immunotherapy for the treatment of brain tumors failed in the absence of activation of Toll-like receptor 2 (TLR2), a pattern recognition receptor necessary for innate immunity. We further found that release of HMGB-1 from dying tumor cells was the endogenous activator of TLR2.5 We were thus able to show that an immunotherapeutic approach based theoretically on the reconstitution of the adaptive immune system within the brain, depended on endogenous activation of the innate immune receptor TLR2. Could a similar component be missing from currently implemented immunotherapies?
We have now discovered that galectin-1 is a powerful tumor-derived inhibitor of anti-glioma natural killer (NK) responses. Reduced glioma cell galectin-1 expression unleashed the capacity of NK cells to eradicate malignant gliomas in mice and rats.6 NK cells infiltrating galectin-1-depleted gliomas expressed higher levels of the cytotoxic protein granzyme B, and galectin-1-depleted glioma cells were ∼3.5 times more sensitive to NK-mediated lysis in vitro. In fully immune competent mice, the rapid elimination of gliomas by NK cells occurred in the absence of T cell activation.6
Microarray database detection of high galectin-1 expression in glioma cells taken together with its role in the migration of cancer cells and its positive correlation with brain cancer malignancy led us to explore the role of galectin-1 in glioma cell migration along the perivascular compartment.7,8,9 In our study, we found that sh-RNA mediated knockdown of galectin-1 expression in glioma cells did not have an adverse effect on glioma cell growth in vitro. To avoid potential T cell responses, glioma cell migration in vivo was initially tested in RAG1−/− mice (B6.129S7-Rag1tm1Mom/J). Galectin-1 depleted glioma cells failed to grow when implanted into RAG1−/− mice, due to widespread apoptosis of glioma cells, thereby permitting the long-term survival of host mice. We therefore initially concluded that perivascular migration was inhibited by downregulation galectin-1, which led to glioma cell death in vivo. The eventual result of these experiments was the serendipitous discovery of the powerful inhibitory effect of galectin-1 on antitumor NK cell function.6
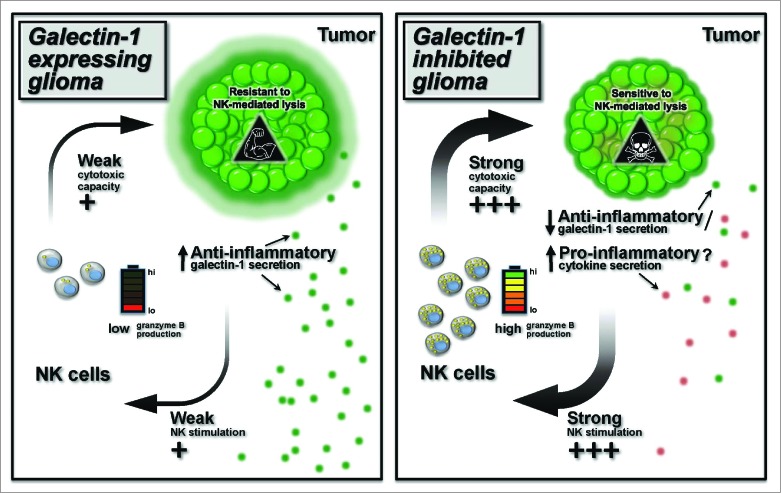
However, though RAG1−/− mice do not contain mature T and B cells they still contain a fully functional innate immune system. NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) on the other hand, are devoid of mature NK cells and contain functionally impaired macrophages. Thus, to discount any effects of innate immunity on glioma progression, we next examined the growth of galectin-1 deficient glioma in NSG mice. To our surprise the galectin-1 deficient tumors grew normally in NSG mice. This experiment suggested that cells of the innate immune system, likely NK cells, play a role in eliminating galectin-1-deficient tumors. If this hypothesis were true, in the absence of NK cells, glioma cells deficient in galectin-1 could now grow. To test this hypothesis, we performed a series of experiments designed to determine whether NK cells were responsible for eliminating glioma tumors deficient in galectin-1. We found that removal of the spleen or thymus in tumor-bearing RAG1−/− mice did not inhibit glioma eradication by NK cells. Circulating NK cells, however, were able to kill glioma cells in vitro without the need of additional ex vivo stimulation. It is therefore most likely that NK cells able to eradicate brain tumors are present in the circulation.6 (see Fig. 1 for an overall schematic view).
Figure 1.
Reducing malignant glioma galectin-1 expression effects the capacity of circulating NK cells to eradicate tumors. The left panel shows wild type tumors expressing their normal levels of galectin-1 (large green halo). Galectin-1 inhibits the capacity of natural killer (NK) cells to kill glioma tumors, and reduces the sensitivity of glioma cells to NK killing. The panel on the right depicts glioma tumors expressing reduced galectin-1 (small green halo). In glioma cells expressing reduced galectin-1 levels there is reduced secretion of galectin-1, and potential increased secretion of pro-inflammatory cytokines. This licenses NK cells to kill glioma cells, and leads to tumor eradication by NK cells in vivo. The early NK response against gliomas suggests that implementation of similar approaches for the treatment of human malignant glioma tumors may make substantial contributions to extending patients’ lives.
As galectin-1 can kill activated T cells10, we examined whether the adaptive arm of the immune system contributed to the elimination of galectin-1-deficient tumors in immunocompetent mice. To address this, we repeated our analyses with immunocompetent mice, (bear fully functional T, B, and NK cells ) and found that glioma tumors expressing reduced galectin-1 were eliminated in less than 7 days whereas tumors expressing normal levels of galectin-1 grew. Surprisingly, in wild-type animals that rejected glioma tumors expressing reduced galectin-1, glioma specific T cell responses were not activated. However, when NK cells were immuno depleted with specific antibodies, tumor specific T cell responses increased.6 Thus, we conclude that the powerful inhibitory effect of galectin-1 on innate immune responses carries a substantial clinical weight as NK cells deploy their antitumor effects much earlier than T cells, which become activated 7–10 days following antigen presentation. In summary, we have identified an immune inhibitory effect of tumor-derived galectin-1 on innate anti-tumor immunity involving NK cells. Given the powerful killing of glioma tumors by NK cells, it is possible that brain tumors use a multiplicity of mechanisms to block NK attack.10
In conclusion, our results should help to invigorate the study of glioma-mediated regulation of innate immune antitumor activity, as we demonstrate the powerful inhibition of NK cell-mediated tumor cell killing by glioma-derived galectin-1. The search for further glioma-derived molecules that modulate NK function will offer further clinical insights to improve the treatment of brain tumors using immunotherapies, and provide a fertile area for neuro-oncology research. We propose that future immunotherapies ought to incorporate mechanisms to stimulate innate immune antitumor activity. Stimulation of NK cell tumor killing may be the missing link needed to make brain cancer immunotherapies clinically effective.
References
- 1. Holdhoff M, Grossman SA. Controversies in the adjuvant therapy of high-grade gliomas. Oncologist 2011; 16:351-8; PMID:; http://dx.doi.org/ 10.1634/theoncologist.2010-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bregy A, Wong TM, Shah AH, Goldberg JM, Komotar RJ. Active immunotherapy using dendritic cells in the treatment of glioblastoma multiforme. Cancer Treat Rev 2013; 39:891-907; PMID:; http://dx.doi.org/ 10.1016/j.ctrv.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 3. Reardon DA, Wucherpfennig KW, Freeman G, Wu CJ, Chiocca EA, Wen PY, Curry WT, Jr, Mitchell DA, Fecci PE, Sampson JH, Dranoff G. An update on vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines 2013; 12:597-615; http://dx.doi.org/ 10.1586/erv.13.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz Y, Edwards MR, Michelsen KS, Kroeger KM, Liu C, et al. . HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 2009; 6:e10; published: January 13, 2009; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker GJ, Yadav VN, Motsch S, Koschmann C, Calinescu A, Mineharu Y, Carmelo-Piragua S, Orringer D, Bannykh S, Nicholas WS, et al. (2014) Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization and resistance to anti-angiogenic therapy. Neoplasia 2014; 16: 543-61; PMID:; http://dx.doi.org/ 10.1016/j.neo.2014.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker GJ, Chockley P, Yadav VN, Doherty R, Ritt M, Sivaramakrishnan S, Castro MG, Lowenstein PR.. Natural killer cells eradicate galectin-1 deficient glioma in the absence of adaptive immunity. Cancer Res. 2014 Sep 15; 74(18):5079-90; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toussaint LG, 3rd, Nilson AE, Goble JM, Ballman KV, James CD, Lefranc F, Kiss R, Uhm JH. Galectin-1, a gene preferentially expressed at the tumor margin, promotes glioblastoma cell invasion. Mol Cancer, 2012; 11:32; http://dx.doi.org/ 10.1186/1476-4598-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper D, Ilarregui JM, Pesoa SA, Croci DO, Perretti M, Rabinovich GA. Multiple functional targets of the immunoregulatory activity of galectin-1: Control of immune cell trafficking, dendritic cell physiology, and T-cell fate. Methods Enzymol, 480:199-244; PMID:; http://dx.doi.org/ 10.1016/S0076-6879(10)80011-4 [DOI] [PubMed] [Google Scholar]
- 9. Crane CA, Austgen K, Haberthur K, Hofmann C, Moyes KW, Avanesyan L, Fong L, Campbell MJ, Cooper S, Oakes SA, Parsa AT, Lanier LL. Proc Natl Acad Sci U S A. 2014 Sep 2;111(35):12823-8; http://dx.doi.org/ doi: 10.1073/pnas.1413933111. Epub 2014 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy KA, Erickson JR, Johnson CS, Seiler CE, Bedi J, Hu P, Pluhar GE, Epstein AL, Ohlfest JR. CD8+ T cell-independent tumor regression induced by Fc-OX40L and therapeutic vaccination in a mouse model of glioma. J Immunol, 2014; 192:224-33; http://dx.doi.org/ 10.4049/jimmunol.1301633 [DOI] [PMC free article] [PubMed] [Google Scholar]