Abstract
Recent studies of human pancreatic cancer challenge the mouse model-derived notion that the pancreas is a site of immune privilege. A heavy infiltration of CD8+ T cells expressing programmed cell death 1 (PD-1) and smaller numbers of myeloid cells and regulatory T cells provides rationale for the clinical evaluation of immune checkpoint inhibition as a pancreatic cancer therapeutic strategy.
Keywords: IDO, immune checkpoints, myeloid cells, PD-1, regulatory T cells
Introduction
Pancreatic ductal adenocarcinoma (PDA) is widely recognized as one of the deadliest of human malignancies. Inflammation appears to play a role in the pathogenesis of PDA, and the evolving immune response in the context of chronic inflammation may facilitate subsequent invasion and metastasis. Successful immunotherapeutic approaches to the treatment of PDA remain elusive. Therefore, developing a greater understanding of the immunobiology of the disease is of critical importance.
The most widely accepted paradigm shaping immunotherapeutic approaches for PDA suggests that pancreatic adenocarcinoma cells exist in an immune privileged environment. Importantly, this hypothesis is based upon data gleaned from genetically engineered mouse models.1,2 Murine tumors have been found to recruit immunosuppressive myeloid cells that inhibit T-cell infiltration and function, thus therapies that ultimately allow effector T cells to infiltrate these tumors lead to tumor regression. Depletion of myeloid cells has therefore become a focus of preclinical research and therapeutic development.
Is human PDA a site of immune privilege?
Mouse models have dramatically enhanced our understanding of the molecular biology and stromal-epithelial interactions in PDA; however, several publications have demonstrated that the immune response in mice does not precisely parallel that of the human disease. De Monte et al. previously used immunohistochemistry to analyze the expression of the helper T cell-associated transcription factors GATA-3, expressed by T helper type 2 (Th2), and T-bet, preferentially expressed by T helper type 1 (Th1), to show that T cells heavily infiltrated PDA tumors and that higher Th2:Th1 ratios were associated with worse survival.3 Furthermore, Ino et al. demonstrated that PDA tumors are infiltrated by a combination of T cells and myeloid cells, and also showed that determining the predominant subtypes of each could provide prognostic information.4 Our recent in-depth characterization of the immune infiltrate in human PDA has demonstrated that it has a robust T cell-predominant immune infiltration that appears to be attenuated by several immunosuppressive mechanisms.5
Using immunohistochemistry, we found that T cells were distributed throughout the tumor microenvironment and were often in contact with epithelial carcinoma cells.5 Although CD11b+ myeloid cells were also found within the tumors, their presence did not inhibit T-cell infiltration. Since there were large numbers of CD8+ T cells, we used multiparameter flow cytometry of freshly disaggregated tumors to gain insight into the functional capacity of these cells. We demonstrated that most CD8+ tumor-infiltrating T cells have a memory phenotype, based upon their expression of CD45RO, and retain the capacity to produce interferon γ (IFNγ).5 In fact, intratumoral CD8+ T cells were more likely than peripheral blood CD8+ T cells to be positive for CD45RO or IFNγ. These data from primary human PDA tumors can reasonably be interpreted as a demonstration that the pancreatic tumor microenvironment is far from immune privileged.
Pancreatic cancer follows the rules: immune activation triggers immunosuppressive mechanisms
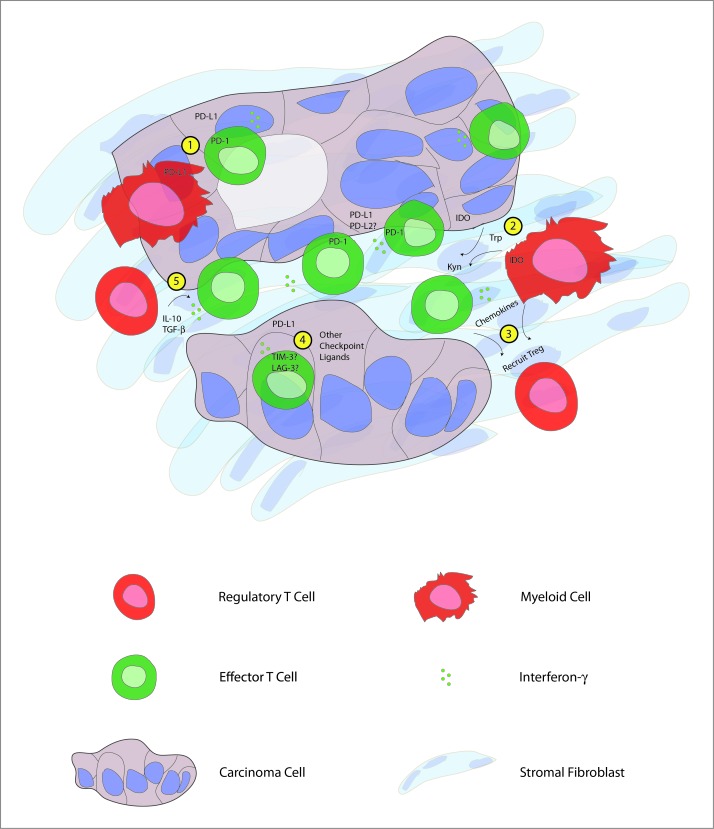
FOXP3+ putative regulatory T cells (Tregs) have previously been shown to infiltrate PDA tumors,6 and we confirmed the presence of CD4+FOXP3+CD25+CD127− Treg in our samples with flow cytometry. The presence of Tregs in the tumor microenvironment provided one potential mechanism through which effector T cell function might be attenuated, especially since FOXP3+ cells were significantly more prevalent in poorly differentiated tumors that are known to be associated with worse survival. Interestingly, we found that a majority of the intratumoral CD8+ T cells expressed high levels of the immune checkpoint molecule programmed cell death-1 (PDCD1, better known as PD-1), providing an additional mechanism through which T-cell activation may be regulated by tumor cells or immunosuppressive myeloid cells. Since PD-1 expression has recently been shown to identify antigen-specific, tumor reactive CD8+ T cells,7 our findings suggest that PDA may be the target of a concerted immune response. Furthermore, the production of IFNγ by CD8+ T cells infiltrating pancreatic tumors may drive adaptive immune resistance mechanisms, as has been shown in melanoma immune escape (Fig. 1).8,9
Figure 1.
Human pancreatic cancer contains a complex immune infiltrate with both effector and regulatory elements. Based upon our findings, we hypothesize that carcinoma-specific effector CD8+ T cells present throughout the tumor produce interferon γ (IFNγ) that drives immunosuppressive counter-regulatory mechanisms by regulatory T cells (Tregs), myeloid cells, and carcinoma cells. Potential mechanisms of immunosuppression that may serve as therapeutic targets include: 1) inhibition of T-cell activation by stimulation of the negative checkpoint regulatory molecule programmed cell death 1 (PD-1) via binding to PD-1 ligands (PD-L1, PD-L2) expressed on cancer cells or myeloid cells; 2) malignant cell and myeloid cell production of the enzyme indoleamine 2,3-dioxygenase (IDO), which catalyzes the breakdown of tryptophan (Trp) to kynurenine (Kyn); 3) chemokine-mediated recruitment of regulatory T cells; 4) binding of additional T cell co-inhibitory receptors, such as T cell immunoglobulin mucin-3 (TIM-3), lymphocyte activation gene-3 (LAG-3) to ligands displayed by carcinoma cells; 5) production of IL-10 and transforming growth factor β (TGFβ) by Tregs.
Neoadjuvant therapy selectively ablates immunosuppressive cells
We hypothesized that successful multimodal neoadjuvant therapy would be accompanied by alterations in the local immune response that might serve to tip the balance in favor of antitumor immunity. Concordant with this hypothesis, we found that tumors from patients undergoing aggressive multidrug chemotherapy followed by chemoradiotherapy prior to surgical resection have dramatically lower numbers of Tregs than do tumors from untreated patients.5 Myeloid cells and CD8+ T cells were somewhat less prevalent in neoadjuvant treated tumors. However, there was no difference in total T cells or CD4+ T cells, and the ratio of CD8+:FOXP3+ cells was significantly higher in the treated group. Therefore, immune activation may play a role in the antitumor effects of neoadjuvant therapy. Alternatively, our observations could simply be due to direct antitumor effects of the therapy and resultant changes in the immune infiltrate.
Conclusions
Human PDA is infiltrated by large numbers of potentially tumor-reactive effector T cells that show evidence of prior activation. Most of these cells express the co-inhibitory molecule PD-1, however, blockade of its ligand, PD-L1, was unsuccessful in a small number of patients with advanced stage PDA.10 The lack of clinical efficacy of PD-L1 blockade in pancreatic cancer patients suggests that it may be necessary to address the immunosuppressive effects of other PD-1 ligands or block additional co-inhibitory pathways such as lymphocyte activation gene-3 (LAG-3) or T cell immunoglobulin mucin-3 (TIM-3). Furthermore, tumor-immune interactions in PDA liver metastases may differ from those in primary tumors. Our ongoing studies of human tumors will address these questions, possibly strengthening the rationale for immune checkpoint blockade in combination with other measures to unleash local immune responses against pancreatic cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Vonderheide RH, Bayne LJ.. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol 2013; 25:200-5; PMID:; http://dx.doi.org/ 10.1016/j.coi.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. . CD40 Agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612-6; PMID:; http://dx.doi.org/ 10.1126/science.1198443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP., Intratumor T. helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011; 208:469-78; PMID:; http://dx.doi.org/ 10.1084/jem.20101876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N.. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013; 108:914-23; PMID:; http://dx.doi.org/ 10.1038/bjc.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibuya KC, Goel VK, Xiong W, Sham JG, Pollack SM, Leahy AM, Whiting SH, Yeh MM, Yee C, Riddell SR, et al. . Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One 2014; 9:e96565; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0096565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hiraoka N, Onozato K, Kosuge T, Hirohashi S.. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006; 12:5423-34; PMID: [DOI] [PubMed] [Google Scholar]
- 7. Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. . PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-59; PMID:; http://dx.doi.org/ 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. . Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF.. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma tumor microenvironment is driven by CD8 +T Cells. Sci Transl Med 2013; 5:200ra116-6; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. . Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]