Abstract
We designed a phase 1 study using dendritic cells (DCs) pulsed with a mixture of three types of Wilms’ tumor 1 (WT1) peptides, including MHC class I/II restricted epitopes (DC/WT1-I/II). Our recent work reveals that the combination of DC/WT1-I/II and chemotherapy induced long-term WT1-specific CD4+ and CD8+ T cell responses.
Keywords: dendritic cell, helper T cell, MHC class II, WT1
Tumor-associated antigens (TAAs)-specific CD8+ cytotoxic T lymphocytes (CTLs) can eradicate tumor cells expressing TAAs in the context of MHC class I (MHC-I) molecules. Whereas, CD4+ T cells recognize antigenic peptides in association with MHC class II (MHC-II) molecules on antigen presenting cells (APCs) and required for priming and maintenance of TAAs-specific CD8+ CTLs. DCs have been pulsed with various MHC-I-restricted TAAs-derived peptides to induce antigen-specific CD8+ CTLs in preclinical studies because most tumor cells are positive for MHC-I, but negative for MHC-II. However, the clinical responses using MHC-I-restricted peptide pulsed DCs were not as vigorous as in preclinical settings.
It is well known that CD4+ T cells are essential for the priming phase and help CD8+ CTLs to develop by activating APCs through CD40-CD40L interaction and/or production of cytokines such as interleukin (IL)-2 and interferon (IFN)γ.1 Moreover, CD4+ helper T cells also required for maintenance of CD8+ CTLs and the infiltration of CD8+ CTLs at the tumor site.2 A recent study has demonstrated that a single infusion of a clonal population of NY-ESO-1-specific CD4+ T cells resulted in durable complete regression of the tumor.3 We have reported that adoptive transfer of MUC1-specific CD4+ T cells to tumor bearing Rag2−/− mice resulted in prevention of lung metastasis.4 Therefore, it might be essential for designing cancer vaccine targeting both CD4+ helper and CD8+ T cells.
The WT1 has been reported one of the excellent TAAs for the target of immunotherapy, resulting from a ranking based on specificity, oncogenicity, immunogenicity, and therapeutic function.5 Therefore, WT1 has been used for the target of immunotherapy. Interestingly, gemcitabine, standard chemotherapy used most often to treat patients with pancreatic cancer, sensitized the human pancreatic cancer cell lines with WT1-specific CTL responses,6 supporting the significance of the chemoimmunotherapy. We and other groups have shown that WT1-specific immune responses could be induced by WT1-specific and MHC-I restricted peptide combined with chemotherapeutic agents, such as gemcitabine and S-1 for patients with pancreatic cancer.7,8 In the clinical trial, we found the significant association between longer survival and positive delayed-type hypersensitivity (DTH) to WT1 peptide.7 Patients with advanced pancreatic cancer have an especially poor prognosis, with a median survival of 4–6 mo. Therefore, there is great need for a novel therapeutic approach. We recently conducted a phase 1 clinical trial of a combination with chemotherapy and DCs pulsed with a mixture of three types of WT1 peptides, including MHC-I or -II (HLA type of A*02:01, A*02:06, A*24:02, DRB1*04:05, DRB1*08:03, DRB1*15:01, DRB1*15:02, DPB1*05:-01, or DPB1*09:01) restricted epitopes (DC/WT1-I/II) to assess whether chemoimmunotherapy targeting WT1-specific CD4+ and CD8+ T cell responses can induce efficient clinical responses in patients with advanced pancreatic cancer (Fig. 1).9 We detected WT1-specific DTH positive reactions in four of seven patients received DC/WT1-I/II; however, zero of four patients received DC/WT1-I or -II showed the DTH. Moreover, upon vaccination with DC/WT1-I/II, all patients developed circulating highly functional WT1-specific CD4+ and CD8+ T cells that maintained their IFNγ-secreting potential. Moreover, WT1-specific CTLs induced by the DC/WT1-I vaccine were not maintained for the entire duration of the treatment protocol. In contrast, all DTH-positive patients vaccinated with DC/WT1-I/II maintained WT1-specific CTLs during the entire treatment period. Importantly, DC/WT1-I/II vaccinations not only stimulated WT1-specific CTLs but also maintained long-term WT1-specific memory CD8+ T cells. The maintenance of the WT1-specific CTLs may be associated with prolonged survival of patients with pancreatic cancer. On the other hand, the WT1-specific CTLs generated by vaccination with DC/WT1-I may be functionally impaired, resulting in short-lived WT1-specific immune responses. When we compared the clinical outcome of pancreatic cancer patients vaccinated with DC/WT1-I/II with control patients treated with DC/WT1-I or -II, WT1-specific DTH-positive patients who received DC/WT1-I/II exhibited a significant increase in progression-free time (PFT) and overall survival (OS). These findings support the hypothesis that the co-activation of WT1-specific helper T cells upon DC/WT1-I/II stimulates the proliferation and maintenance of functional WT1-specific CTLs, resulted in stable disease (SD) of patients with metastatic pancreatic cancer. In our clinical trial, no complete response (CR) or partial response (PR), but long-term SD was observed. More stable stimulation by DC/WT1-I/II may elicit effector, effector memory, and central memory T cells, which are capable of long-lived WT1 recognition and therefore associate with long-term SD. The response ratio of gemcitabine in advanced pancreatic cancer patients is approximately 10%. Therefore, the long-term SD might be a unique characteristic of DC/WT1-I/II.
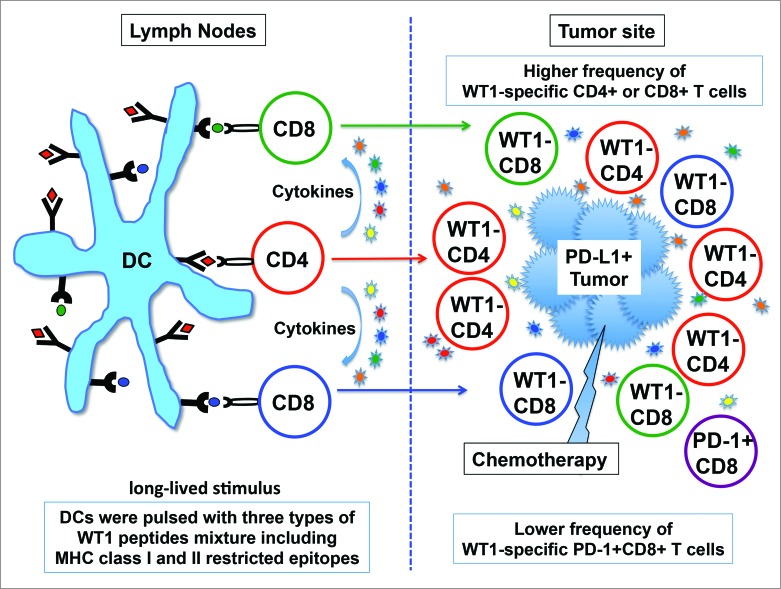
Figure 1.
Induction of WT1-specific CD4+ and CD8+ T cell responses by dendritic cells pulsed with MHC class I/II restricted multiple WT1 peptides. To activate WT1-specific CD4+ helper and CD8+ cytotoxic T cells simultaneously, dendritic cells (DCs) are pulsed with three types of WT1 peptide mixtures restricted by MHC class I/II (DC/WT1-I/II). After injection of DC/WT1-I/II, DC/WT1-I/II migrate to regional lymph nodes, reside in the T cell area, closely interact with T cells, and activate WT1-specific CD4+ and CD8+ T cells. WT1-specific CD4+ T cells provide help to the WT1-specific CD8+ T cells by secreting several cytokines. Programmed death-ligand 1 (PD-L1) on tumor cells is induced by IFNγ, which is predominantly produced by CD4+ helper type 1 cells. The overexpression of PD-L1 on tumor cells and the lower frequency of WT1-specific programmed cell death protein 1 (PD-1)+ CD8+ T cells may be associated with long-term survival.
The best characterized signal for programmed death-ligand 1 (PD-L1) induction is IFNγ, which is predominantly produced by CD4+ helper type 1 cells.10 We observed strong PD-L1 expression of tumor cells in all 2 DTH-strong-positive patients received DC/WT1-I/II examined. The overexpression of PD-L1 on tumor cells and the WT1-specific PD-1+ CD8+ T cells at a little lower frequency that was observed in the super-responders received DC/WT1-I/II may be associated with long-term survival. Chemotherapy, such as gemcitabine treatment, can augment the antitumor effects of cancer vaccines by depleting Tregs and MDSCs, which can potentially enhance the antitumor immune responses. Moreover, an immune checkpoint blockade with antibodies targeting inhibitory immune receptors, such as PD-1 and PD-L1, was used to successfully treat patients with advanced melanoma.10 Therefore, the blockade of multiple immune regulatory checkpoints by antibodies such as PD-1/PD-L1 combined with chemoimmunotherapy targeting WT1 using MHC-I/II restricted peptides may be effective in treating patients with advanced pancreatic cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol 2000; 164:3095-101; PMID:; http://dx.doi.org/ 10.4049/jimmunol.164.6.3095 [DOI] [PubMed] [Google Scholar]
- 2.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, Scott B. Tumor-specific CD4+ T cells have a major "post-licensing" role in CTL mediated anti-tumor immunity. J Immunol 2000; 165:6047-55; PMID:; http://dx.doi.org/ 10.4049/jimmunol.165.11.6047 [DOI] [PubMed] [Google Scholar]
- 3.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C; Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 2008; 358: 2698-703; PMID:; http://dx.doi.org/ 10.1056/NEJMoa0800251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koido S, Enomoto Y, Apostolopoulos V, Gong J. Tumor regression by CD4+ T cells primed with dendritic/tumor fusion cell vaccines. Anticancer Res 2014; 34:8:3917-24; PMID:; http://ar.iiarjournals.org/content/34/8/3917.long [PubMed] [Google Scholar]
- 5.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323-37; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahara A, Koido S, Ito M, Nagasaki E, Sagawa Y, Iwamoto T, Komita H, Ochi T, Fujiwara H, Yasukawa M, et al. Gemcitabine enhances Wilms' tumor gene WT1 expression and sensitizes human pancreatic cancer cells with WT1-specific T-cell-mediated antitumor immune response. Cancer Immunol Immunother 2011; 60:1289-97; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishida S, Koido S, Takeda Y, Homma S, Komita H, Takahara A, Morita S, Ito T, Morimoto S, Hara K, et al. Wilms tumor gene 1 (WT1) peptide-based cancer vaccine combined with gemcitabine for patients with advanced pancreatic cancer. J Immunother 2014; 37:105-14; PMID:; http://dx.doi.org/ 10.1097/CJI.0000000000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaida M, Morita-Hoshi Y, Soeda A, Wakeda T, Yamaki Y, Kojima Y, Ueno H, Kondo S, Morizane C, Ikeda M, et al. Phase 1 trial of Wilms tumor 1 (WT1) peptide vaccine and gemcitabine combination therapy in patients with advanced pancreatic or biliary tract cancer. J Immunother 2011; 34:92-9; PMID:; http://dx.doi.org/ 10.1097/CJI.0b013e3181fb65b9 [DOI] [PubMed] [Google Scholar]
- 9.Koido S, Homma S, Okamoto M, Takakura K, Mori M, Yoshizaki S, Tsukinaga S, Odahara S, Koyama S, Imazu H, et al. Treatment with chemotherapy and dendritic cells pulsed with multiple Wilms’ tumor 1 (WT1)-specific MHC class I/II-restricted epitopes for pancreatic cancer. Clinical Cancer Res 2014; 20: 4228-39; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0314 [DOI] [PubMed] [Google Scholar]
- 10.Zitvogel L, Kroemer G. Targeting PD-1/PD-L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1:1223-5; PMID:; http://dx.doi.org/ 10.4161/onci.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]