Abstract
Tumor antigen (TA)-targeting monoclonal antibody (mAb)-based treatments are considered to be one of the most successful strategies in cancer therapy. Besides targeting TAs and inducing tumor cell death, such antibodies interact with immune cells through Fc-dependent mechanisms to induce adaptive memory immune responses. However, multiple inhibitory/immunosuppressive pathways can be induced by tumor cells to limit the establishment of an efficient antitumor response and consequently a sustained clinical response to TA-targeting mAbs. Here, we provide an overview on how TA-targeting mAbs in combination with conventional cancer therapies and/or inhibitors of key immunosuppressive pathways might represent promising approaches to achieve long-term tumor control.
Keywords: combined therapies, immunomodulation, immunosuppressive pathways, TA-targeting mAbs, vaccine-like effects
Abbreviations: TA, tumor antigen; mAb, monoclonal antibody; FDA, food and drug administration; CTLA4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death 1; ADCC, antibody-dependent cell cytotoxicity; ADCP, antibody-dependent cell phagocytosis; CDC, complement-dependent cytotoxicity; NK, natural killer; FcRn, neonatal Fc receptor; B-NHL, B-cell non-Hodgkin's lymphoma; DC, dendritic cell; ICD, immunologic cell death; Treg, regulatory T cell; MDSC, myeloid-derived suppressor cell; IFNγ, interferon γ; HMGB1, high-mobility group box 1; IDO, indoleamine 2, 3-dioxygenase
Introduction
Cancer immunotherapy relies on several strategies, including antibodies, cytokines, and adoptive cell transfer, to promote tumor cell destruction and develop memory immune responses with the final aim of ensuring the elimination of residual cancer cells and avoiding tumor relapse.1 Among these strategies, advances in antibody engineering and the development of chimeric, humanized and fully human monoclonal antibody (mAbs) have led to the explosive increase of therapeutic antibodies in cancer, particularly of tumor antigen (TA)-targeting mAbs.2 Antibody-based therapies offer many advantages due to the long half-life, good tolerance and broad extracellular fluid bio-distribution of these biomolecules. Since the introduction of the first effective chimeric therapeutic mAbs in oncology (the anti-CD20 antibody rituximab and the HER2 antagonist trastuzumab), mAb-based antitumor treatments have been playing a major role in cancer care and mAbs have been the biggest class of new drugs approved for the treatment of cancer during the last decade.3,4 The market of mAb-based products is currently one of the fastest growing sectors within the biopharmaceutical industry. Indeed, in addition to the 13 antibodies already approved by the FDA for various oncological indications, a pipeline of 165 new anticancer mAbs are in clinical trials: 89 (54%) in phase I, 64 (39%) in phase II and 12 (7%) in phase III studies.5
The immune system has the remarkable ability to detect, eliminate and also “remember” cancer cells and an endogenous immune response against tumor cells has regularly been observed in patients. However, generally, this endogenous antitumor immune response cannot prevent a dramatic clinical outcome. Thus, for curative treatment, immunotherapies must not only limit cancer growth by killing tumor cells, but also strengthen the endogenous immune response in order to establish a long-lasting cancer-specific immunity. Interestingly, several in vivo pre-clinical models support the concept of vaccine-like effects induced by TA-targeting mAb treatments (see below). Moreover, recent observations in patients who have received TA-targeting mAbs indicate that such treatment can immunomodulate the innate and adaptive immunity, leading to immune-mediated tumor cell elimination, in addition to the well-known direct cytotoxic effects (for a review see ref).6 The current challenges are now to precisely understand how TA-targeting mAbs potentiate the immune system and to identify the mechanisms that may limit their immunomodulatory effects in order to better exploit the potential synergy of TA-targeting mAbs in association with other therapeutic agents. In this context, the field of cancer immunotherapy turned a corner in 2011 with the significant clinical success of immune checkpoint blockers (the anti-CTLA4 antibody ipilimumab7 and the anti-PD-1 antibodies nivolumab and lambolizumab8,9) in patients with metastatic melanoma. These results not only demonstrate the crucial role of immune cells within the tumor microenvironment in controlling tumor development, but also better define the inhibitory mechanisms leading to tumor immune escape.
In this review, we will focus on TA-targeting mAb therapy and will discuss the potential of such mAbs to eliminate tumor cells and interact with the endogenous immune system. We will then consider some of the most promising strategies in which the immunomodulatory potential of TA-targeting mAbs is combined with other conventional treatments, such as immune checkpoint blockers or chemotherapy, to achieve synergistic effects and generate a sustained and long-term protective antitumor immune response.
TA-targeting mAbs: more than just direct effects
The idea behind TA-targeting mAb-based immunotherapy is to eliminate cancer cells without harming normal tissues and, therefore, with no or very few side effects. TA-targeting mAbs are composed of two distinct functional units: the antigen binding fragment (Fab) that binds to its specific target molecule expressed on tumor cells, and the constant fragment (Fc) that can initiate the host immune response through interaction with Fc-receptors. For several years, investigators mainly focused on the ability of TA-targeting antibodies to induce tumor cell lysis by engaging well-known immune effector mechanisms, such as antibody-dependent cell cytotoxicity (ADCC),10 antibody-dependent cell phagocytosis (ADCP)11 and complement-dependent cytotoxicity (CDC).12 These mechanisms are crucial for the direct effects of mAbs, particularly for ADCC involving natural killer (NK) cells, macrophages and probably granulocytes. Experimental evidence in Fcγ receptor-deficient mice supports the view that at least part of the antitumor effects of clinically relevant antibodies, such as rituximab (MabThera®), trastuzumab (Herceptin®) and cetuximab (Erbitux®), is mediated via ADCC.13 Based on these observations, strong efforts have been made to manipulate the Fc region. For instance, antibody glyco-engineering, to improve their ADCC and cytotoxicity, and protein-engineering, to increase the Fc domain affinity for the neonatal Fc receptor (FcRn) and thus the antibody half-life, are promising approaches to optimize the direct therapeutic effects of mAbs.14
However, a new concept has recently emerged. In parallel to their direct short-term effects, mAbs are now also considered immunomodulatory molecules that can recruit Fc-receptor-expressing innate immune cells to induce a long-term endogenous adaptive immune response (vaccine-like effect) that is responsible for the better and sustained control of tumor development observed in some patients.
Several clinical observations made in patients with B-cell non-Hodgkin's lymphoma (B-NHL) treated with rituximab argue in favor of such vaccine-like effects. First, the better efficacy of rituximab in patients carrying the high affinity variant of the IgG FcγRIIIa, which displays increased ADCC, compared to those with the low affinity variant, strongly suggests that host immune components contribute to the mAb protective effects.15,16 Then, a phase II clinical study on the effect of rituximab alone or combined with interferon α-2a showed that this combination might improve the rate of long-term molecular complete remission and prolong relapse-free survival.17 Moreover, rituximab-induced lysis of lymphoma cells promotes the uptake and cross-presentation of lymphoma-cell peptides, leading to the generation of a cytotoxic T lymphocyte response in vitro.18 This finding reinforces the hypothesis that the efficacy of TA-targeting mAbs may rely on their capacity to activate the adaptive effector immune response.
Nevertheless, the proof of concept for a vaccine-like effect induced by TA-targeting mAb treatments was obtained in preclinical mouse models. Mouse xenograft models are generally used for the preclinical evaluation of mAbs; however, they cannot take into account the participation of the adaptive immunity to the therapeutic effects of mAbs because of the immunodeficiency of the mouse strains used. Thus, the first demonstration that the endogenous adaptive immunity was essential for tumor regression after TA-targeting mAb treatment was provided by Stagg et al. in immunocompetent BALB/c mice bearing established TUBO (mammary cancer cells) tumors and treated with anti-TRAIL-R2 and/or anti-ErbB-2 mAbs.19 In this model, depletion of CD8+ T cells resulted in primary and secondary tumor relapse, demonstrating the role of the mAb-induced antitumor adaptive immunity in the control of tumor development. Another study showed that an initial treatment with anti-CD20 mAbs induces Fc-dependent protection against human EL4A cells that express CD20 and allows immunocompetent mice to survive after a second tumor challenge.20 Using this model, the authors clearly demonstrated that the long-term protective effect of the mAb therapy requires CD4+ T cells both at the beginning of the treatment and at the time of the new tumor challenge. A third study, using immunocompetent mice bearing HER2/neu-positive tumors, showed that treatment with an anti-neu mAb induces immunological memory and protection against a new tumor challenge that rely on the contribution of the cytotoxic response by IFNγ+ tumor-specific CD8+ T lymphocytes.21 Other works focused on mAbs that target infected cells, rather than TA like in the previous studies, in a retroviral-induced leukemia mouse model. Specifically, Gros et al. showed that short treatment of infected animals with an anti-Env neutralizing mAb leads to the induction of long-lasting (>1 year) protective antiviral immunity, allowing the treated mice to survive and to resist to a new viral challenge a long time after the therapeutic mAb has disappeared. The survival of such mice depends on the endogenous protection based on both humoral22,23 and cellular24 antiviral responses. The development of these immune responses is not the result of the direct reduction of the viral load by the mAb that would permit the immune system reaction, but of a bona fide immunomodulatory effect of the mAb through its interaction with the IgG Fc-receptors.25,26 Indeed these long-term protective effects depend on two Fc-dependent mechanisms: (i) the activation of dendritic cells (DC) by cellular immune complexes composed of the administered mAb and infected cells26 and (ii) the inhibition of the regulatory T cell expansion, which is normally observed in untreated animals.27
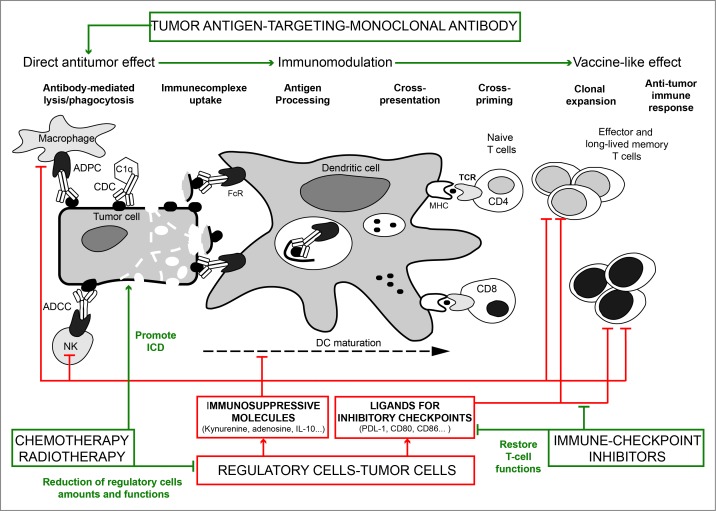
Altogether, these data suggest that mAbs targeting TA or viral antigens in infected cells can interact with the host immune system in a Fc-dependent manner to induce an adaptive memory immune response for long-lasting protection in vivo (Table 1). These results represent a real shift in our understanding of the mechanisms of action of therapeutic TA-targeting mAbs and open exciting perspectives for their use in combination with classical anticancer therapies, such as chemotherapy, radiotherapy and/or specific anticancer drugs (see below). Indeed, anticancer mAbs should no longer be considered as therapeutics with only immediate and direct effects, but should rather be qualified as “immunobiomodulators” based on their ability to recruit cells of the immune system and create a favorable environment at the tumor site to stimulate the adaptive immunity (Fig. 1). Although several preclinical models convincingly demonstrated that long-lasting antitumor protection can be achieved in vivo, these observations need now to be extended with new experimental and clinical data. Particularly, the precise cellular and molecular mechanisms involved in the achievement of the long-term effects following prophylactic or curative immunotherapies with TA-targeting mAbs need to be better understood. The concept that the therapeutic efficacy of different anticancer therapies relies also on their capacity to re-stimulate the patient's immune surveillance and not only on their tumor cell killing efficiency has already been demonstrated for chemotherapeutics agents, although their design and selection was initially based only on their direct cytostatic and cytotoxic effects toward malignant cells.28,29 The current understanding of the immune surveillance mechanisms leads us to speculate that, depending on the tumor type and the targeted TA, a key step for the establishment of long-lasting protective effects following treatment with TA-targeting mAbs might be their capacity to induce tumor cell death in such a way to make the dying cells visible to the immune system. The notion of immunogenic cell death (ICD) has been very well described in response to chemo- and radio-therapy treatments; however, to our knowledge, it has never been investigated in the context of TA-targeting mAbs.
Table 1.
Evidence of vaccine-like effects after TA-targeting mAb treatment and involved mechanisms.
| Evidence of vaccine-like effects | |
| Humoral response induced and maintained | (22, 25, 26) |
| Cellular response induced and maintained | (20, 21, 24, 26) |
| Long-lasting protection against challenge | (20, 21, 24, 26) |
| Adoptive transferable protection | (20–23) |
| Cellular mechanisms involved | |
| Fc-mediated and isotype-dependent | (20, 21, 25, 26) |
| Tregs inhibition | (27) |
| T cell recruitment in the tumor | (21) |
| Modification of tumor environment | (21) |
| Cellular immune complex formation | (26) |
| Specific CTL functions improved | (21, 24, 26) |
| Cell populations involved | |
| Macrophages (early stage) | (19) |
| CD4+pos (early stage and late protection) | (20) |
| CD8+pos (late protection) | (19–21, 24) |
| Dendritic cells (early stage) | (26) |
Abbreviations: CTL, cytotoxic T lymphocytes; mAb, monoclonal antibody; TA, tumor-antigen; Tregs, regulatory T cells.
Figure 1.
Combinatorial strategies based on an immunotherapy using TA-targeting mAbs for the development of long-term antitumor immunity. TA-targeted mAbs form immune complexes with tumor cells and recruit innate immune cells through Fc/FcγR interaction. This leads to the lysis and elimination of tumor cells by ADCC, CDC and ADPC via recruitment of NK cells, complement and macrophages, respectively, and to the release of cellular immune complexes (cIC) composed of tumor cell debris and TA-targeting mAbs. Together with danger signals, uptake of these cIC by DCs improves antigen presentation and cross-priming and ultimately leads to the generation of tumor-specific effector T cells. Both innate and adaptive immune responses in the tumor are potentially impaired by immunosuppressive/inhibitory mechanisms. Immunosuppressive molecules, such as IL-10 or adenosine, directly inhibit T cell proliferation, the cytolytic functions of NK cells and CD8+ T cells and DC survival. Kynurenine and adenosine also promote the recruitment of regulatory cells, such as Tregs, M2 macrophages or MDSC in the tumor. Expression by tumor cells of ligands (PDL-1, CD8+0, CD8+6…) for inhibitory receptors or checkpoints (PD-1, CTLA-4…) down-modulate the amplitude of T cell activation and limit the antitumor immune response of effector T cells. Thus, neutralizing immunosuppressive molecules and blocking the immune checkpoints represent key strategies to restore the effector functions of T cells and achieve a long-lasting antitumor immune response following TA-targeting mAb treatment. Another combinatorial approach is based on the use of ICD-promoting therapies, such as chemotherapy and radiotherapy. The release of danger signals, such as ATP, HMGB1 and expression of calreticulin, may synergize with TA-targeting mAbs to favor antigen presentation, DC maturation, cross-priming and development of tumor-specific effector T cells. Through their capacity to reduce the amount or inhibit the functions of regulatory cells, some chemotherapeutic agents will also facilitate the establishment of a protective antitumor response. TA, tumor antigen; mAb monoclonal antibody; ADCC, antibody-dependent cell cytotoxicity; ADCP, antibody-dependent cell phagocytosis; CDC, complement-dependent cytotoxicity; NK, natural killer; CTLA4, cytotoxic T-lymphocyte-associated protein 4; PD-1, programmed cell death 1; cIC; cellular immune complexes; Fc/FcγR, Fc/Fcγ receptor; ICD, immunologic cell death; DC, dendritic cell; MDSC, myeloid-derived suppressor cell; Tregs, regulatory T cells; HMGB1, high-mobility group box 1; MHC, major histocompatibility complex; TCR, T cell receptor; PDL-1, programmed death ligand 1; IL-10, interleukin 10.
Furthermore, as tumor cells can elicit immunosuppressive and inhibitory signals to escape the endogenous antitumor immunity, the long-term antitumor immune protection induced by TA-targeting mAbs could be limited also by their inability to overcome the tumor immune escape mechanisms. Thus, it will be essential to determine the impact of TA-targeting mAbs on the immunosuppressive and inhibitory mechanisms known to limit the endogenous immune responses. Indeed, although it does not seem to be the case in the pre-clinical models described above, such mechanisms may constrain the antitumor immune response initiated by TA-targeting immunotherapy. This particular phase of tumor immune escape is characterized by an increase in immunosuppressive signals from regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSC) and M2-polarized macrophages, but also by the presence of poorly functional or exhausted T cells that specifically express inhibitory receptors (or immune checkpoints).30,31 Thus, it will be essential to determine whether the neutralization of some of these immunosuppressive signals is required for the long-lasting effects of TA-targeting mAbs, for instance by combining agents to target both tumor and immune cells within the tumor microenvironment.
Combining therapeutic strategies to achieve long-term control of tumor growth with TA-targeting mAbs
The goals of mAb-based immunotherapy are the destruction of tumor cells and the establishment of an efficient antitumor immunological memory response. The main reasons of the poor long-term efficacy of immunotherapy are mostly the lack of tumor immunogenicity and the strong inhibitory/immunosuppressive signals from the tumor environment. To overcome these two key factors of cancer progression, TA-targeting mAb treatments may need to be combined with other therapeutics.
Specifically, TA-targeting mAbs could be associated with treatments that induce ICD to strengthen the stimulation of the innate immunity. For this purpose, chemo- and radio-therapy appear to be good candidates. Indeed, it has been recently highlighted that they can stimulate the antitumor immunity through the release or the expression of damage-associated molecular signals (HMGB1, Calreticulin, ATP) that favor the recruitment and differentiation of antigen-presenting cells32 and optimal TA presentation.33-35 For instance, the efficacy of trastuzumab (anti-HER-2 mAb) and cetuximab (anti-EGFR mAb), which induce antigen presentation through the formation of immune complexes,36,37 is enhanced when combined with radio- or chemo-therapy, thus supporting the concept of a combinatorial therapeutic approach.38 Several trials have also demonstrated that rituximab (anti-CD20 mAb) is more efficient and safe when combined with the CHOP (cyclophosphamide, hydroxy doxorubicin, vincristine, prednisone) chemotherapy regimen (R-CHOP) for the treatment of diffuse large B cell lymphoma.39,40 Another interesting combination might be with vemurafenib, a specific BRAF inhibitor recently approved for treatment of some melanomas. Vemurafenib enhances the expression of several TAs, thus enabling their immune recognition by T lymphocytes.41 Therefore, it may have interesting synergistic effects in combination with TA-targeted mAbs.
However, whether associating chemotherapy and mAbs really improves the antitumor immune response remains unclear and controversial. For example, the combination of cetuximab or panitumumab (anti-EGFR mAb) with oxaliplatin, as first-line treatment of colorectal cancer in patients with wild type KRAS, does not improve the survival benefit and response rate.42 This suggests that combined therapies are much more than just the addition of two separate effects and that each drug could affect the efficacy and/or toxicity of the other. Interestingly, using BALB/c mice bearing TUBO tumors, Park et al. demonstrated that depending on the timing of administration, chemotherapeutic drugs have a different impact on the antitumor immunity induced by treatment with TA-targeting mAbs. Specifically, they found that paclitaxel and anti-HER2/neu mAbs have a synergistic effect only if chemotherapy is administered before and not after the mAb treatment.21 Indeed, when administered one day before the mAbs, paclitaxel not only synergized with the anti-HER2/neu mAbs to control the primary tumor, but also preserved the long-lasting protection. Conversely, mice, in which paclitaxel was administered after the mAbs, were less resistant to a subsequent tumor challenge. As the half-life of paclitaxel is rather short, the authors speculated that there might only be a window of time when this chemotherapeutic drug may effectively reduce the tumor burden without inhibiting the antibody-induced immunity. Altogether, these observations underline that more preclinical studies are needed to identify which type of chemical drugs can be combined with TA-targeting mAbs as well as their dosage and timing of administration to obtain synergistic effects.
A second strategy is based on targeting the inhibitory pathways (immune checkpoints) that limit the antitumor immune response. The mAb against CTLA4 (a transmembrane receptor expressed predominantly by T cells) was the first successful immunotherapy drug to target a T cell inhibitory signaling pathway approved by the FDA in 2011 for the treatment of patients with advanced melanoma.7 Recent preclinical findings offer evidence that the anti-CTLA4 mAb synergizes with several chemotherapeutic agents (ixabepilone, paclitaxel, etoposide and gemcitabine) in various mouse tumor models (fibrosarcoma, breast, lung and colon cancer). These synergistic effects are associated with an increase of activated CD4+ and CD8+ T cells and a decrease of MDSCs within tumor-draining lymph nodes, indicating a potential effect on the antitumor immune response.43 Other immune inhibitory receptors, such as PD1 and TIM-3, have been identified and the efficacy of mAbs against these factors has been validated in preclinical tumor models.44-46 Both PD-1 and Tim-3 are involved in dysfunction or suppression of the cytotoxic CD8+ T-cell-mediated immune response.47,48 The PD-1/PD-L1 axis is also involved in tumor-specific CD4+ effector cell tolerization.49 Interestingly, it has been shown that chemotherapy-induced inflammation suppresses CD4+ T-cell response through the PD1/PD-L1 axis and that disrupting this pathway augments the efficacy of chemotherapy with durable antitumor effects.50 Several studies also support the relevance of Tim3 blockade for treatment of various cancer types and remarkable synergistic effects have been observed in combined therapies that target Tim3 and PD-1 (for a review see ref).51 These interesting observations raise the question of whether antibodies/inhibitors targeting CTLA4, PD-1/PD-L1, Tim3 or other inhibitory checkpoints might also increase the therapeutic efficacy of TA-targeting mAb-based immunotherapy. To our knowledge only one study suggests that it might be the case. Indeed Stagg J et al. recently demonstrated that associating trastuzumab with an anti-PD-1 mAb enhances the benefits of the immunomodulatory effect of the anti-HER2/ErbB2 mAb in the MMTV-ErbB-2 transgenic pre-clinical tumor model.52
A limitation of TA-targeting mAb treatment efficiency might also come from the presence of regulatory cell populations within the tumor microenvironment. Although mAb-mediated targeting of infected cells in the retroviral-induced leukemia model described above can limit the expansion of Tregs,27 it is obvious that Tregs and other immunosuppressive cells can limit the long-term efficacy of mAb treatments through inhibition of the antitumor immune response. These immunosuppressive mechanisms, usually observed in highly aggressive cancers, may indeed counteract the immune cell network activated by TA-targeting mAbs and impair their immunomodulatory functions. Consequently, the exhaustive identification of immunosuppressive pathways is a necessary step for designing therapeutic combinations to achieve durable antitumor protective immunity. In this context, one strategy that definitively deserves further investigations is the blockade of molecules within immunosuppressive enzymatic pathways, such as the tryptophan catabolic enzyme indoleamine 2, 3-dioxygenase (IDO) and the CD39/CD73 ectonucleotidases.
IDO is expressed by tumor cells, but also by cells of the immune system, such as macrophages and DCs.53 It has been proposed to be a key contributor of immunosuppression in many malignancies, including melanoma, colorectal, pancreatic, gastric, brain, lung, bladder and ovarian cancer,54 and clinical studies indicate that high IDO expression by tumor cells correlates with poor clinical outcome. IDO catabolizes the essential amino acid tryptophan, leading to the production of the bioactive metabolite kynurenine that inhibits effector T cells and activates Tregs.55 Therefore, blocking IDO activity represents an interesting therapeutic strategy to increase the antitumor response and hinder tumor progression. Indeed, pharmacological inhibition of IDO with 1-methyl-tryptophan (1MT) stimulates the T-cell dependent antitumor response in mouse models and delays tumor outgrowth, but cannot trigger complete tumor regression on its own.56 However, when combined with chemotherapeutic drugs or radiotherapy, the antitumor effect is more pronounced. Whether IDO inhibitors may also favor the antitumor immune response primed by TA-targeted mAb immunotherapy has never been tested. Finally, recent data demonstrated that IDO deficiency/inhibition and blockade of immune checkpoints, such as CTLA-4 and PD-1/PD-L1, synergize to control tumor outgrowth and enhance the overall survival in different tumor models.57,58 Based on these results, clinical trials are currently assessing the efficacy of such combined therapies for the treatment of melanoma (see https://ClinicalTrials.gov identifier NCT01604889).
CD39 is the main ectonucleotidase expressed by selected cell populations, such as Tregs and myeloid-derived suppressive cells,59 and contributes to their immunosuppressive functions. CD39 hydrolyzes extracellular ATP and ADP into AMP.60,61 AMP is then processed into adenosine, a critical regulator of both innate and adaptive immune responses, by the ecto-5′-nucleotidase CD73. Upon binding to A2A receptors expressed by effector CD8+ T lymphocytes, NK cells, DC, macrophages and granulocytes, adenosine induces the accumulation of intracellular cAMP and inhibits the effector functions of all these cells.62-64 Adenosine also limits the therapeutic effectiveness of anti-CTL-4 mAbs.65 Furthermore, extracellular ATP, released by dying tumor cells following chemotherapy, is essential for the recruitment and differentiation of DC precursors into mature DCs with the capacity of presenting tumor-associated antigens that will initiate the antitumor immune response. Interestingly, chemotherapeutic agents fail to elicit an immunogenic response in cancer cells that overexpress CD39, if CD39 is not blocked by pharmacologic inhibitors.66 In summary, as CD39 inhibition can induce both immunogenic tumor cell death and prevent adenosine-mediated immunosuppression by immunoregulatory cells, the association of CD39 inhibitors with TA-targeting mAbs could be an attractive strategy to strengthen the mAb-mediated antitumor immune response and ultimately consolidate the clinical response. It is important to note that inhibition of the adenosine pathway with a selective inhibitor of CD73 or of the A2A receptor significantly improves the antitumor immune response and markedly inhibits tumor growth in mice treated with anti-CTLA-465 or anti-PD-1 mAbs.67 This latest finding clearly shows that these two pathways are not overlapping and that adenosine limits the therapeutic efficacy of anti-CTLA-4 mAbs.
Such synergistic effects are encouraging in view of optimizing strategies with TA-targeting mAbs. We expect that activation of innate immune cells by immune complexes formed by mAbs and tumor cells could be enhanced by the concomitant action of molecules that target several immunosuppressive pathways and of ICD-promoting therapies. This could favor a better antigen presentation, leading to a faster and stronger early immune response responsible for the activation and expansion of specific antitumor T lymphocytes and the establishment of an antitumor memory response that reduces the risk of relapse.
Conclusions
Tumor antigen-specific immune responses result from a complex dynamic interplay between the host immune cells and tumor cells. Often, escape mechanisms developed by tumor cells lead to the lack of a sustained clinical response in patients following TA-targeting mAb-based immunotherapy. An efficient antitumor immune response is certainly linked to the capacity of treatments to avoid concurrent inhibitory mechanisms. Therefore, combinatory therapies may be required for optimal therapeutic effect (Fig. 1). “Combination therapy is the way of the future,” said Marc Mansour, chief operating officer at the Canadian vaccine developer Immunovaccine, in November 2013. The clear clinical success of the so-called “immune checkpoint” blockers, a new type of immunotherapeutics that can circumvent the inhibitory tumor-dependent pressure, in patients with melanoma opens obvious perspective for combined anticancer therapies. One of the main challenges will be to combine therapeutic approaches that disable the immunological brake with TA-targeting mAbs to recruit effector cells in the tumor environment and induce activation of the immune system. The use of chemotherapeutic drugs to stimulate the immunogenicity of tumor cells targeted by mAbs should also be considered, because it has already been shown that such combination improves the efficacy of TA-targeting mAb treatments in some pre-clinical mouse models. These promising combinatorial approaches should offer strong opportunities to increase the effectiveness of biomolecules through synergistic effects that ultimately generate a sustained antitumor immunity and vaccine-like antitumor effects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported by institutional grants from INSERM and Université de Montpellier 1 and by a specific grant from the LabEx MabImprove. H-A Michaud is supported by a fellowship from the Fondation pour la Recherche Médicale (FRM).
References
- 1. DeVita VT, Jr., Rosenberg SA. Two hundred years of cancer research. N Engl J Med 2012; 366:2207-14; PMID:; http://dx.doi.org/10.1056/NEJMra1204479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol 2006; 6:343-57; PMID:; http://dx.doi.org/10.1038/nri1837 [DOI] [PubMed] [Google Scholar]
- 3. Reichert JM. Antibodies to watch in 2014. MAbs 2014; 6:5-14; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Monoclonal antibodies in cancer therapy. Oncoimmunology 2013; 2:e22789; PMID:; http://dx.doi.org/10.4161/onci.22789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reichert JM, Dhimolea E. The future of antibodies as cancer drugs. Drug Discov Today 2012; 17:954-63; PMID:; http://dx.doi.org/10.1016/j.drudis.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 6. Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:; http://dx.doi.org/10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 7. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:; http://dx.doi.org/10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:; http://dx.doi.org/10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013; 369:134-44; PMID:; http://dx.doi.org/10.1056/NEJMoa1305133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hubert P, Amigorena S. Antibody-dependent cell cytotoxicity in monoclonal antibody-mediated tumor immunotherapy. Oncoimmunology 2012; 1:103-5; PMID:; http://dx.doi.org/10.4161/onci.1.1.17963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winiarska M, Glodkowska-Mrowka E, Bil J, Golab J. Molecular mechanisms of the antitumor effects of anti-CD20 antibodies. Front Biosci (Landmark Ed) 2011; 16:277-306; PMID:; http://dx.doi.org/10.2741/3688 [DOI] [PubMed] [Google Scholar]
- 12. Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res 2010; 20:34-50; PMID:; http://dx.doi.org/10.1038/cr.2009.139 [DOI] [PubMed] [Google Scholar]
- 13. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:; http://dx.doi.org/10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 14. Monnet C, Jorieux S, Souyris N, Zaki O, Jacquet A, Fournier N, Crozet F, de Romeuf C, Bouayadi K, Urbain R, et al. Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody. MAbs 2014; 6:422-36; PMID:; http://dx.doi.org/10.4161/mabs.27854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002; 99:754-8; PMID:; http://dx.doi.org/10.1182/blood.V99.3.754 [DOI] [PubMed] [Google Scholar]
- 16. Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003; 21:3940-7; PMID:; http://dx.doi.org/10.1200/JCO.2003.05.013 [DOI] [PubMed] [Google Scholar]
- 17. Kimby E, Jurlander J, Geisler C, Hagberg H, Holte H, Lehtinen T, Ostenstad B, Hansen M, Osterborg A, Linden O, et al. Long-term molecular remissions in patients with indolent lymphoma treated with rituximab as a single agent or in combination with interferon alpha-2a: a randomized phase II study from the Nordic Lymphoma Group. Leuk Lymphoma 2008; 49:102-12; PMID:; http://dx.doi.org/10.1080/10428190701704647 [DOI] [PubMed] [Google Scholar]
- 18. Selenko N, Majdic O, Jager U, Sillaber C, Stockl J, Knapp W. Cross-priming of cytotoxic T cells promoted by apoptosis-inducing tumor cell reactive antibodies? J Clin Immunol 2002; 22:124-30; PMID:; http://dx.doi.org/10.1023/A:1015463811683 [DOI] [PubMed] [Google Scholar]
- 19. Stagg J, Sharkey J, Pommey S, Young R, Takeda K, Yagita H, Johnstone RW, Smyth MJ. Antibodies targeted to TRAIL receptor-2 and ErbB-2 synergize in vivo and induce an antitumor immune response. Proc Natl Acad Sci U S A 2008; 105:16254-9; PMID:; http://dx.doi.org/10.1073/pnas.0806849105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood 2010; 116:926-34; PMID:; http://dx.doi.org/10.1182/blood-2009-10-248609 [DOI] [PubMed] [Google Scholar]
- 21. Park S, Jiang Z, Mortenson ED, Deng L, Radkevich-Brown O, Yang X, Sattar H, Wang Y, Brown NK, Greene M, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010; 18:160-70; PMID:; http://dx.doi.org/10.1016/j.ccr.2010.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gros L, Dreja H, Fiser AL, Plays M, Pelegrin M, Piechaczyk M. Induction of long-term protective antiviral endogenous immune response by short neutralizing monoclonal antibody treatment. J Virol 2005; 79:6272-80; PMID:; http://dx.doi.org/10.1128/JVI.79.10.6272-6280.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gros L, Pelegrin M, Plays M, Piechaczyk M. Efficient mother-to-child transfer of antiretroviral immunity in the context of preclinical monoclonal antibody-based immunotherapy. J Virol 2006; 80:10191-200; PMID:; http://dx.doi.org/10.1128/JVI.01095-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gros L, Pelegrin M, Michaud HA, Bianco S, Hernandez J, Jacquet C, Piechaczyk M. Endogenous cytotoxic T-cell response contributes to the long-term antiretroviral protection induced by a short period of antibody-based immunotherapy of neonatally infected mice. J Virol 2008; 82:1339-49; PMID:; http://dx.doi.org/10.1128/JVI.01970-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasser R, Pelegrin M, Michaud HA, Plays M, Piechaczyk M, Gros L. Long-lasting protective antiviral immunity induced by passive immunotherapies requires both neutralizing and effector functions of the administered monoclonal antibody. J Virol 2010; 84:10169-81; PMID:; http://dx.doi.org/10.1128/JVI.00568-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Michaud HA, Gomard T, Gros L, Thiolon K, Nasser R, Jacquet C, Hernandez J, Piechaczyk M, Pelegrin M. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog 2010; 6:e1000948; PMID:; http://dx.doi.org/10.1371/journal.ppat.1000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nasser R, Pelegrin M, Plays M, Gros L, Piechaczyk M. Control of regulatory T cells is necessary for vaccine-like effects of antiviral immunotherapy by monoclonal antibodies. Blood 2013; 121:1102-11; PMID:; http://dx.doi.org/10.1182/blood-2012-06-432153 [DOI] [PubMed] [Google Scholar]
- 28. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:; http://dx.doi.org/10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 29. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014; 21:15-25; PMID:; http://dx.doi.org/10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011; 331:1565-70; PMID:; http://dx.doi.org/10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 31. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013; 14:1014-22; PMID:; http://dx.doi.org/10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity 2013; 39:211-27; PMID:; http://dx.doi.org/10.1016/j.immuni.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 33. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID:; http://dx.doi.org/10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- 34. Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 2012; 31:1062-79; PMID:; http://dx.doi.org/10.1038/emboj.2011.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:; http://dx.doi.org/10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 36. Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, Bestoso E, Apollinari S, Mannucci S, Marra M, et al. Cetuximab +/- chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer 2012; 130:1577-89; PMID:; http://dx.doi.org/10.1002/ijc.26181 [DOI] [PubMed] [Google Scholar]
- 37. Kim PS, Armstrong TD, Song H, Wolpoe ME, Weiss V, Manning EA, Huang LQ, Murata S, Sgouros G, Emens LA, et al. Antibody association with HER-2/neu-targeted vaccine enhances CD8+ T cell responses in mice through Fc-mediated activation of DCs. J Clin Invest 2008; 118:1700-11; PMID:; http://dx.doi.org/10.1172/JCI34333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006; 354:567-78; PMID:; http://dx.doi.org/10.1056/NEJMoa053422 [DOI] [PubMed] [Google Scholar]
- 39. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood 2010; 116:2040-5; PMID:; http://dx.doi.org/10.1182/blood-2010-03-276246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell R, Jaeger U, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011; 12:1013-22; PMID:; http://dx.doi.org/10.1016/S1470-2045(11)70235-2 [DOI] [PubMed] [Google Scholar]
- 41. Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213-9; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-10-0118 [DOI] [PubMed] [Google Scholar]
- 42. Zhou SW, Huang YY, Wei Y, Jiang ZM, Zhang YD, Yang Q, Xie DR. No survival benefit from adding cetuximab or panitumumab to oxaliplatin-based chemotherapy in the first-line treatment of metastatic colorectal cancer in KRAS wild type patients: a meta-analysis. PLoS One 2012; 7:e50925; PMID:; http://dx.doi.org/10.1371/journal.pone.0050925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jure-Kunkel M, Masters G, Girit E, Dito G, Lee F, Hunt JT, Humphrey R. Synergy between chemotherapeutic agents and CTLA-4 blockade in preclinical tumor models. Cancer Immunol Immunother 2013; 62:1533-45; PMID:; http://dx.doi.org/10.1007/s00262-013-1451-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spranger S, Gajewski T. Rational combinations of immunotherapeutics that target discrete pathways. J Immunother Cancer 2013; 1:16; PMID:; http://dx.doi.org/10.1186/2051-1426-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ngiow SF, Teng MW, Smyth MJ. Prospects for TIM3-Targeted Antitumor Immunotherapy. Cancer Res 2011; 71:6567-71; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-11-1487 [DOI] [PubMed] [Google Scholar]
- 46. Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-gamma-mediated antitumor immunity and suppresses established tumors. Cancer Res 2011; 71:3540-51; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-11-0096 [DOI] [PubMed] [Google Scholar]
- 47. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 2010; 207:2175-86; PMID:; http://dx.doi.org/10.1084/jem.20100637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fourcade J, Sun Z, Pagliano O, Chauvin JM, Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S, et al. PD-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8(+) T cells induced by melanoma vaccines. Cancer Res 2014; 74:1045-55; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-13-2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, Munn DH, Zhou G. Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood 2012; 120:2229-39; PMID:; http://dx.doi.org/10.1182/blood-2011-12-398321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ding ZC, Lu X, Yu M, Lemos H, Huang L, Chandler P, Liu K, Walters M, Krasinski A, Mack M, et al. Immunosuppressive Myeloid Cells Induced by Chemotherapy Attenuate Antitumor CD4+ T-Cell Responses through the PD-1-PD-L1 Axis. Cancer Res 2014; 74:3441-53; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-13-3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Anderson AC. Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res 2014; 2:393-8; PMID:; http://dx.doi.org/10.1158/2326-6066.CIR-14-0039 [DOI] [PubMed] [Google Scholar]
- 52. Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 2011; 108:7142-7; PMID:; http://dx.doi.org/10.1073/pnas.1016569108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9:1269-74; PMID:; http://dx.doi.org/10.1038/nm934 [DOI] [PubMed] [Google Scholar]
- 54. Sucher R, Kurz K, Weiss G, Margreiter R, Fuchs D, Brandacher G. IDO-Mediated Tryptophan Degradation in the Pathogenesis of Malignant Tumor Disease. Int J Tryptophan Res 2010; 3:113-20; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest 2007; 117:1147-54; PMID:; http://dx.doi.org/10.1172/JCI31178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med 2005; 11:312-9; PMID:; http://dx.doi.org/10.1038/nm1196 [DOI] [PubMed] [Google Scholar]
- 57. Castro MG, Baker GJM, Lowenstein PR. Blocking immunosuppressive checkpoints for glioma therapy: the more the merrier! Clin Cancer Res 2014; 20:5147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013; 210:1389-402; PMID:; http://dx.doi.org/10.1084/jem.20130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 2013; 32:1743-51; PMID:; http://dx.doi.org/10.1038/onc.2012.269 [DOI] [PubMed] [Google Scholar]
- 60. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257-65; PMID:; http://dx.doi.org/10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, Centonze D, Bernardi G, Dell'Acqua ML, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007; 110:1225-32; PMID:; http://dx.doi.org/10.1182/blood-2006-12-064527 [DOI] [PubMed] [Google Scholar]
- 62. Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013; 13:842-57; PMID:; http://dx.doi.org/10.1038/nrc3613 [DOI] [PubMed] [Google Scholar]
- 63. Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 1997; 90:1600-10; PMID: [PubMed] [Google Scholar]
- 64. Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res 2006; 66:7758-65; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-06-0478 [DOI] [PubMed] [Google Scholar]
- 65. Iannone R, Miele L, Maiolino P, Pinto A, Morello S. Adenosine limits the therapeutic effectiveness of anti-CTLA4 mAb in a mouse melanoma model. Am J Cancer Res 2014; 4:172-81; PMID: [PMC free article] [PubMed] [Google Scholar]
- 66. Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology 2012; 1:393-5; PMID:; http://dx.doi.org/10.4161/onci.19070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013; 19:5626-35; PMID:; http://dx.doi.org/10.1158/1078-0432.CCR-13-0545 [DOI] [PubMed] [Google Scholar]