Abstract
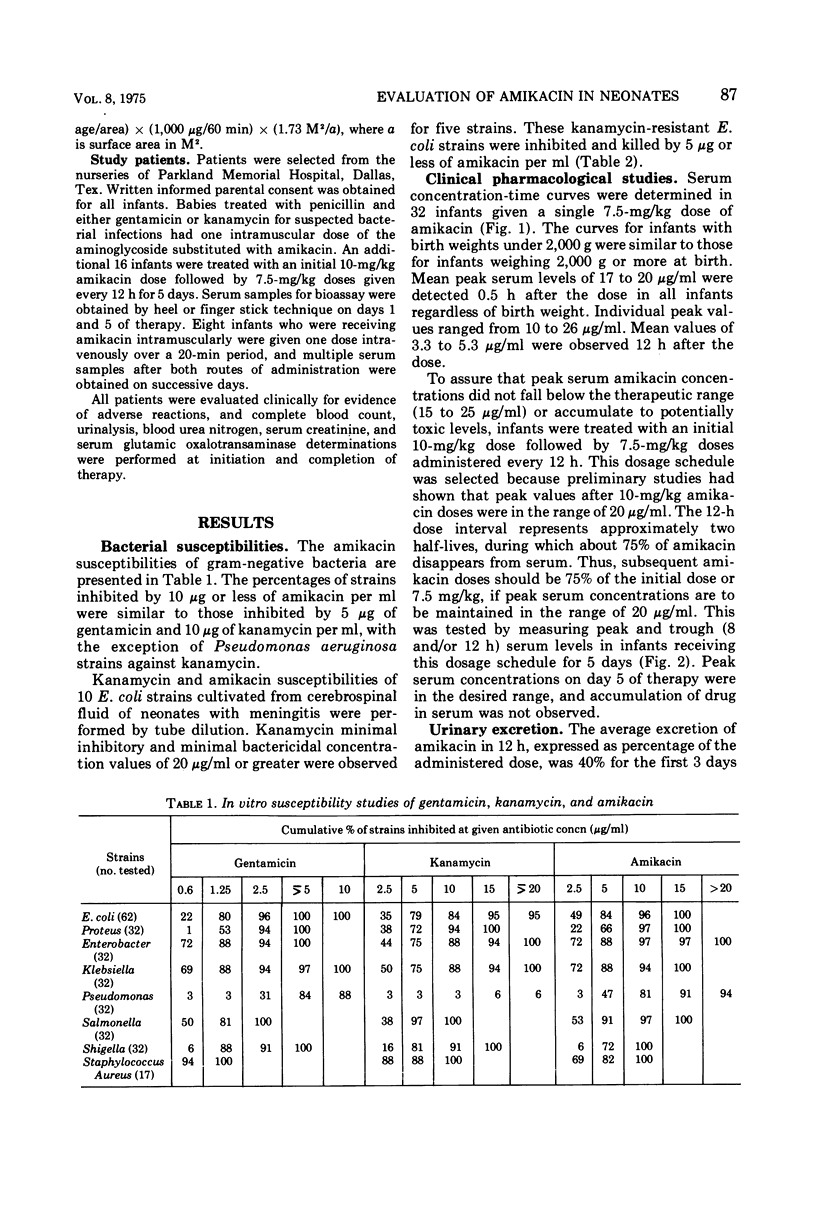
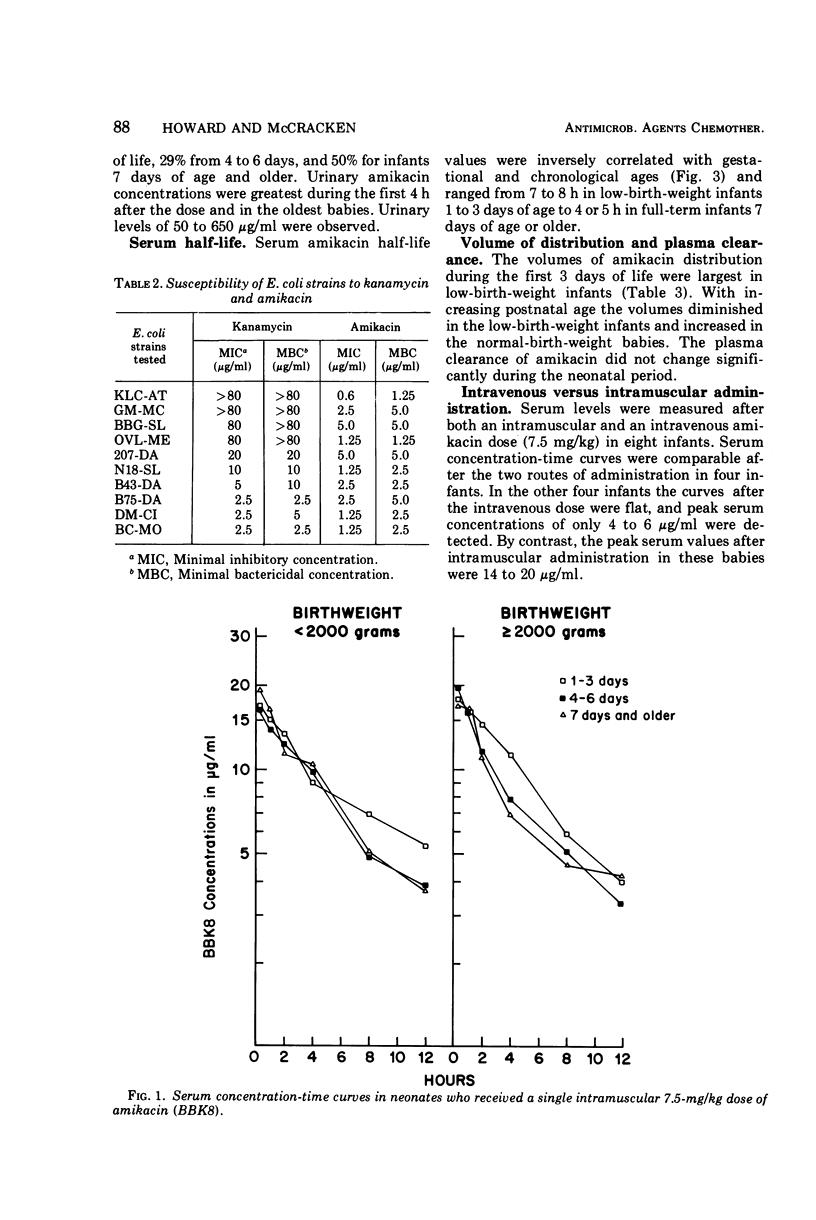
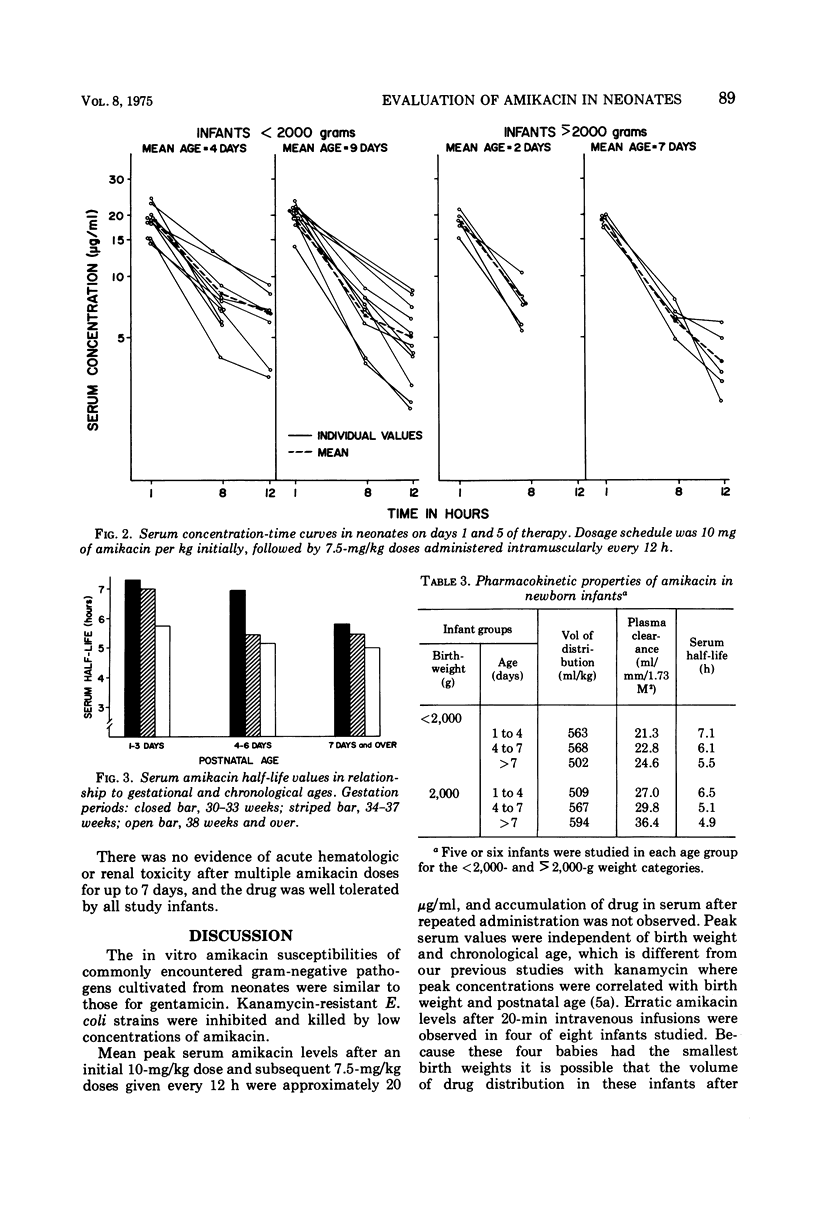
The in vitro bacterial susceptibilities and pharmacokinetic properties of amikacin (BB-K8) were studied in newborn infants. Gram-negative bacteria and Staphylococcus aureus isolated from neonates were uniformly susceptible to 10 μg or less of amikacin per ml, and five Escherichia coli strains resistant to kanamycin were inhibited and killed by 5 μg or less of amikacin per ml. Mean peak serum concentrations of 17 to 20 μg/ml were observed 30 min after 7.5-mg/kg amikacin doses, and accumulation of drug in serum was not detected after repeated doses for 5 to 7 days. Intravenous infusion of amikacin over a 20-min period resulted in extremely low peak serum levels in four of eight infants studied. Serum half-life values were correlated inversely with postnatal age and renal clearances of amikacin. The volumes of drug distribution indicate that amikacin remains primarily in the extracellular fluid space of neonates. The lack of efficacy and safety data preclude the use of amikacin in neonates at this time.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassady G. Bromide space studies in infants of low birth weight. Pediatr Res. 1970 Jan;4(1):14–24. doi: 10.1203/00006450-197001000-00002. [DOI] [PubMed] [Google Scholar]
- Cassady G. Plasma volume studies in low birth weight infants. Pediatrics. 1966 Dec;38(6):1020–1027. [PubMed] [Google Scholar]
- Clarke J. T., Libke R. D., Regamey C., Kirby W. M. Comparative pharmacokinetics of amikacin and kanamycin. Clin Pharmacol Ther. 1974 Jun;15(6):610–616. doi: 10.1002/cpt1974156610. [DOI] [PubMed] [Google Scholar]
- Haltalin K. C., Markley A. H., Woodman E. Agar plate dilution method for routine antibiotic susceptibility testing in a hospital laboratory. Am J Clin Pathol. 1973 Sep;60(3):384–394. doi: 10.1093/ajcp/60.3.384. [DOI] [PubMed] [Google Scholar]
- Howard J. B., McCracken G. H., Jr Reappraisal of kanamycin usage in neonates. J Pediatr. 1975 Jun;86(6):949–956. doi: 10.1016/s0022-3476(75)80234-4. [DOI] [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P. K., Washington J. A., 2nd Comparative in vitro activity of three aminoglycosidic antibiotics: BB-K8, kanamycin, and gentamicin. Antimicrob Agents Chemother. 1973 Aug;4(2):133–139. doi: 10.1128/aac.4.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]


