Abstract
Objective
to investigate whether there is a relationship between plasmatic levels of nitrate, body temperature, and blood pressure values in patients with sepsis, severe sepsis and septic shock.
Method
prospective observational study performed in a Brazilian hospital; data were collected from July to December 2009. Thirty blood samples were obtained from a total of 29 patients. Blood samples (10ml) were collected for subsequent laboratory analysis to determine nitrate levels in the plasma.
Results
nitric oxide synthesis is increased in patients with septic shock, and is inversely correlated to the body temperature values.
Conclusion
these data show that the measurement of body temperature and the observation of hypothermic conditions in septic patients could be important to guide the nursing regarding the evolution of individuals with sepsis to septic shock.
Keywords: Sepsis, Endotoxemia, Body Temperature, Nitric Oxide Synthase
Introduction
In intensive care units, Septic shock is a serious and progressive complication which has a very high mortality rate (30-90%). Its main characteristic is peripheral arteriolar vasodilatation, which causes decreased systemic vascular resistance, high cardiac output, hypotension, and inadequate tissue perfusion, as a consequence of bacterial infection(1).
Nurses working in areas such as clinical care, surgical care and intensive care, require specific training to recognize symptoms of sepsis before they progress to the most common complication, septic shock. Dedicated monitoring can help prevent this potential risk in the medical-surgical settings, especially through the evaluation of vital signs. This practice is recommended by current consensus practice guidelines, as well as evidence-based practice(2).
The pathophysiology of sepsis, severe sepsis and septic shock, includes a complex role played by cytokines - reactive oxygen species, such as nitric oxide (NO). It has been established by Salomao and colleagues that NO generation is increased in septic shock when compared to septic or severe septic patients, and its persistence is associated with a poor outcome(3).
Septic shock has been frequently associated with body temperature disturbances and with a sharp drop in blood pressure, partly explained by the induction of vasoactive enzymes. The enzyme inducible nitric oxide synthase (iNOS) is rapidly expressed in the vascular smooth muscle component of arteries and veins(1). Under these conditions, very high levels of the vasodilator gas nitric oxide (NO) are formed locally, rendering the vessels hyporesponsive to the constrictor mechanisms(1). Thermoregulatory responses to sepsis can induce a febrile state, hypothermia, or a mixture of hypothermia and a febrile state. Fever is the most common thermoregulatory symptom of sepsis, however, hypothermia may also occur in the critical state of shock and it is believed that this significantly aggravates the prognosis of the patient(4).
Thermoregulatory alterations have been attributed to the synthesis of nitric oxide (NO) throughout the body, which is driven by the nitric oxide synthase enzyme (NOS). The first function attributed to NO as a biological mediator was its capability to produce vasodilatation - denominated endothelium-derived relaxing factor(5). However, numerous studies have suggested other possible functions for NO; one of them being its involvement in the control of body temperature during physiological and pathological conditions. In experiments with rats and rabbits, NO has demonstrated its participation as a pyretic mediator in LPS-induced fever, as the intravenous administration of L-NAME (N G-nitro-L-arginine methyl ester, a non-specific NOS inhibitor) produced a decrease in body temperature and the suppression of the febrile state(6). Conversely, the intracerebroventricular administration of NO from donors, reduced the fever in rabbits, which could suggest an antipyretic role for NO acting in the central nervous system (CNS)(7).
There are nursing studies regarding how patients respond to diseases or adapt to changes, utilizing data and observations derived from the clinical practice and clinical studies. However, further studies are needed to clarify the hypothesis that NO may help control body temperature and arterial blood pressure in humans during sepsis. Therefore, regarding the participation of NO in the control of cardiovascular function and thermoregulation during sepsis, the goal of this study was to correlate the NO plasmatic levels with body temperature and blood pressure in patients with sepsis, severe sepsis and septic shock.
Materials and methods
Patients
The study was approved by the Ethics Committee of the Hospital das Clínicas, University of Sao Paulo, Ribeirao Preto, Brazil (protocol number HCRP 7433/2008). A total of 29 patients with a clinical diagnosis of sepsis, severe sepsis or septic shock were enrolled in this vital signs monitoring study, following the criteria of the American College of Chest Physicians/Society of Critical Care Medicine - Consensus Conference(1). The patients in the first 72 hours of sepsis symptoms, or 48 hours after the first organ dysfunction (severe sepsis) or refractory hypotension with intravenous fluid infusion (septic shock) were included. Thirty samples were used in this study: seven patients with sepsis, five with severe sepsis and eighteen with septic shock. Some patients were not included in the study for the following reasons: less than 18 years of age, in the terminal stage of a disease (i.e., cancer or AIDS), receiving experimental therapy, with a perceived imminent threat of death, or dying. The primary sources of infection were: lungs (15), urinary tract (3) abdomen (2), and others (9). Sixteen patients died: 3 with sepsis, 1 with severe sepsis and 12 with septic shock.
Data collection
Blood tests were performed using 10ml blood samples taken from venous or arterial access; in the case of patients that did not have an adequate venous network, the blood was taken from the radial, brachial or femoral arteries. In some patients the blood was collected through the invasive blood pressure device so as not to require a puncture. Three samples were collected for each patient, with 12 hour intervals between each collection. Only one patient progressed from severe sepsis to septic shock. Thus, for this patient, six blood samples were collected (three samples at the stage of sepsis and three samples at the stage of severe sepsis), also considering the period of 12 hours between collections.
All patients were in contact isolation for some resistant microorganisms. An apron, goggles and gloves were used when handling blood collection or evaluating vital signs. According to positive blood cultures, the patients were treated with specific antibiotics (some of them with more than one antibiotic). The majority of the patients (22) were under continuous sedation and had an endotracheal tube or plastic tracheostomy tube for mechanical ventilation; patients who were in septic shock were also receiving vasoactive drugs (noradrenaline, dopamine or dobutamine) to maintain their blood pressure levels.
In addition to the diagnosis of sepsis, severe sepsis or septic shock, there were other diagnoses. One patient had a confirmed diagnosis of influenza A (H1N1) virus. Sixteen of the thirty patients died.
The vital signs were checked at the same time that the blood was collected. A thermometer (manual mercury column - Incoterm) was used to measure the body temperature. The thermometer was placed in the axillary region and remained in place for approximately five minutes to measure the body temperature. Other parameters, such as heart rate, oxygen saturation and blood pressure, were obtained through the patients' cardiac monitors; the monitor brands were Datex-Ohmeda and Dixtal DX 2010.
Plasma nitrate measurement
To specifically control the synthesis of NO, the plasma nitrate levels were measured, and blood samples were taken from a peripheral vein each time the body temperature was measured. A volume of 10ml of blood was collected in plastic tubes containing heparin, centrifuged for 20 min at 2000 g at 4ºC for plasma separation then stored in eppendorfs tubes at -70ºC prior to dosage.
Total nitrate was determined using the Sievers Nitric Oxide Analyzer system. Plasma samples were deproteinized using cold absolute ethanol and were injected into a reaction vessel containing vanadium trichloride (VCl3), which converts nitrate to NO. The NO produced was detected by ozone induced by chemiluminescence. Plasma peak values of NO samples were determined using a standard curve constructed with sodium nitrate solutions of various concentrations (5, 10, 25, 50, 100 and 1000 µM).
Data analysis
Results were expressed as means (SD). A statistical analysis was performed on these data using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparisons test. The Pearson correlation analysis was used to measure the correlations between HOMA-IR and nitrate plasma concentration. Values of p<0.05 were considered to be significant.
Results
In this study 29 patients were included with a total of 30 samples (100% samples). Of the 30 samples, 7 (22.58%) were diagnosed with sepsis, 5 (19.35%) with severe sepsis and 18 (58.06%) with septic shock.
Figure 1 shows the body temperature values (1a) and plasma nitrate levels (1b) of the three groups: sepsis, severe sepsis and septic shock. No significant difference was found between patients with sepsis, severe sepsis, and septic shock. However, nitrate plasma levels were significantly higher in septic shock patients (p<0.05) when compared to patients with sepsis and severe sepsis.
Figure 1.
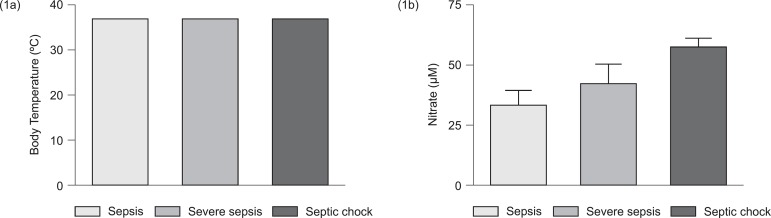
Body temperature values (1a) and plasma nitrate levels (1b) of the three groups: sepsis, severe sepsis, and septic shock
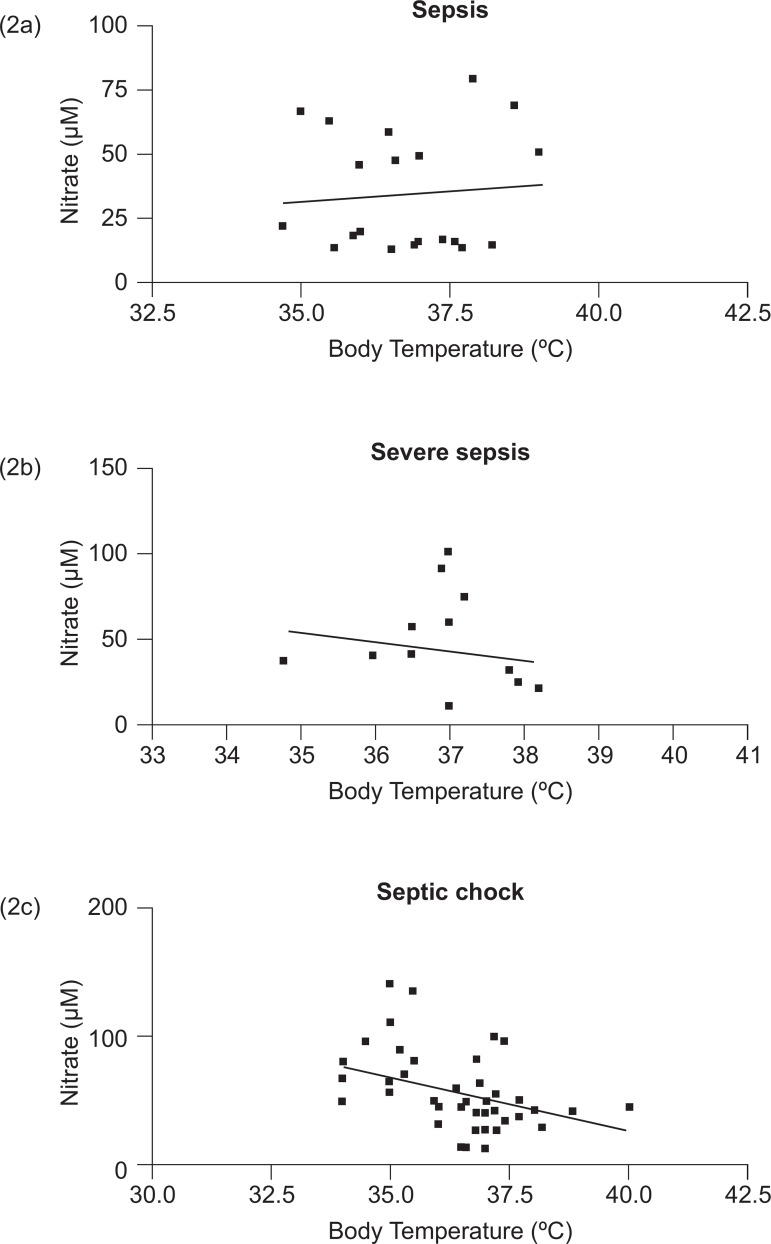
Figure 2 shows the correlation between body temperature and nitrate levels in patients with sepsis (2a), severe sepsis (2b), and septic shock (2c). No correlation was found between body temperature and nitrate plasma levels in septic and severe septic patients. However, there was a significant correlation between these parameters when the patients with septic shock were analyzed (Pearson coefficient -0.3991; p=0.0037 and r2 =0.1593).
Figure 2.
Correlation between body temperature and nitrate levels in patients with sepsis (2a), severe sepsis (2b), and septic shock (2c)
No significant difference was found in blood pressure among individuals of the three groups (sepsis, severe sepsis and septic shock). However, a tendency toward decreased blood pressure was observed in the septic shock group.
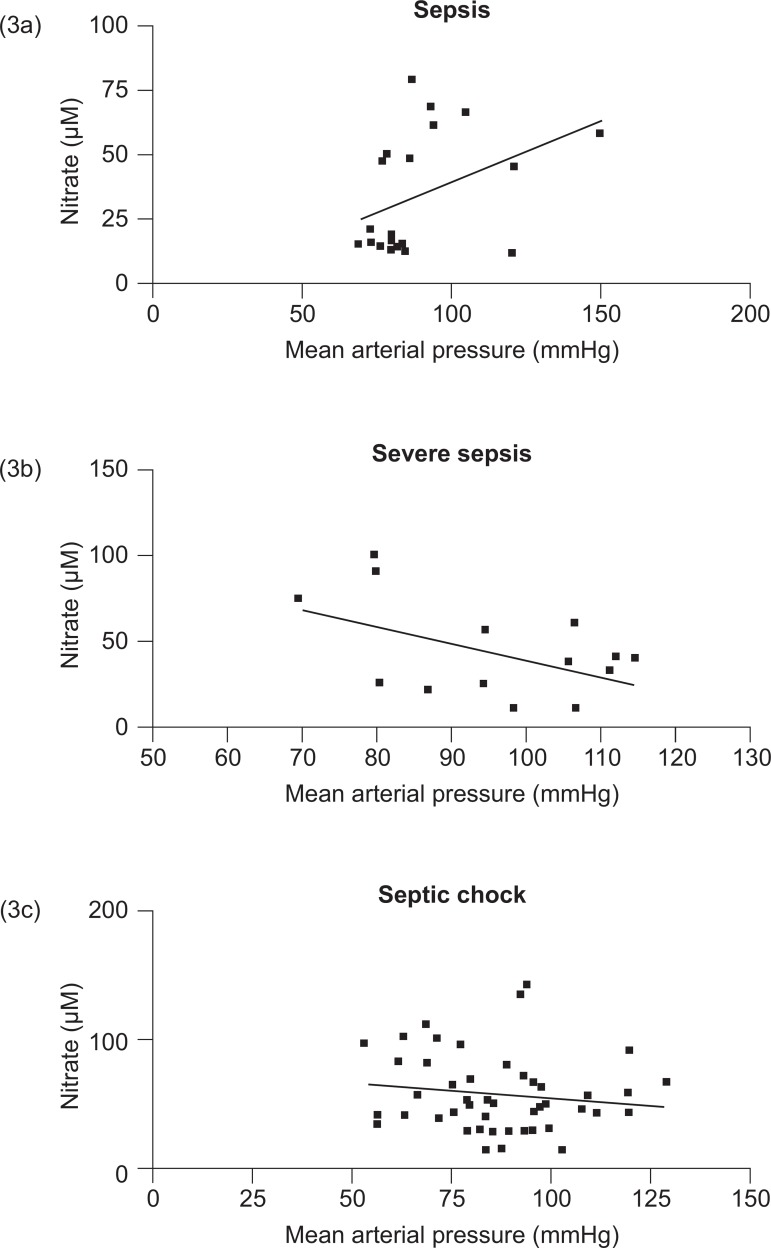
Figure 3 shows the correlation between mean arterial pressure and nitrate levels in patients with sepsis (3a), severe sepsis (3b), and septic shock (3c). No significant correlation was found between these parameters in sepsis, severe sepsis, or septic shock patients.
Figure 3.
Correlation between mean arterial pressure and nitrate levels in patients with sepsis (3a), severe sepsis (3b), and septic shock (3c)
Discussion
This study demonstrated the negative correlation between body temperature values and plasma nitrate levels in patients diagnosed with septic shock. The monitoring of patients with endotoxemia requires the participation of nurses with the ability to recognize the signs and symptoms of sepsis before it progresses to the septic shock diagnosis. Careful monitoring can prevent potential risk, mainly through the monitoring of vital sign values. This practice is widely recommended by recent clinical practice(2).
Septic shock results from a conflict between the pathogen and the immune system of the host(8). This conflict induces an intense inflammatory response, culminating in the synthesis of excessive nitric oxide, which has both beneficial and detrimental effects on the body(8). It is known that nitric oxide has extensive bactericidal activity. When NO is produced through the activation of inducible nitric oxide synthase enzyme (iNOS) - present mainly in immune cells (such as macrophages and neutrophils), it can lead to the nitrosylation of the bacterial membrane(9). In addition to its action in the immune system, nitric oxide can be synthesized in other tissues of the body through the action of other subtypes of the nitric oxide synthase enzyme on its main substrate, L-arginine(4). The first function attributed to nitric oxide as a biological mediator was demonstrated through its ability to induce vasodilation, initially designated as endothelium-derived relaxing factor(5).
During sepsis, the excessive production of nitric oxide by immune cells can induce severe hypotension resistant to vasoconstriction therapy, resulting in generalized organ failure(1). However, recent studies have revealed another function of nitric oxide when participating in body temperature control in physiological and pathophysiological conditions. Scammell demonstrated that nitric oxide acts as a mediator in endotoxin-induced fever in rats. The administration of nitric oxide synthesis inhibitors reduced the body temperature and as a consequence diminished the febrile response in these animals(6). Conversely, the administration of drugs capable of donating nitric oxide (such as sodium nitroprusside), when given within the lateral cerebral ventricle, can reduce fever, suggesting a central antipyretic role for nitric oxide(7).
Sepsis and septic shock are associated with changes in the body temperature balance. Thermoregulatory responses to sepsis can induce fever, hypothermia or a combination of both. Fever is a well-known and expected response during sepsis; however, hypothermia, which can occur in cases of shock, is believed to be an aggravating factor in the patient's prognosis(4). Beneficial effects of fever include: a) reduction of bacterial growth, b) reduced viral replication, c) enhanced host response by increasing the activity of leukocyte infiltration and activation of natural killer cells, d) activation of T cells, and e) the production of cytokines by mononuclear cells. Fever also decreases hemoglobin's affinity for oxygen, which can facilitate the distribution of oxygen to tissues. Furthermore, fever can mitigate the effects of the endotoxin(10).
Despite the fact that hypothermia has a neuroprotective effect in some patients, the protection mechanism and deleterious effects are not fully understood, particularly in cases of infection. Several authors suggest that hypothermia, in cases of severe infection, denotes the inability of the body to continue its fight against the infective agent. However, other studies suggest that hypothermia is the body's controlled response, in which the regulation point of the hypothalamus is changed, thus promoting decreased production and increased loss of heat(6).
Final considerations
This study demonstrated that individuals with septic shock have a plasma concentration of nitrate significantly higher than those with a diagnosis of sepsis. It also suggested an increase in nitric oxide synthesis, induced by the action of the immune system. A tendency to decrease the body temperature in patients with septic shock was also observed, although it was not statistically significant. It is believed that this reflects the use of antipyretic drugs in febrile individuals. Conversely, no correlation between arterial blood pressure and nitric oxide plasma concentration was observed. When the correlation between body temperature values and plasma nitrate levels was analyzed, it was observed that there was a significant negative correlation between these two parameters in individuals diagnosed with septic shock. The lower the body temperature, the greater the concentration of nitric oxide produced by the immune system of these patients.
The main conclusion of this study is that body temperature is inversely correlated with concentrations of nitric oxide in patients with septic shock. That is, the lower the body temperature, the higher the concentrations of nitric oxide. This biological regulator is a highly cytotoxic free radical; therefore, a rise in body temperature could be an indication that the patient is worsening. It is hoped that this study will encourage the assessment and evaluation of vital signs in these patients, with this guidance being particularly directed toward the nursing team.
References
- 1.Bone RC, Sibbald WJJ, Sprung C. The ACCP-SCCM Definitions of sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano KK. Continuous physiologic monitoring and the identification of sepsis: what is the Evidence supporting current clinical practice? AACN Adv Crit Care. 2006;17:215–223. [PubMed] [Google Scholar]
- 3.Santos SS, Brunialty MK, Rigato O, Machado FR, Silva E, Salomão R. Generation of nitric oxide and reactive oxygen species by neutrophis and monocytes from septic patients and association with outcomes. Shock. 2012;38:18–23. doi: 10.1097/SHK.0b013e318257114e. [DOI] [PubMed] [Google Scholar]
- 4.Theilen H, Ragaller M. Therapy of hyperthermia and septic shock. Necessary or injurious? Anaesthesiology. 2007;6:949–952. doi: 10.1007/s00101-007-1211-z. [DOI] [PubMed] [Google Scholar]
- 5.Moncada S, Palmer RMJ, Higgs EA. Nitric oxide: physiology, pathophysiology and pharmacology.[Review] Pharmacology. 1991;43:109–142. [PubMed] [Google Scholar]
- 6.Scammell TE, Elmquist JK, Saper CB. Inhibition of nitric oxide synthase produces hypothermia and depresses lipopolysaccharide fever. Am J Physiol. 1996;271:R333–R338. doi: 10.1152/ajpregu.1996.271.2.R333. [DOI] [PubMed] [Google Scholar]
- 7.Saia RS, Oliveira-Pelegrin GR, da Silva ME, Aguila FA, Antunes-Rodrigues J, Rocha MJ, Cárnio EC. Neonatal endotoxin exposure changes neuroendocrine, cardiovascular function and mortality during polymicrobial sepsis in adult rats. Regul Pept. 2011;169:21–30. doi: 10.1016/j.regpep.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Groeneveld AB, Tacx AN, Bossink AW, van Mierlo GJ, Hack CE. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin Immunol. 2003;106:106–115. doi: 10.1016/s1521-6616(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 9.Victor VM, Espulges JV, Hernandez-Mjiares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets. 2009;9:376–389. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- 10.Fuhong S, Nam DN, Zhen W, Ying C, Peter R, Jean-Louis V. Fever control in septic shock: beneficial or harmful? Shock. 2005;23:516–520. [PubMed] [Google Scholar]