Abstract
CAR therapy has shown promise in treating cancer, but at the cost of unexpected toxicity against normal tissues, not predicted by preclinical testing. We are working to generate more physiologically relevant models for preclinical CAR toxicity testing, and in doing so, have discovered that CAR therapy induces immunogenic cell death, with the potential for cures.
Keywords: adoptive cell therapy, cancer immunology, chimeric antigen receptor, gene-engineering, glioblastoma, tumor immunotherapy
Glioblastoma (GBM) is the most common primary malignant brain tumor and is a universally fatal disease, with a dismal prognosis of less than 15 months of survival from diagnosis.1 The current standard of care includes tumor resection, radiation, and chemotherapy. This conventional treatment is inherently nonspecific and is profoundly detrimental to patient quality of life. Curative treatments that drastically limit or prevent toxicity altogether remain the ultimate goal, and this objective has only recently gained traction as a clinically pragmatic approach for GBM with the emergence of immunotherapy.
Adoptive cell therapy (ACT) of lymphocytes targeting tumor antigens have been remarkably effective in so-called ‘liquid tumors’, including chronic lymphocytic leukemia and acute lymphoblastic leukemia.2 The treatment of solid cancers, however, presents additional challenges, including immunosuppressive tumor microenvironments and physical barriers to tumor access behind ‘immune privileged’ sites. Chimeric antigen receptors (CARs) represent an emerging ACT-based technology that circumvents several mechanisms of tumor immune evasion. They are not prone to MHC downregulation by tumors, and the latest generation of CARs can elicit costimulatory signaling without the need for exogenous ligands, which are frequently absent in malignant tumors. Although CARs have already proven their clinical promise against several cancers, early patient trials have also shed light on their potent side-effects and deadly potential when targeting antigens that are not exclusively expressed by tumors.3 The difference between administering a naked antibody and of tying a T-cell effector function to the same antibody became tragically apparent when the first patient to receive HER2/ERBB2 CAR engineered T cells suffered rapid and catastrophic multi-organ failure.4 This outcome stands in stark contrast to the thousands of patients who have been treated with adjuvant anti-HER2 mAbs with low incidence of side-effects, proving the potency of T cell responses to even relatively low levels of target antigen expressed on normal cells. These early observations have proven the critical importance of target selection, and that currently, the identification of tumor-specific targets remains the biggest impediment to the safe and effective application of CARs against a wider spectrum of cancers. This is perhaps of greatest importance when designing novel therapies against tumors residing in the brain.
The type III variant of the epidermal growth factor receptor, EGFRvIII, is considered a truly tumor-specific antigen that is commonly expressed on the cell surface of GBM, lung, breast, and ovarian cancers, but is completely absent on healthy tissues.1,5 We recently developed murine and human third-generation (CD3ζ.CD28.4-1BB) CARs targeting the EGFRvIII mutation (EGFRvIII+ CARs) to evaluate their ability to traffic to the brain, localize to invasive tumor deposits, and treat intracranial (IC) gliomas.6-8 One useful way of studying this therapy for translational purposes is to take advantage of xenogeneic model systems, wherein primary human GBM tumors can be implanted orthotopically into immune-deficient NOD/SCID/common gamma-chain−/- (NSG) mice. In a recent publication, we described the in vivo growth characteristics of the D-270MG xenograft, which demonstrates the microscopic development of GBM more accurately than commonly used glioma cell lines such as U87MG.7 Whereas U87MG produce IC tumors with well-defined boundaries, D-270MG tumors exhibit expanding borders, radiating cells from masses, and subarachnoid infiltration of tumor cells. We used this model to treat IC D270MG tumor-bearing NSG mice systemically with EGFRvIII+ CARs. We monitored localization of both tumor and EGFRvIII+ CARs using substrate-specific luciferase imaging in vivo, and confirmed our findings with immunohistochemistry on fixed specimens. Our data demonstrated that systemically delivered CARs can efficiently traffic into the CNS, migrate to areas of invasive tumor, and mediate killing to enhance overall survival compared to controls.7
The D-270MG/NSG mouse model provided a proof-of-principle supporting CAR therapy against tumors residing in the brain, putting to rest the conventional notion that the presence of the blood-brain barrier and ‘immune-privileged’ state of the CNS would impede CAR-mediated tumor killing. Based on this data, we evaluated CAR therapy in a syngeneic tumor model system, which afforded us the opportunity to study the impact of EGFRvIII+ CARs in the context of an intact endogenous immune system.
In a manuscript recently published in Clinical Cancer Research, we utilized the VM/Dk immune-competent mouse model with orthotopic implants of SMA560.EGFRvIII tumor, a spontaneously arisen murine astrocytoma cell line.8 We treated 3-5 day established subcutaneous and IC tumors with systematically delivered EGFRvIII+ CARs in a dose-dependent manner, and observed cures at the highest doses. We found this treatment required prior host lymphodepletion (5Gy irradiation), and could be blocked in vivo by systemic delivery of excess soluble EGFRvIII peptide. Although EGFRvIII is a tumor-specific mutation, our use of soluble peptide remains an important proof-of-strategy for abrogating off-target toxicity for CARs directed against targets co-expressed in normal tissues, making it a potentially useful clinical safety feature.
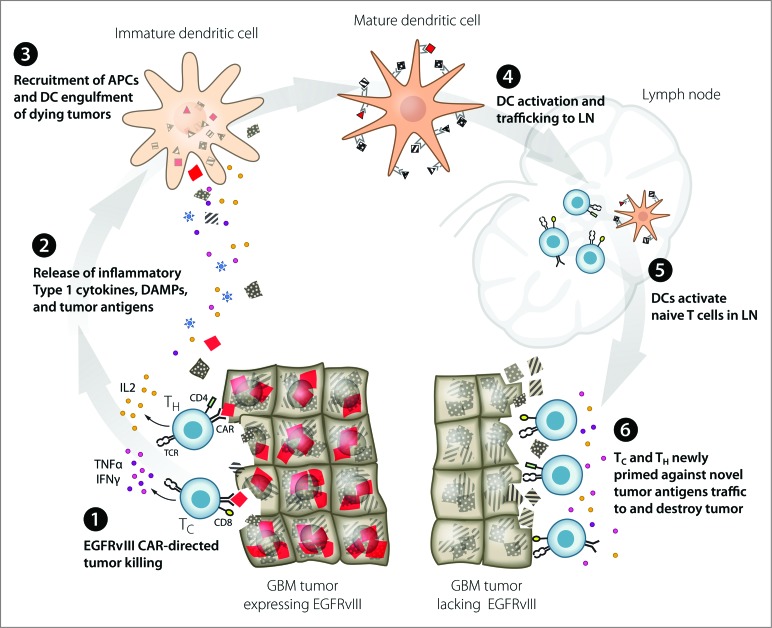
Our most intriguing finding, however, was the discovery that cured CAR-treated mice were able to mount a protective immune response, not only to rechallenge with the same tumor, but also to the EGFRvIII-negative parental tumor.8 This suggests that CAR therapy in an immune-intact subject is sufficient to generate additional de novo immunity against additional tumor antigens, a phenomenon referred to as epitope spreading.9 Inherent in this finding is the implication of CAR-mediated immunogenic tumor cell death (ICD). ICD is believed to result in the release of proinflammatory cytokines and production of danger signals to stimulate an endogenous immune response to additional tumor antigens obtained from dying tumor cells (Fig. 1).10 This finding demonstrates CAR T-cell therapy could be a potentially curative treatment for patients with cancer.
Figure 1.
Proposed model: EGFRvIII CAR modified T-cell immunotherapy of GBM leads to immunogenic cell death and epitope spreading. EGFRvIII+ CAR targeted destruction of GBM [1] results in release of immunostimulatory cytokines by T cells and antigens and DAMPs by dying tumor cells [2]. APCs are recruited to the site of tumor destruction where they engulf dying tumor cells and become activated by DAMPs [3]. Mature DCs expressing multiple MHC-restricted tumor antigens traffic to the LN [4] where they prime naïve T cells recognizing novel tumor antigens [5]. Newly activated T cells traffic to tumor and destroy GBM based upon recognition of novel tumor antigens [6]. APC, antigen presenting cell; CAR, chimeric antigen receptor; DAMP, danger associated molecular pattern; DC, dendritic cell; EGFRvIII, epidermal growth factor receptor variant 3; GBM, glioblastoma multiforme; IFNɣ, interferon gamma; IL2, interleukin-2; LN, lymph node; TC, cytolytic T cell; TH, helper T cell; TNFα, tumor necrosis factor α.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Chandramohan V, Mitchell DA, Johnson LA, Sampson JH, Bigner DD. Antibody, T-cell and dendritic cell immunotherapy for malignant brain tumors. Future Oncol 2013; 9:977-90; PMID: ; http://dx.doi.org/10.2217/fon.13.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 2014; 123:2625-35; PMID: ; http://dx.doi.org/10.1182/blood-2013-11-492231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013; 39:49-60; PMID: ; http://dx.doi.org/10.1016/j.immuni.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther: J Am Soc Gene Ther 2010; 18:843-51; PMID: ; http://dx.doi.org/10.1038/mt.2010.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi BD, Archer GE, Mitchell DA, Heimberger AB, McLendon RE, Bigner DD, Sampson JH. EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol 2009; 19:713-23; PMID: ; http://dx.doi.org/10.1111/j.1750-3639.2009.00318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, Johnson LA, Davis JL, Zheng Z, Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum Gene Ther 2012; 23:1043-53; PMID: ; http://dx.doi.org/10.1089/hum.2012.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miao H, Choi BD, Suryadevara CM, Sanchez-Perez L, Yang S, De Leon G, Sayour EJ, McLendon R, Herndon II JE, Healy P, et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PloS One 2014; 9:e94281; PMID: ; http://dx.doi.org/10.1371/journal.pone.0094281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson JH, Choi BD, Sanchez-Perez L, Suryadevara CM, Snyder DJ, Flores CT, Schmittling RJ, Nair SK, Reap EA, Norberg PK, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res: Off J Am Assoc Cancer Res 2014; 20:972-84; PMID: ; http://dx.doi.org/10.1158/1078-0432.CCR-13-0709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilon SA, Kelly C, Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol 2003; 170:1202-8; http://dx.doi.org/10.4049/jimmunol.170.3.1202 [DOI] [PubMed] [Google Scholar]
- 10.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID: ; http://dx.doi.org/10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]