Abstract
RIG-I-like helicases (RLH) are cytosolic sensors for viral RNA inducing type I interferon production. We found that pancreatic cancer cells express functional RLH and are susceptible to RLH-induced apoptosis via intrinsic and extrinsic pathways. Tumor cells displayed features of immunogenic cell death resulting in dendritic cell activation, enhanced antigen cross-presentation and efficient tumor control in vivo.
Keywords: fas, immunogenic cell death, pancreatic cancer, RIG-I-like helicases, type I IFN
Abbreviations: ATP, adenosine triphosphate; CD, cluster of differentiation; CTL, cytotoxic T lymphocytes; CXCL10, C-X-C motif chemokine 10; DC, dendritic cell; HMGB1, high-mobility group box protein I; hsp70, heat shock protein 70; IFN, interferon; IRF, interferon regulatory factor; MAPK, mitogen-activated protein kinase; MDA5, melanoma differentiation-associated protein 5; MDSC, myeloid-derived suppressor cell; RAGE, receptor for advanced glycation endproducts; RIG-I, retinoic acid-inducible gene I; RLH, RIG-I-like helicase; TLR, toll-like receptor
Hallmarks of pancreatic cancer are early metastasis, therapy resistance and an immunosuppressive microenvironment, impairing the function of antigen-presenting cells and effector T cells. Immunotherapy has shown promise for several tumor entities, including pancreatic cancer, but the key challenge is to break immunosuppression for tipping the balance toward antitumor immunity.1 In recent years, it became evident that the type of cell death that is induced by specific therapeutics dictates whether tumors are recognized or ignored by the immune system.2 Immunogenic cell death leads to adaptive immune responses and has been described for certain chemotherapeutic drugs, such as anthracyclines and oxaliplatin. Proposed mechanisms for immune activation include the release of “danger signals” to the cell surface (calreticulin) or the extracellular space (heat shock proteins, HMGB1, DNA, RNA, ATP) by dying cells, facilitating antigen uptake, and activation of dendritic cells (DC) for efficient T cell stimulation.
Since immune responses against viruses and tumors share essential features including type I IFN-dependence and cytotoxic T cells, mimicking a viral infection with immune activating RNA species is an interesting novel strategy for tumor immunotherapy. Retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA5) are two ubiquitously expressed cytosolic helicases that recognize viral RNA species and can be activated by synthetic RNA; these include dsRNA for MDA5 and 5′-triphosphate RNA for RIG-I. Upon RNA binding, a signaling cascade mediated by IRFs, MAPK, and NF-κB is initiated that leads to the production of type I IFN and other innate immune response genes.3 Importantly, tumor cells are highly susceptible to RIG-I-like helicase (RLH)-induced cell death via intrinsic apoptosis providing a rationale for evaluating RLH ligands as anticancer drugs.4-6 We previously observed in a mouse model of pancreatic cancer that treatment with RIG-I ligands induced efficient tumor control, which was accompanied by activation of DC and required CD8+ T cells as effector cells.7 This led us to speculate that RLH activation is connected to some kind of immunogenic cell death triggering adaptive immunity.
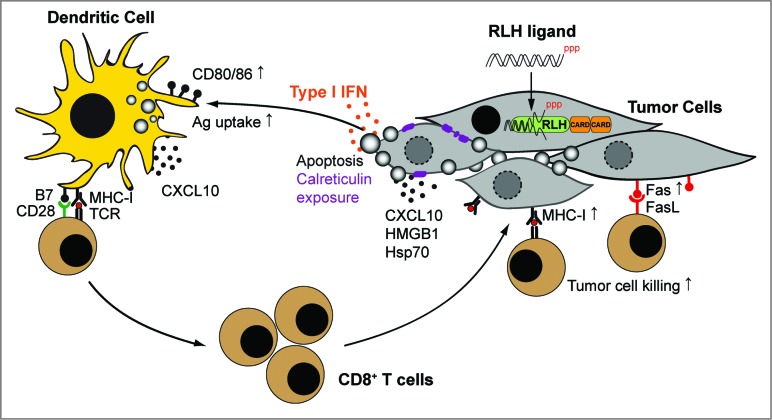
In a recent study published in Cell Death and Differentiation, we demonstrated that RLH activation in tumor cells induces typical features associated with immunogenic cell death.8 Pancreatic cancer cells treated with RLH ligands underwent intrinsic apoptosis, translocated calreticulin to the outer cell membrane and released danger molecules, such as HMGB1 and hsp70. In addition, they produced type I IFN and CXCL10, a chemokine attracting T cells and other immune cells to sites of inflammation. Tumor cells also upregulated expression of MHC-I and the death receptor Fas (CD95) on the cell surface, sensitizing them toward T cell-mediated lysis. To assess immunological consequences of RLH-induced cell death, tumor cells were cocultured with primary DC populations. Exposure of CD8α+ DC to the tumor cells induced upregulation of costimulatory molecules, enhanced antigen uptake and cross-presentation of tumor-associated antigen to naïve CD8+ T cells (Fig. 1). We further demonstrated that RLH ligands induce adaptive antitumor immunity in vivo. Vaccination with RLH-activated apoptotic tumor cells mediated tumor protection. In addition, local administration of RLH ligands into the tumor led to tumor regression and prolonged survival in two different pancreatic cancer models. Thus, RLH-induced cell death fulfills key criteria defining immunogenic cell death.2
Figure 1.
Mechanisms of immunogenic tumor cell death induced by RLH ligands. Delivery of RIG-I-like helicase (RLH) ligands into the tumor induces the release of type I interferon (IFN), proinflammatory cytokines and chemokines, as well as intrinsic apoptosis. Translocation of calreticulin from the endoplasmic reticulum to the cell surface may facilitate antigen uptake of apoptotic cells by dendritic cells (DC) in draining lymph nodes. Type I IFN and other danger signals promote DC activation and cross-presentation of tumor antigen to CD8+ T cells, leading to their clonal expansion. Local CXCL10 production facilitates the recruitment of lymphocytes into the tumor. Enhanced MHC-I and Fas (CD95) expression on tumor cells sensitize them toward CTL-mediated killing, linking innate with adaptive immunity (modified from Ref. 8).
But how does cell death in response to RLH signaling differ from other immunogenic cell death inducers, such as cytotoxic drugs, which usually fail to confer antitumor immunity in pancreatic cancer? In fact, our study revealed that apoptosis of pancreatic cancer cells induced with oxaliplatin neither induced DC activation nor facilitated antigen cross-presentation, despite effective antigen release and uptake by DC. To our surprise we found that, in contrast to previous reports, DC activation was independent on TLR, RAGE or inflammasome signaling. Applying different approaches we could identify type I IFN as the key feature of immunogenic cell death induced by RLH ligands. Possibly, the immunosuppressive microenvironment found in pancreatic cancer forms a hurdle that cannot be overcome by cytotoxic drugs, whereas type I IFN acts as a double-sided sword; on the one side it alerts the immune system to danger (i.e. a viral infection) leading to activation of DC and effector cells, and on the other side it releases the brake of immunosuppressive cells, such as regulatory T cells and myeloid-derived suppressor cells (MDSC), which are massively recruited in pancreatic cancer.9,10 In line with this hypothesis ex vivo analysis of RLH-treated tumors showed increased expression of type I IFN and Th1 cytokines, recruitment of CD8+ T cells, decreased numbers of MDSC and strong DC activation.7,8
We propose that turning RLH-mediated anti-viral immune signaling against tumors offers new therapeutic options for hard to treat tumors. In fact, we could show that primary human pancreatic cancers express both RIG-I and MDA5 (7 and unpublished data) and that RLH-mediated apoptosis induction is independent on the p53 mutational status of the tumor cells.4,7,8 The emergence of apoptosis resistance, which is frequently observed for chemotherapeutic drugs or radiation, is thus less likely. We envision that RLH-induced immunogenic cell death, e.g., via ultra-sound guided injection of RLH ligands into pancreatic cancer prior to surgery, may facilitate the induction of antitumor immune responses carrying the potential to control tumor recurrence.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft SCHN 664/3-1, SCHN 664/3-2 and GK 1202 to MS.
References
- 1. Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology 2013; 144(6): 1230-40; PMID:; http://dx.doi.org/10.1053/j.gastro.2012.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Ann Rev Immunol 2013; 31: 51-72; PMID:; http://dx.doi.org/10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 3. Loo YM, Gale M, Jr. Immune signaling by RIG-I-like receptors. Immunity 2011; 34(5): 680-92; PMID:; http://dx.doi.org/10.1016/j.immuni.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Besch R, Poeck H, Hohenauer T, Senft D, Hacker G, Berking C, Hornung V, Endres S, Ruzicka T, Rothenfusser S, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. J Clin Invest 2009; 119(8): 2399-411; PMID:; http://dx.doi.org10.1172/JCI37155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meng G, Xia M, Xu C, Yuan D, Schnurr M, Wei J. Multifunctional antitumor molecule 5'-triphosphate siRNA combining glutaminase silencing and RIG-I activation. Int J Cancer 2014; 134(8): 1958-71; PMID:; http://dx.doi.org/10.1002/ijc.28416 [DOI] [PubMed] [Google Scholar]
- 6. Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, et al. 5'-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med 2008; 14(11): 1256-63; PMID:; http://dx.doi.org/10.1038/nm.1887 [DOI] [PubMed] [Google Scholar]
- 7. Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, et al. Therapeutic efficacy of bifunctional siRNA combining TGF-beta1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 2013; 73(6): 1709-20; PMID:; http://dx.doi.org/10.1158/0008-5472.CAN-11-3850 [DOI] [PubMed] [Google Scholar]
- 8. Duewell P, Steger A, Lohr H, Bourhis H, Hoelz H, Kirchleitner SV, Stieg MR, Grassmann S, Kobold S, Siveke JT, et al. RIG-I-like helicases induce immunogenic cell death of pancreatic cancer cells and sensitize tumors toward killing by CD8 T cells. Cell Death Differ 2014; PMID:; http://dx.doi.org/10.1038/cdd.2014.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pace L, Vitale S, Dettori B, Palombi C, La Sorsa V, Belardelli F, Proietti E, Doria G. APC activation by IFN-alpha decreases regulatory T cell and enhances Th cell functions. JImmunol 2010; 184(11): 5969-79; PMID:; http://dx.doi.org/10.4049/jimmunol.0900526 [DOI] [PubMed] [Google Scholar]
- 10. Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res 2011; 17(7): 1765-75; PMID:; http://dx.doi.org/10.1158/1078-0432.CCR-10-2672 [DOI] [PubMed] [Google Scholar]