Abstract
Apoptotic cells have long been considered as intrinsically tolerogenic or unable to elicit immune responses specific for dead cell-associated antigens. However, multiple stimuli can trigger a functionally peculiar type of apoptotic demise that does not go unnoticed by the adaptive arm of the immune system, which we named “immunogenic cell death” (ICD). ICD is preceded or accompanied by the emission of a series of immunostimulatory damage-associated molecular patterns (DAMPs) in a precise spatiotemporal configuration. Several anticancer agents that have been successfully employed in the clinic for decades, including various chemotherapeutics and radiotherapy, can elicit ICD. Moreover, defects in the components that underlie the capacity of the immune system to perceive cell death as immunogenic negatively influence disease outcome among cancer patients treated with ICD inducers. Thus, ICD has profound clinical and therapeutic implications. Unfortunately, the gold-standard approach to detect ICD relies on vaccination experiments involving immunocompetent murine models and syngeneic cancer cells, an approach that is incompatible with large screening campaigns. Here, we outline strategies conceived to detect surrogate markers of ICD in vitro and to screen large chemical libraries for putative ICD inducers, based on a high-content, high-throughput platform that we recently developed. Such a platform allows for the detection of multiple DAMPs, like cell surface-exposed calreticulin, extracellular ATP and high mobility group box 1 (HMGB1), and/or the processes that underlie their emission, such as endoplasmic reticulum stress, autophagy and necrotic plasma membrane permeabilization. We surmise that this technology will facilitate the development of next-generation anticancer regimens, which kill malignant cells and simultaneously convert them into a cancer-specific therapeutic vaccine.
Keywords: ATP release, autophagy, calreticulin, endoplasmic reticulum stress, HMGB1, immunotherapy
Abbreviations: APC, antigen-presenting cell; ATF6, activating transcription factor 6; BAK1, BCL2-antagonist/killer 1; BAX, BCL2-associated X protein; BCL2, B-cell CLL/lymphoma 2 protein; CALR, calreticulin; CTL, cytotoxic T lymphocyte; Δψm, mitochondrial transmembrane potential; DAMP, damage-associated molecular pattern; DAPI, 4′,6-diamidino-2-phenylindole; DiOC6(3), 3,3′-dihexyloxacarbocyanine iodide; EIF2A, eukaryotic translation initiation factor 2A; ER, endoplasmic reticulum; FLT3LG, fms-related tyrosine kinase 3 ligand; G3BP1, GTPase activating protein (SH3 domain) binding protein 1; GFP, green fluorescent protein; H2B, histone 2B; HMGB1, high mobility group box 1; HSP, heat shock protein; HSV-1, herpes simplex virus type I; ICD, immunogenic cell death; IFN, interferon; IL, interleukin; MOMP, mitochondrial outer membrane permeabilization; PDIA3, protein disulfide isomerase family A; member 3; PI, propidium iodide; RFP, red fluorescent protein; TLR, Toll-like receptor; XBP1, X-box binding protein 1
Introduction
Cell death can be classified based on several parameters, including morphological manifestations, biochemical features, kinetic considerations and functional outcomes.1-7 This said, how cell death has been investigated and conceived since its pristine descriptions (dating back to the mid-19th century)8 has obviously evolved along with the technological advances that have been made throughout the last one and a half centuries.9,10 Thus, morphology-based classifications postulating the existence of 3 cell death subroutines (i.e., type I, type II and type III cell death)2,11-14 have been progressively abandoned in favor of definitions that rely on objectively quantifiable functional features.3,15-19 Alongside, the long-standing conception according to which distinct types of cell death like apoptosis and necrosis would constitute mutually exclusive and diametrically opposed entities has been refuted. In particular, throughout the past 2 decades it has become clear that: (1) apoptosis is not the sole type of regulated cell death that contributes to (post-) embryonic development and adult tissue homeostasis;20 (2) similar to apoptosis, necrosis can occur in a regulated fashion, i.e., it can involve a genetically encoded molecular machinery;4,5,21 (3) similar to their necrotic counterparts, apoptotic cells can sometimes be detected by the immune system and elicit an adaptive immune response specific for dead cell-associated antigens.6,7,22,23 Thus, although apoptosis as a physiological process involved in (post-)embryonic development and tissue homeostasis invariably fails to engage the adaptive branch of the immune system,24,25 specific stimuli can promote an immunogenic variant of regulated cell death that manifests with both morphological and biochemical features of apoptosis.2,3,6 Of note, defects in the clearance of apoptotic cells by professional phagocytes have been associated with autoimmune conditions such as systemic lupus erythematosus and chronic inflammation.26,27 However, it remains unclear whether this reflects the immunogenic potential of intact apoptotic corpses or the insurgence of secondary necrosis.
Back in 2005, we were the first to report the unexpected finding that murine colorectal carcinoma CT26 cells as well as murine fibrosarcoma MCA205 cells exposed to a lethal dose of doxorubicin in vitro are capable of vaccinating syngeneic mice against a subsequent challenge with living cells of the same type.22 We dubbed such a functionally peculiar variant of cellular demise, manifesting with an apoptotic morphology and depending on the activity of apoptotic caspases, “immunogenic cell death” (ICD).22 It turned out that the unsuspected ability of doxorubicin (an anthracycline employed for the treatment of various carcinomas) to trigger ICD as a standalone intervention, hence converting dying cancer cells into a vaccine that is efficient in the absence of adjuvants, is shared by a relatively restricted set of lethal triggers.28-33 These include, but perhaps are not limited to, mitoxantrone and epirubicin (2 other anthracyclines currently used in the clinic),34-37 bleomycin (a glycopeptide antibiotic endowed with antineoplastic properties),38 oxaliplatin (a platinum derivative generally employed against colorectal carcinoma),39-42 cyclophosphamide (an alkylating agent approved for the treatment of neoplastic and autoimmune conditions),43-48 etoposide (a topoisomerase inhibitor currently used for the treatment of several neoplasms) combined with the chemical inhibitor of glycolysis 2-deoxyglucose,49,50 patupilone (a microtubular inhibitor that has not yet been approved for use in humans),51-53 septacidin (an antifungal antibiotic produced by Streptomyces fibriatus)54,55 specific forms of radiation therapy,34,56-64 photodynamic therapy (a clinically approved anticancer intervention that involves the administration of a photosensitizing agent followed by light irradiation),65-73 high hydrostatic pressure,74 multiple oncolytic viruses,75-83 replication-defective viral vectors encoding a potentially cytotoxic product (e.g., thymidine kinase from herpes simplex virus type I, HSV-1) combined with viruses expressing an immunostimulatory molecule (e.g., fms-related tyrosine kinase 3 ligand, FLT3LG),84 the clinically employed proteasomal inhibitor bortezomib,85-87 shikonin (a component of Chinese herbal medicine),88 a monoclonal antibody specific for the epidermal growth factor receptor (EGFR),89 capsaicin (a neurotoxic derivative of homovanillic acid found in chili peppers),90,91 and perhaps the n3-polyunsaturated fatty acid docosahexaenoic acid,92 as well as the transgene-driven expression of SMAC mimetics.93,94 In addition, some interventions are capable of converting non-immunogenic instances of cell death into bona fide ICD. These maneuvers include the administration of cardiac glycosides, which are particularly powerful in this respect as they promote per se all major manifestations of ICD (see below),95-97 or zoledronic acid (a bisphosphonate currently approved to treat osteoporosis and to prevent skeletal fractures in cancer patients with bone metastases),98,99 as well as the provision of co-stimulatory signals via CD40.100 This said, it should be kept in mind that the capacity of a given agent to cause ICD or exacerbate the immunogenicity of apoptosis cannot be predicted yet from its structural or chemical properties, since molecules as similar to each other as oxaliplatin and cisplatin do not share this functional profile.39,40
The notion that apoptotic cancer cells do not always go undetected by the immune system has profound clinical repercussions.101 First, it implies that the immune system, at least under specific circumstances, can mount an adaptive immune response against (self) malignant cells, hence mediating antineoplastic effects or contributing to the therapeutic activity of conventional anticancer regimens. This concept represents the theoretical foundation of modern tumor immunology and anticancer immunotherapy.22,102,103 As a matter of fact, many chemotherapeutics that have been successfully used in the clinic throughout the past century have recently been discovered to mediate off-target immunostimulatory effects, ICD being one of the underlying mechanisms (though not the sole).104-106 Second, it implies that a large number of parameters reflecting the immunological competence of the host, including the type, quantity and localization of tumor-infiltrating lymphoid and myeloid cells,107-113 the amount of blood-borne memory T cells that are able to recognize antigens associated with apoptotic cancer cells,114 the circulating levels of various ICD-associated biomarkers, including the non-histone chromatin-binding protein high mobility group box 1 (HMGB1),46,115-117 as well as genetic polymorphisms affecting virtually all facets of the immune response,41,108,118,119 may be endowed with a robust prognostic or predictive value. This notion has already been demonstrated in several ICD-related clinical scenarios. Thus, the relative abundance of tumor-infiltrating CD8+ cytotoxic T lymphocytes (CTLs) and CD4+CD25+FOXP3+ regulatory T cells reportedly predicts the propensity of breast carcinoma patients to benefit from anthracycline- or oxaliplatin-based chemotherapy, respectively.52,120 Along similar lines, single nucleotide polymorphisms in the genes coding for ICD-relevant receptors such as Toll-like receptor 4 (TLR4) and purinergic receptor P2X, ligand-gated ion channel, 7 (P2RX7) have been shown to influence disease outcome among breast carcinoma patients treated with anthracycline-based chemotherapy.41,119 Taken together, these observations demonstrate that the induction of ICD is a therapeutically relevant objective, calling for the identification of novel ICD inducers and molecules that improve the immunogenicity of conventional variants of apoptosis.
After summarizing the main molecular and cellular determinants that underlie ICD, we discuss the assays that are currently available for the detection of surrogate ICD markers and how these methods can be combined into a platform that is compatible with high-content, high-throughput applications. We surmise that this methodological approach will accelerate the discovery and development of therapeutic regimens that kill malignant cells in an immunogenic fashion.
Immunogenic Cell Death Signaling
According to current models, ICD relies on the ability of specific stimuli to kill cells while provoking the spatiotemporally coordinated emission of immunogenic signals.7,121-129 Such signals are conveyed by damage-associated molecular patterns (DAMPs), i.e., molecules that are not accessible by the immune system in physiological conditions but are released or exposed on the outer leaflet of the plasma membrane during cytoprotective stress responses or upon cell death.103,130-133 Similar to their microbial counterparts, many (but not all) DAMPs exert robust immunostimulatory effects upon binding to pattern recognition receptors (PRRs) expressed by immune cells.121 So far, 3 DAMPs have been attributed a key role in the immunogenic potential of virtually all ICD inducers: the endoplasmic reticulum (ER) chaperone calreticulin (CALR),34,65,126,134-136 ATP,66,124,137-143 and HMGB1.41,46,115,116,144-147 In addition, many DAMPs have been shown to contribute to the immunogenicity of cell death in a limited amount of experimental scenarios. These include immunostimulatory cytokines like interferon α (IFNα),148,149 various chaperones of the heat-shock protein (HSP) family, notably heat shock 70kDa protein 1A (HSPA1A, best known as HSP70) and heat shock protein 90kDa α (cytosolic), class A member 1 (HSP90AA1, best known as HSP90),65,71,85,90,145,150-153 sphingomyelin metabolites (e.g., ceramide and sphingosine-1-phosphate),154 a plethora of mitochondrial products (e.g., mitochondrial DNA, N-formylated peptides, cardiolipin),155-157 cytosolic components like urate and F-actin,158-161 as well as products of the breakdown of the extracellular matrix (e.g., hyaluronan fragments).162,163
CALR gets exposed on the cell surface early in the course of ICD, i.e., before the apoptosis-associated shuffling of phosphatidylserine between the inner and outer leaflet of the plasma membrane.34,164,165 The molecular mechanisms underlying this ICD hallmark have been dissected in detail and appear to involve 3 distinct signaling modules: (1) an ER stress module centered around the phosphorylation of eukaryotic translation initiation factor 2A (EIF2A) and the resultant arrest in protein synthesis; (2) an apoptotic module involving the activation of caspase-8 and the consequent cleavage of B-cell receptor-associated protein 31 (BCAP31), as well as the pro-apoptotic Bcl-2 family members BCL2-associated X protein (BAX) and BCL2-antagonist/killer 1 (BAK1); and (3) an exocytosis module requiring the actin cytoskeleton as well as vesicle-associated membrane protein 1 (VAMP1) and synaptosomal-associated protein, 25 kDa (SNAP25), 2 proteins involved in intracellular vesicular trafficking.36 Moreover, in some (but not all) models of ICD,67 CALR obligatorily translocates to the cell surface together with another ER chaperone, protein disulfide isomerase family A, member 3 (PDIA3).36,37 Upon binding to low density lipoprotein receptor-related protein 1 (LRP1, also known as CD91), membrane-exposed CALR delivers a major phagocytic signal to professional antigen-presenting cells (APCs) such as dendritic cells, de facto improving their capacity to take up dead cells and their corpses.66,91,166-173 Interestingly, the phagocytosis-stimulatory effects of CALR is robustly counterbalanced by CD47, which is highly expressed by a large panel of solid and hematopoietic tumors.166 This latter observation suggests that various neoplasms benefit from avoiding the effects of CALR exposure, perhaps as this prevents the elicitation of an adaptive immune response against the malignant cells that “physiologically” succumb in the course of oncogenesis and tumor progression. Alternatively, the phagocytosis-inhibitory activity of CD47 may confer tumors with an advantage by increasing the local availability of macromolecules derived from their spontaneous demise and degradation of some of their cellular constituents. This possibility has not yet experimentally addressed.
The ICD-associated release of ATP proceeds through a complex mechanism that involves (1) the apparent relocalization of vesicular ATP stores from lysosomes to autolysosomes; (2) the redistribution of lysosomal-associated membrane protein 1 (LAMP1) to the plasma membrane; (3) Rho-associated, coiled-coil containing protein kinase 1 (ROCK1)-mediated and myosin II-dependent cellular blebbing; and (4) the opening of pannexin 1 (PANX1) channels, 4 processes that rely on caspases.140,142,174 In a vast majority of models, the secretion of ATP by cells exposed to ICD inducers requires an intact autophagic machinery.83,138,139,175 In these settings, the genetic or pharmacological inhibition of autophagy limits ATP release by cells succumbing to ICD and hence negatively influences their ability to elicit an adaptive immune response upon inoculation in immunocompetent syngeneic mice.60,138,139 Along similar lines, the chemical inducer of autophagy STF-62247 increases the immunostimulatory potential of ICD as triggered by chlorin-e6-based photodynamic therapy (MK, unpublished observations). However, this does not seem to apply to all ICD inducers.68 Thus, the ability of hypericin-based photodynamic therapy to induce the secretion of ATP does not appear to change in autophagy-deficient versus autophagy-proficient cells.68,70,176 Moreover, the former respond to hypericin-based photodynamic therapy by exposing higher amounts of CALR on the plasma membrane than the latter, hence exhibiting a superior immunogenic potential.68,70,176 Possibly, this reflects the incapacity of autophagy-deficient cells to clear oxidized proteins, resulting in an aggravation of the ER stress response that underlies CALR exposure in the course of ICD.68,70,176 Irrespective of these variations, extracellular ATP operates as a strong chemoattractant and promotes not only the recruitment of immune cells to sites of ICD, but also their differentiation, an effect that depends on purinergic receptor P2Y, G-protein coupled, 2 (P2RY2).141,177-179 Moreover, extracellular ATP promotes the activation of the NLR family, pyrin domain containing 3 (NLRP3) inflammasome in APCs, hence stimulating the processing and release of interleukin (IL)-1β and IL-18.119,180-189 In line with this notion, the immunogenic potential of cells succumbing to ICD can be significantly reduced by pharmacological or genetic interventions that limit the availability of ATP in the pericellular space, such as the administration of recombinant apyrase (an ATP-degrading enzyme) or the transfection-enforced overexpression of ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1, best known as CD39), which converts ATP into ADP and AMP.190 Intriguingly, CD39 and 5′-nucleotidase, ecto (NT5E, best known as CD73), which transforms AMP into adenosine, are often overexpressed by malignant tissues. This expression pattern reflects the advantage that these enzymes confer to cancer cells by the degradative conversion of extracellular ATP, which promotes immunosurveillance, into adenosine, which exerts potent immunosuppressive effects.191-197 Of note, autophagy is also important for the perception of cell death as immunogenic because it contributes to several aspects of cellular immune responses, including the differentiation, survival and activation of myeloid and lymphoid cells.198-200
The release of HMGB1 from cells succumbing to ICD requires the permeabilization of both the nuclear and plasma membranes, de facto constituting a post-mortem event.3,41 Although autophagy has been proposed to contribute to the release of HMGB1 from dying cells, at least under some circumstances,201 the molecular machinery that underlies this crucial manifestation of ICD has not yet been elucidated in detail. This said, extracellular HMGB1 is well known to mediate robust pro-inflammatory effects upon binding to several receptors on the surface of immune cells, including TLR2, TLR4 and advanced glycosylation end product-specific receptor (AGER, best known as RAGE).202-210 Moreover, extracellular HMGB1 reportedly exerts a chemotactic activity by forming a complex with chemokine (C-X-C motif) ligand 12 (CXCL12) that signals via chemokine (C-X-C motif) receptor 4 (CXCR4).211 Finally, at least under some circumstances, endogenous HMGB1 appears to promote autophagy by interfering with the mutually inhibitory interaction between the central autophagic regulator beclin 1 (BECN1) and the anti-apoptotic protein B-cell CLL/lymphoma 2 (BCL2).212-214 It is therefore tempting to speculate, yet remains to be formally demonstrated, that the nuclear release of HMGB1 may contribute to the autophagic response of cells succumbing to ICD inducers. Of note, the biological activity of extracellular HMGB1 appears to be regulated by its redox state.215-221 Moreover, HMGB1 binds not only to TLR2, TLR4 and RAGE, but also to hepatitis A virus cellular receptor 2 (HAVCR2, best known as TIM-3), hence mediating immunosuppressive (as opposed to immunostimulatory) effects.222-224 Taken together, these observations suggest that the biological activity of HMGB1 exhibits a consistent degree of context-dependency. Nonetheless, HMGB1-deficient malignant cells exposed to ICD inducers fail to elicit adaptive immune responses upon inoculation into immunocompetent syngeneic mice, a defect that can be corrected by the co-administration of synthetic TLR4 ligands.225-228 Together with the notion that Tlr4−/- mice fail to perceive anthracycline-treated syngeneic cells as immunogenic,41,229 this observation demonstrates the importance of the HMGB1-TLR4 signaling axis for ICD.
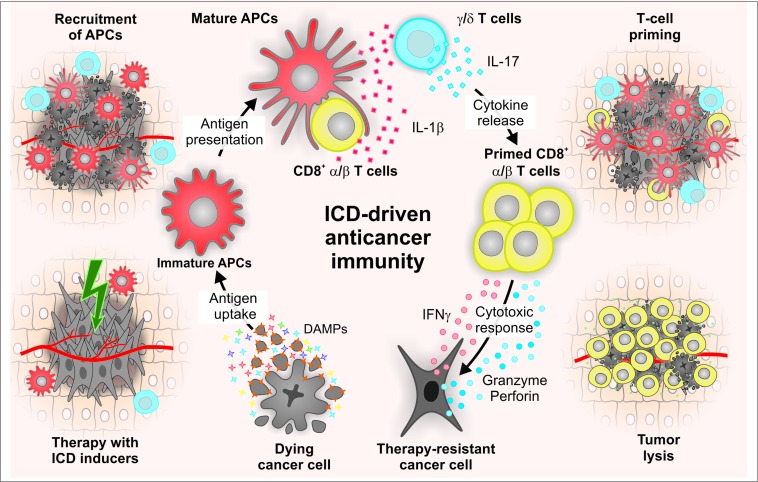
In summary, the spatiotemporally coordinated emission of specific DAMPs promotes the recruitment of APCs to sites of ongoing ICD, their ability to take up dead cell-derived particulate material, as well as their capacity to prime an adaptive immune response.6 This generally proceeds in 2 phases, involving the sequential recruitment and activation of IL-17-secreting γδ T cells and αβ CTLs.31,230 The latter not only mediate direct antineoplastic effects, mostly by secreting interferon γ (IFNγ) and via the granzyme-perforin pathway, but also underlie the establishment of protective immunological memory (Fig. 1).231
Figure 1.
Molecular and cellular mechanisms of immunogenic cell death. Cancer cells succumb to specific stimuli (e.g., anthracyclines, oxaliplatin, some forms of radiation therapy, photodynamic therapy) while emitting a spatiotemporally ordered combination of damage-associated molecular patterns (DAMPs). These signals include (but are not limited to) the pre-apoptotic exposure of the endoplasmic reticulum chaperone calreticulin (CALR) on the surface of dying cells, the secretion of ATP during the blebbing phase of apoptosis, and the release of the nuclear protein high mobility group box 1 (HMGB1) upon plasma membrane permeabilization. Upon binding to specific receptors, immunogenic cell death (ICD)-associated DAMPs promote the recruitment of antigen-presenting cells (APCs) and stimulate their ability to take up particulate material and cross-present dead cell-associated antigens to CD8+ cytotoxic T lymphocytes (CTLs) while secreting interleukin (IL)-1β. The consequent adaptive immune response also involves γδ T lymphocytes that produce IL-17. Both γδ T cells and αβ CTLs mediate direct antineoplastic effects by secreting interferon γ (IFNγ) and via the granzyme-perforin pathway. In addition, some CTLs acquire a memory phenotype, underlying the establishment of long-term immunological protection.
Gold-Standard Methods to Monitor ICD
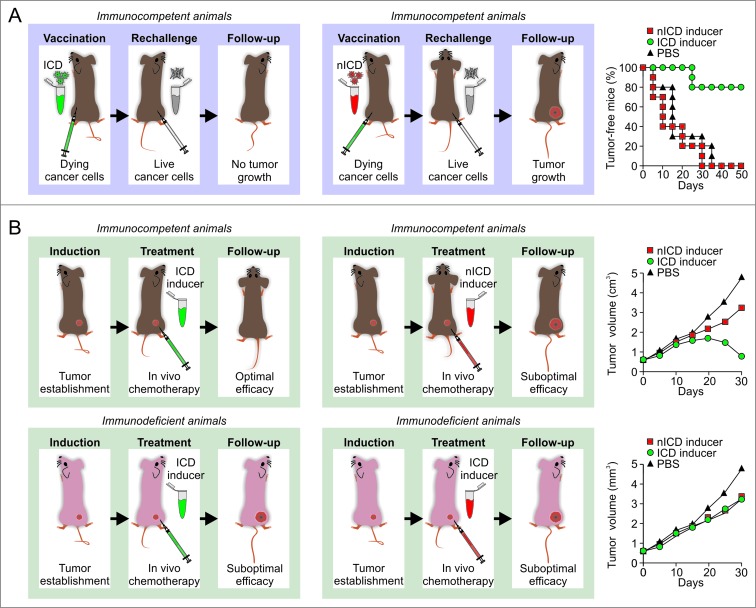
As it stands, the gold-standard approach to evaluate the ability of a specific stimulus to cause bona fide ICD relies on vaccination assays.6,22,30 In this setting, malignant cells are exposed in vitro to the lethal stimulus of choice, thoroughly washed (to remove the stimulus), resuspended in an adequate volume of PBS, and then inoculated subcutaneously into the flank of immunocompetent syngeneic mice. One week later, living cells of the same type are introduced subcutaneously into the opposite flank, and mice are routinely monitored for the appearance of a palpable neoplastic lesion (Fig. 2A). The proportion of mice that do not develop subcutaneous tumors reflects the degree of immunogenicity of cell death as induced by the lethal trigger under evaluation. As a note, murine cells succumbing to prototypic inducers of ICD such as doxorubicin and mitoxantrone effectively vaccinate 80% of mice.34,95,232
Figure 2.
Assays for the evaluation of immunogenic cell death in vivo. (A) Vaccination assays. Murine cancer cells of choice are exposed in vitro to a putative inducer of immunogenic cell death (ICD), 1 μM mitoxantrone (positive control) or 50 μM cisplatin (negative control) for a predetermined time (normally 6–24 hours), then washed, resuspended in PBS, and eventually injected s.c. into one flank (vaccination site) of immunocompetent syngeneic mice (ideally 5–10 per group). One week later, mice are challenged with living cancer cells of the same type, which are inoculated s.c. into the contralateral flank (challenge site). Tumor incidence and growth are routinely monitored at both injection sites over a 1-2 months period. The development of neoplastic lesions at the vaccination site indicates that the stimulus under investigation is unable to cause cell death (under the circumstances under investigation) to a degree that is compatible with the elicitation of adaptive immunity. Conversely, in the absence of tumors at the vaccination site, the ability of the experimental maneuver under evaluation to promote bona fide ICD inversely correlates with the number of neoplastic lesions developed at the challenge site. As an indication, neoplastic cells exposed in vitro to 1 μM mitoxantrone for 6 hours and maintained in culture for additional 18 hours vaccinate approximately 80% of mice against a challenge with living cells of the same type. (B) Therapeutic assays. Immunocompetent and immunodeficient syngeneic mice bearing grafted, genetically-driven or chemically-induced subcutaneous or orthotopic tumors are treated with a putative ICD inducer, mitoxantrone (positive control) or cisplatin (negative control) at therapeutic doses, followed by the monitoring of tumor size over a 1–3 weeks period. In this setting, bona fide ICD inducers mediate optimal antineoplastic effects in immunocompetent, but not in immunodeficient, mice. Since this is also the case of therapeutic interventions that exert off-target immunostimulatory effects, this assay cannot be employed alone to discriminate between ICD and non-immunogenic cell death (nICD). Please note that all curves represented in this figure do not depict primary data but have been created for the sake of exemplification.
As a confirmatory assay, putative ICD inducers can be assessed for their ability to mediate immune system-dependent therapeutic effects against established neoplastic lesions.6,34,233 In this scenario, grafted, genetically-driven or chemically-induced subcutaneous or orthotopic tumors are established in both immunocompetent and immunodeficient mice. Malignant lesions are then allowed to progress until a pre-determined size or time point, beyond which tumor-bearing mice are treated with the compound under evaluation (Fig. 2B). In this experimental setup, bona fide ICD inducers mediate optimal therapeutic effects in immunocompetent, but not in immunodeficient, mice.34,41,95,119,233 Importantly, this latter approach is suitable to validate the results of vaccination experiments but cannot be employed alone to determine the capacity of a specific intervention to cause ICD. Indeed, even the activity of antineoplastic regimens that fail to render dying cells immunogenic but induce other immunostimulatory effects is negatively affected by the absence of a functional immune system.104,105 Among other molecules, this applies to the microtubular inhibitor paclitaxel and the nucleoside analog gemcitabine.104,105
The main drawbacks of these assays relate to the use of rodents: the need for a tightly controlled sterile facility (which is mandatory for working with immunodeficient animals), prolonged times for the establishment/growth of neoplastic lesions, and significant costs. Moreover, vaccination and therapeutic tests for the detection of ICD are limited by the relatively restricted number of syngeneic tumor models that are currently available. Thus, although they constitute the gold-standard approach for the detection of ICD, vaccination assays relying on immunocompetent mice and syngeneic cancer cells are intrinsically incompatible with large screening campaigns. To circumvent this issue, various techniques that allow for the detection of one or more ICD manifestations in vitro and in vivo have been developed.6,234 Monitoring the immunostimulatory activity of lead compounds (be it linked to the induction of ICD or reflecting other mechanisms) early in the drug discovery pipeline may indeed speed up significantly the development of novel anticancer agents.104
Detection of Surrogate ICD Biomarkers
A relatively ample panel of ICD-associated phenomena can be monitored in vitro to obtain insights into the ability of a specific intervention to provoke ICD (Table 1).
Table 1.
Assays for the detection of immunogenic cell death-associated processes in vitro
| Process | Parameter | Platform | Main advantage | Main disadvantage | Notes |
|---|---|---|---|---|---|
| Cell death | BAX activation | Flow cytometry Fluorescence microscopy Immunoblotting |
Compatible with real-time detection |
Real-time detection requires transgenic cell lines | Based on conformation-specific antibodies or cell lines expressing GFP-tagged BAX |
| Δψm dissipation | Flow cytometry Fluorescence microscopy Fluorometry |
Early process in the cascade of events leading to cell death | The Δψm can be dissipated in the course of cell death-unrelated processes |
Several Δψm-sensitive probes with different spectral and biochemical properties are available, including DiOC6(3) and CMTMRos | |
| Caspase activation | Flow cytometry IF microscopy Fluorescence microscopy Fluorometry Immunoblotting |
Directly involved in CALR exposure |
Some caspases get activated in the course of cell death-unrelated processes |
Antibodies specific for active caspases or their substrates, as well as self-quenched peptides that emit upon cleavage are available |
|
| Nuclear pyknosis | Fluorescence microscopy | Compatible with simultaneous assessments |
Prone to underestimation, owing to the detachment of cells from the substrate |
Based on chromatinophilic dyes like Hoechst 33342 or cell lines expressing RFP-tagged variants of H2B | |
| PMP | Flow cytometry Fluorescence microscopy Light microscopy |
Straightforward and very reliable indicator of cell death | End-stage measurement | Several exclusion dyes with different spectral properties are available, including trypan blue, DAPI and PI |
|
| Surface-exposed PS | Flow cytometry Fluorescence microscopy |
Compatible with simultaneous assessments |
PS exposure does not always accompany cell death |
Based on fluorochrome-tagged variants of the protein annexin A5 |
|
| CALR exposure | Surface-exposed CALR |
Flow cytometry Fluorescence microscopy Native gels |
Compatible with real- time detection and simultaneous assessments |
Real-time detection requires transgenic cell lines | Based on CALR-specific antibodies, cell lines expressing HaloTag™-tagged CALR variants, GFP-tagged CALR variants, or GFP-tagged PDIA3 variants, or the quantification of cell surface proteins upon biotinylation |
| ER stress | Phosphorylation of EIF2A or EIF2A kinases | IF microscopy Immunoblotting |
EIF2A phosphorylation is required in CALR exposure | Incompatible with high-throughput platforms | Based on phosphoneoepitope- specific antibodies |
| XBP1 splicing | Fluorescence microscopy | Compatible with real-time detection |
Incomplete assessment of the ER stress response |
Based on cell lines expressing a fluorescent variant of XBP1 |
|
| ATF6 activation | Fluorescence microscopy | Compatible with real-time detection |
Incomplete assessment of the ER stress response |
Based on cell lines expressing a fluorescent variant of ATF6 |
|
| Formation of stress granules | Fluorescence microscopy | Compatible with real-time detection |
Not specific for ER stress | Based on cell lines stably expressing a GFP-tagged variant of G3BP1 |
|
| ATP secretion | Extracellular ATP | Luminometry HPLC-MS |
Very sensitive and compatible with real-time detection | Extracellular ATP is exposed to several ectonucleotidases | Extracellular ATP can be monitored in culture supernatants or in cells stably expressing luciferase on their surface |
| Cytosolic ATP | Fluorescence microscopy Luminometry HPLC-MS |
Cytosolic ATP is more stable than its extracellular counterpart | Indirect indication of ATP secretion |
Residual cytosolic ATP can be monitored upon cell lysis or in cells expressing ATP-sensitive FRET-based probes |
|
| Vesicular ATP | Flow cytometry Fluorescence microscopy |
Compatible with real-time detection |
Indirect indication of ATP secretion |
Based on the fluorescent probe quinacrine |
|
| Autophagy | Autophagosome formation | Fluorescence microscopy Immunoblotting Other techniques |
Can be monitored with a large panel of techniques |
Autophagy is not always required for the secretion of ATP in the course of ICD | Cell lines stably expressing GFP-LC3 offer a means to monitor the formation of autophagic vacuoles in real-time |
| HMGB1 release | Extracellular HMGB1 | ELISA Immunoblotting Mass spectroscopy |
Very sensitive and compatible with real-time detection | Relatively expensive | ELISA kits for the detection of HMGB1 are available from commercial providers |
| Intracellular HMGB1 | Fluorescence microscopy Immunoblotting |
Compatible with real-time detection |
Indirect indication of HMGB1 release |
Based on HGMB1-specific antibodies or cell lines expressing fluorescent variants of HMGB1 |
Abbreviations: ATF6, activating transcription factor 6; BAX, BCL2-associated X protein; CALR, calreticulin; CMTMRos, chloromethyltetramethylrosamine; Δψm, mitochondrial transmembrane potential; DAPI, 4′,6-diamidino-2-phenylindole; DiOC6(3), 3,3′-dihexyloxacarbocyanine iodide; EIF2A, eukaryotic translation initiation factor 2A; ELISA, enzyme-linked immunosorbent assay; ER, endoplasmic reticulum; FRET, fluorescence resonance energy transfer; G3BP1, GTPase activating protein (SH3 domain) binding protein 1; GFP, green fluorescent protein; H2B, histone 2B; HGMB1, high mobility group box 1; HPLC, high-performance liquid chromatography; ICD, immunogenic cell death; IF, immunofluorescence; MS, mass spectrometry; PDIA3, protein disulfide isomerase family A, member 3; PI, propidium iodide; PMP, plasma membrane permeabilization; PS, phosphatidylserine; RFP, red fluorescent protein; XBP1, X-box binding protein 1.
Cell death
By definition, ICD inducers must be cytotoxic and provoke cell death above a minimal threshold level. Cancer cells emit indeed a wide panel of DAMPs in response to non-lethal perturbations of homeostasis. However, such DAMPs differ in both qualitative and quantitative terms from those emitted by cells of the same type dying in response to the same stimulus applied with a lethal intensity and duration. Living cells are less likely to be taken up by APCs and ignite an adaptive immune response than their dying counterparts. Moreover, if the fraction of dying cells is excessively low, neoplastic lesions develop at the vaccination site and protective immunity cannot be established.34,95 Thus, agents that stimulate all the key manifestations of ICD including CALR exposure, ATP secretion and HMGB1 release, but fail to exert robust cytotoxic effects cannot be considered as authentic ICD inducers. This is the case of cardiac glycosides including digoxin and digitoxin, which nonetheless are powerful at converting non-immunogenic instances of cell death into bona fide ICD, hence operating as a potent immune adjuvant.95-97,235
Several assays are commercially available to monitor cell death-associated parameters, the most reliable indicator of cell death being end-stage plasma membrane permeabilization.9,236 This can be monitored by so-called exclusion dyes like the DNA-binding chemicals propidium iodide (PI) and 4′,6-diamidino-2-phenylindole (DAPI), which only accumulate in cells with permeabilized plasma membranes. PI and DAPI can be conveniently detected by flow cytometry or fluorescence microscopy (absorption/emission peaks: 535/617 and 358/461 nm, respectively). On flow cytometry, both PI and DAPI can be combined with fluorescence variants of the protein annexin A5 (ANXA5), permitting the detection of phosphatidylserine exposure,9,237,238 as well as with 3,3′-dihexyloxacarbocyanine iodide (DiOC6(3), absorption/emission peaks: 482/504 nm), allowing for the quantification of mitochondrial transmembrane potential (Δψm).239-241 The externalization of phosphatidylserine (a phospholipid normally restricted to the inner leaflet of the plasma membrane) indeed accompanies multiple (though not all) instances of apoptotic cell death,16,242-245 while the permanent dissipation of the Δψm as a result of mitochondrial outer membrane permeabilization (MOMP) constitutes one of the major hallmarks of mitochondrial apoptosis.17,18,246,247 Of note, DiOC6(3) is not compatible with fixation, but other Δψm-sensitive probes are, including chloromethyltetramethylrosamine (absorption/emission peaks: 554/576 nm).248 MOMP is accompanied by the massive activation of caspase-9 and -3, while caspase-8 is required for ICD upstream of MOMP. The activation of caspases can be documented by flow cytometry or fluorescence microscopy, either upon the immunostaining of cells with monoclonal antibodies specific for active caspase fragments, or with cell-permeant caspase substrates that become fluorescent upon cleavage.9,249,250 Alternatively, caspase activation can be detected in a semi-quantitative manner by immunoblotting, with antibodies specific for caspases (which are themselves activated by cleavage) or their substrates.250,251
As MOMP ensues the assembly of BAX/BAK1-containing oligomers across the outer mitochondrial membrane, the process can also be monitored by means of green fluorescent protein (GFP)-BAX chimeras (GFP absorption/emission peaks: 395/509 nm). In this setting, the relocalization of BAX to mitochondria can be followed by fluorescence microscopy as a shift in GFP fluorescence from a diffuse to a punctate or network-like pattern.40,252 Finally, one of the major morphological modifications of apoptosis (and hence of ICD) is nuclear condensation (pyknosis).1,2,95 Also this process can be conveniently monitored by fluorescence microscopy, either in cells that constitutively express a GFP- or red fluorescent protein (RFP)-tagged variant of histone 2B (RFP-H2B, absorption/emission peaks: 584/607 nm) or upon fixation and staining with the chromatinophilic dye Hoechst 33342 (absorption/emission peaks: 361/461 nm).40,95,235
CALR exposure
Several assays are available to directly monitor the ICD-associated translocation of CALR on the outer leaflet of the plasma membrane. For instance, this can be achieved on flow cytometry, by staining non-permeabilized cells with a CALR-specific antibody, or in cells that stably express a CALR-HaloTag™ fusion protein.40,95 In the latter scenario, the HaloTag™ label can be visualized by a cell-impermeant fluorescent chemical, resulting in the specific detection of the CALR molecules that are effectively accessible for ligand binding from the extracellular microenvironment.40,95 In both cases, it is imperative to remove from the analysis dead (PI+ or DAPI+) cells, as the permeabilized plasma membrane allows both the CALR-specific antibody and the normally cell-impermeant HaloTag™ ligand to access intracellular CALR.34,40 Alternatively, CALR exposure can be monitored upon the biotinylation of cell surface proteins (which must be performed in pre-apoptotic conditions, when plasma membranes are intact, to avoid false-positive results owing to intracellular CALR), followed by streptavidin-mediated precipitation, and detection by immunoblotting,34,66,253 or by fluorescence microscopy, in cells that constitutively express a CALR-GFP fusion construct. For the sake of precision, it should be noted that the latter system does not detect CALR-GFP exposure in itself, but the ER perinuclear clustering that invariably accompanies exposure.20,232 We have also successfully employed a PDIA3-specific antibody and flow cytometry as well as PDIA3-GFP-expressing cells and fluorescence microscopy to (indirectly) assess CALR exposure in the course of ICD, as in our models PDIA3 invariably co-translocates with CALR on the surface of cells exposed to ICD inducers.36,37,95 However, this does not apply to all experimental settings,66,67 implying that the PDIA3-GFP fusion is a useful confirmatory tool but cannot be employed as a standalone means to identify all instances of ICD.
In some instances, it may be important to monitor CALR exposure along with the proficiency of the ER stress response. This may indeed allow for the identification of defects in the signaling pathway that leads to the translocation of CALR to the outer leaflet of the plasma membrane. Several assays are currently available for the detection of the different arms of the ER stress response.136,254-256 For instance, the phosphorylation state of EIF2A and/or of the major EIF2A kinases, including EIF2A kinase 1 (EIF2AK1, best known as HRI),257 EIF2AK2 (best known as PKR),258 and EIF2AK3 (best known as PERK),259–261 can be assessed by immunoblotting, flow cytometry or immunofluorescence microscopy with phosphoneoepitope-specific antibodies.260 The splicing status of X-box binding protein 1 (XBP1) mRNA, reflecting the activation of the ER stress sensor endoplasmic reticulum to nucleus signaling 1 (ERN1, best known as IRE1α), can be monitored by quantitative real-time RT-PCR,262 as well as by flow cytometry or fluorescence microscopy, either in cells that express a fluorescently-tagged version of XBP1263 or upon the administration of a self-quenched RNA probe that can be cleaved by IRE1α.264 Finally, the nuclear redistribution of activating transcription factor 6 (ATF6) can be easily evaluated by fluorescence microscopy in cells that constitutively express GFP- or RFP-tagged variants of ATF6.52 As an alternative, ER stress can be indirectly monitored upon the formation of GTPase activating protein (SH3 domain) binding protein 1 (G3BP1)-containing granules in cells genetically modified to express a G3BP1-GFP fusion.40,265 This said, G3BP1 appears to redistribute to granules in response to a wide panel of stressful conditions that are not limited to specific perturbations of reticular homeostasis. Thus, monitoring G3BP1 aggregation can be useful to determine whether cells mount a stress response to a putative inducer of ICD, yet cannot be employed to formally imply the ER in this process.
ATP secretion
The ICD-associated secretion of ATP can be monitored by 2 complementary approaches: directly, by quantification of extracellular ATP,137,180 or indirectly, by the assessment of residual intracellular ATP.137,139 The most employed method currently available for the quantification of ATP levels relies on the ability of eukaryotic luciferases to produce light while oxidizing D-(-)-luciferin (which must be added exogenously) in a ATP-dependent manner.266,267 This can be applied to culture supernatants as well as to cell lysates, and hence is compatible with both the direct and indirect assessment of ATP secretion in the course of ICD. The vesicular pool of ATP can also be visualized by fluorescence microscopy upon staining cells with the ATP-binding fluorochrome quinacrine (absorption/emission peaks: 436/525 nm).268 Alternatively, intracellular ATP can be monitored in living cells by a fluorescence resonance energy transfer (FRET)-based assay involving a yellow fluorescent protein-cyan fluorescent protein (YFP-CFP) fusion containing a domain that changes its conformation upon ATP binding, hence shifting the spectral properties of the probe.269
In some settings, it may be relevant to monitor the autophagic response that generally precedes and is required for ICD-associated ATP release. This can be achieved by a wide panel of techniques, whose detailed discussion goes largely beyond the scope of this set of recommendations.15,270,271 This said, one of the most convenient approaches to obtain insights into the autophagic response of cells exposed to homeostatic perturbations relies on the use of a GFP- or RFP-tagged variant of microtubule-associated protein 1 light chain 3 (MAP1LC3, best known as LC3).272 In the course of autophagy, LC3 gets conjugated to phosphatidylethanolamine, hence acquiring the ability to accumulate into forming autophagosomes.273,274 In line with this notion, GFP-LC3 redistributes from a diffuse to a punctate pattern in cells mounting an autophagic response, a phenomenon that can readily be monitored by fluorescence microscopy.
HMGB1 release
Similar to the secretion of ATP, the release of HMGB1 in the supernatant of cells undergoing ICD can be monitored directly or indirectly, as a function of residual intracellular HMGB1.41,207,275 The former approach relies on the immunoblotting-based assessment of HGMB1 in concentrated cell supernatants, or (most often) on commercially available enzyme-linked immunosorbent assay (ELISA) kits specific for human or murine HMGB1. These kits generally allow for the precise quantification of HMGB1 concentrations in a wide panel of biological specimens, including culture supernatants, serum samples and interstitial fluids, yet may be relatively expensive for use in large-scale screening campaigns.95,147,275 Alternatively, HMGB1 release can be assessed by fluorescence microscopy in cells expressing a GFP-tagged variant of HMGB1, as the loss of colocalization between the GFP signal and a nuclear stain (e.g., Hoechst 33342, H2B-RFP).275 This said, the precise quantification of HMGB1 variants exhibiting differential redox states requires mass spectroscopy.276
High-content, high-throughput platform
Cell death that is not accompanied by CALR exposure, ATP secretion and HMGB1 release is generally not perceived as immunogenic.34,41,119 In other words, the absence of only one such ICD-associated events often entails a consistent decrease in the immunogenicity of cell death, if not its total loss. This implies that the ability of a given intervention to promote ICD can be inferred in vitro only upon the concurrent evaluation of all ICD hallmarks. Indeed, cells succumbing to homeostatic perturbations that stimulate ATP secretion and HMGB1 release but not CALR exposure, such as the administration of cisplatin, fail to elicit adaptive immune responses upon inoculation into immunocompetent mice.34,39,40 This said, a platform that would allow for the simultaneous detection of cell death, CALR exposure, ATP secretion and HMGB1 release in the context of large screening campaigns was missing. To circumvent this obstacle to the identification of novel, perhaps clinically relevant bona fide inducers of ICD, we recently developed a robotized cell biology platform that allows for entirely automated compound handling and multiplex read-out capability (including fluorescence microscopy, flow cytometry and bioluminescence detection) in sterile conditions. We then designed fully automated workflows based on various combinations of the assays described above and including appropriate procedures for data handling/normalization and statistical analysis. This approach is compatible with the high-content, high-throughput screening of large chemical libraries, returning a cumulative score that represents the ability of a specific compound to promote the 4 tenets of ICD. Importantly, this integrated platform does not abolish the need to evaluate putative ICD inducers for their capacity to elicit protective anticancer immune responses in gold-standard vaccination assays. Nonetheless, it allows for the relatively straightforward identification of candidate molecules. By means of this approach, septacidin has been identified as a bona fide ICD inducer.232 Moreover, cardiac glycosides were found to robustly improve the immunogenic potential of cell death.95-97,235 We expect this platform not only to allow for the discovery of other ICD inducers, but also to facilitate the understanding of the molecular pathways that underlie ICD and how these can be modulated for therapeutic purposes.
Concluding Remarks and Future Directions
As described above, the simultaneous detection of cell death, CALR exposure, ATP secretion and HMGB1 release by means of a high-content-, high-throughput-compatible platform is useful for the identification of candidate ICD inducers among large chemical libraries. Nonetheless, vaccination assays involving immunocompetent mice and syngeneic cancer cells do not cease to constitute the gold-standard approach to formally identify bona fide triggers of ICD.
Paradoxically, the major obstacle to the identification and development of clinically relevant ICD inducers appears to be represented by the murine system itself, as rodent and human cells do not necessarily respond to a specific stimulus in a comparable fashion. As a standalone example, mouse cells are highly resistant to the cytotoxic activity of cardiac glycosides, owing to the expression of a mutated subunit of their target, the Na2+/K+ ATPase.95,277 This implies that formally determining whether a given intervention provokes ICD in the human system is complicated. Humanized rodent models, i.e., immunodeficient mice reconstituted with a human immune system,278 may partially circumvent this issue. However, the interaction between human immune cells and the murine microenvironment may be negatively influenced by inter-species molecular variations that compromise the ability of the former to mount an appropriate immune response.279,280 Thus, although attempts are being made to limit such variations,281 experimental models that allow for the proper evaluation of ICD in the human system require further improvements. Finally, the procedure outlined above for the identification of novel ICD inducers assesses the biochemical processes that are required for the immunogenicity of anthracycline-induced cell death. However, ICD might exist in functionally distinct variants, implying that hitherto uncharacterized mechanisms might render cell death immunogenic. This possibility should be actively investigated in future studies.
Irrespective of these caveats, we are confident that the screening of large chemical or small-interfering RNA libraries combined with vaccination assays in the murine model will allow for the identification of novel, therapeutically relevant interventions for the induction or modulation of ICD. Moreover, the immunohistochemical detection of ICD-associated biomarkers in bioptic specimens from cancer patients may convey robust predictive or prognostic indications, at least under some circumstances.282,283 The implementation of well-designed, longitudinal immunomonitoring procedures into the clinical development of antineoplastic agents is required to ascertain the actual prognostic or predictive value of ICD-associated processes among oncological patients.284-286 Of note, a Phase I clinical study has recently been launched to investigate the safety and preliminary therapeutic efficacy of adenoviral vectors genetically modified to trigger ICD, in subjects with malignant glioma and glioblastoma multiforme (NCT01811992). In this setting, serotype 5, replication-defective, first-generation adenoviruses encoding the HSV-1 thymidine kinase and similar vectors coding for FLT3LG are co-infused at the time of surgical tumor resection, followed by valacyclovir (a gancylovir-like prodrug converted by the viral thymidine kinase and cellular kinases into its triphosphate cytotoxic variant)287,288 in the context of current standard-of-care therapy (source https://clinicaltrials.gov/). The results of such a first-in-man study relying on the genetic induction of ICD in cancer patients are urgently awaited.
Acknowledgments
GL and LG are supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; AXA Chair for Longevity Research; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Disclosure of Potential Conflicts of Interest
AH owns a share of Oncos Therapeutics Ltd. (Helsinki, Finland) and TILT Biotherapeutics Ltd. (Helsinki, Finalnd), and is a legal employee of the latter.
References
- 1. Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ 2007; 14:1237-43; PMID:; http://dx.doi.org/ 10.1038/sj.cdd.4402148 [DOI] [PubMed] [Google Scholar]
- 2. Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. . Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ 2009; 16:3-11; PMID:; http://dx.doi.org/ 10.1038/cdd.2008.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deir WS, Fulda S, et al. . Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ 2012; 19:107-20; PMID:; http://dx.doi.org/ 10.1038/cdd.2011.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 2010; 11:700-14; PMID:; http://dx.doi.org/ 10.1038/nrm2970 [DOI] [PubMed] [Google Scholar]
- 5. Vanden Berghe T, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 2014; 15:135-47; PMID:; http://dx.doi.org/ 10.1038/nrm3737 [DOI] [PubMed] [Google Scholar]
- 6. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 7. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860-75; PMID:; http://dx.doi.org/ 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 8. Vogt CI. Untersuchungen über die Entwicklungsgeschichte der Geburtshelferkröte (Alytes obstetricans). Jent, 1842. [Google Scholar]
- 9. Galluzzi L, Aaronson SA, Abrams J, Alnemri ES, Andrews DW, Baehrecke EH, Bazan NG, Blagosklonny MV, Blomgren K, Borner C, et al. . Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ 2009; 16:1093-107; PMID:; http://dx.doi.org/ 10.1038/cdd.2009.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: past, present and future. Cell Death Dis 2014; 5:e1257; PMID:; http://dx.doi.org/ 10.1038/cddis.2013.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26:239-57; PMID:; http://dx.doi.org/ 10.1038/bjc.1972.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest 2005; 115:2679-88; PMID:; http://dx.doi.org/ 10.1172/JCI26390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ericsson JL. Studies on induced cellular autophagy. I. Electron microscopy of cells with in vivo labelled lysosomes. Exp Cell Res 1969; 55:95-106; PMID:; http://dx.doi.org/ 10.1016/0014-4827(69)90462-5 [DOI] [PubMed] [Google Scholar]
- 14. Maximow AA. Studies on the Changes Produced by Roentgen Rays in Inflamed Connective Tissue. J Exp Med 1923; 37:319-40; PMID:; http://dx.doi.org/ 10.1084/jem.37.3.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. . Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008; 4:151-75; PMID:; http://dx.doi.org/ 10.4161/auto.5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 1995; 182:1545-56; PMID:; http://dx.doi.org/ 10.1084/jem.182.5.1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 2010; 11:621-32; PMID:; http://dx.doi.org/ 10.1038/nrm2952 [DOI] [PubMed] [Google Scholar]
- 18. Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev 2007; 87:99-163; PMID:; http://dx.doi.org/ 10.1152/physrev.00013.2006 [DOI] [PubMed] [Google Scholar]
- 19. Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 2008; 9:231-41; PMID:; http://dx.doi.org/ 10.1038/nrm2312 [DOI] [PubMed] [Google Scholar]
- 20. Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 2005; 6:328-40; PMID:; http://dx.doi.org/ 10.1038/nrm1619 [DOI] [PubMed] [Google Scholar]
- 21. Galluzzi L, Kepp O, Krautwald S, Kroemer G, Linkermann A. Molecular mechanisms of regulated necrosis. Semin Cell Dev Biol 2014; 35C:24-32; PMID 24582829; http://dx.doi: 10.1016/j.semcdb.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 22. Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. . Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691-701; PMID:; http://dx.doi.org/ 10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Activation of dendritic cells by tumor cell death. Oncoimmunology 2012; 1:1218-9; PMID:; http://dx.doi.org/ 10.4161/onci.20428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 2006; 27:244-50; PMID:; http://dx.doi.org/ 10.1016/j.it.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 25. Abud HE. Shaping developing tissues by apoptosis. Cell Death Differ 2004; 11:797-9; PMID:; http://dx.doi.org/ 10.1038/sj.cdd.4401455 [DOI] [PubMed] [Google Scholar]
- 26. Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum 2002; 46:191-201; PMID:; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 27. Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 2010; 6:280-9; PMID:; http://dx.doi.org/ 10.1038/nrrheum.2010.46 [DOI] [PubMed] [Google Scholar]
- 28. Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology 2012; 1:179-88; PMID:; http://dx.doi.org/ 10.4161/onci.1.2.19026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2013; 2:e23510; PMID:; http://dx.doi.org/ 10.4161/onci.23510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology 2014; 3:e27878; PMID:; http://dx.doi.org/ 10.4161/onci.27878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011; 71:4809-20; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0753 [DOI] [PubMed] [Google Scholar]
- 32. Cirone M, Garufi A, Di Renzo L, Granato M, Faggioni A, D’Orazi G. Zinc supplementation is required for the cytotoxic and immunogenic effects of chemotherapy in chemoresistant p53-functionally deficient cells. Oncoimmunology 2013; 2:e26198; http://dx.doi.org/ 10.4161/onci.26198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 2014; 21:15-25; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. . Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:; http://dx.doi.org/ 10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 35. Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 2011; 71:4821-33; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-0950 [DOI] [PubMed] [Google Scholar]
- 36. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, et al. . Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 2009; 28:578-90; PMID:; http://dx.doi.org/ 10.1038/emboj.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, et al. . The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 2008; 15:1499-509; PMID:; http://dx.doi.org/ 10.1038/emboj.2009.1x [DOI] [PubMed] [Google Scholar]
- 38. Bugaut H, Bruchard M, Berger H, Derangere V, Odoul L, Euvrard R, Ladoire S, Chalmin F, Végran F, Rébé C, et al. . Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One 2013; 8:e65181; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0065181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. . Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29:482-91; PMID:; http://dx.doi.org/ 10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 40. Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, et al. . Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2011; 30:1147-58; PMID:; http://dx.doi.org/ 10.1038/onc.2010.500 [DOI] [PubMed] [Google Scholar]
- 41. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. . Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID:; http://dx.doi.org/ 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- 42. Gou HF, Huang J, Shi HS, Chen XC, Wang YS. Chemo-immunotherapy with oxaliplatin and interleukin-7 inhibits colon cancer metastasis in mice. PLoS One 2014; 9:e85789; http://dx.doi.org/ 10.1371/journal.pone.0085789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tongu M, Harashima N, Yamada T, Harada T, Harada M. Immunogenic chemotherapy with cyclophosphamide and doxorubicin against established murine carcinoma. Cancer Immunol Immunother 2010; 59:769-77; PMID:; http://dx.doi.org/ 10.1007/s00262-009-0797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT, Belardelli F, et al. . Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res 2011; 71:768-78; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2788 [DOI] [PubMed] [Google Scholar]
- 45. Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 2011; 33:369-83; PMID:; http://dx.doi.org/ 10.1007/s00281-011-0245-0 [DOI] [PubMed] [Google Scholar]
- 46. Stoetzer OJ, Fersching DM, Salat C, Steinkohl O, Gabka CJ, Hamann U, Braun M, Feller AM, Heinemann V, Siegele B, et al. . Circulating immunogenic cell death biomarkers HMGB1 and RAGE in breast cancer patients during neoadjuvant chemotherapy. Tumour Biol 2013; 34:81-90; PMID:; http://dx.doi.org/ 10.1007/s13277-012-0513-1 [DOI] [PubMed] [Google Scholar]
- 47. Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, Wakefield LM, Oppenheim JJ. Effective chemoimmunotherapy with anti-TGFbeta antibody and cyclophosphamide in a mouse model of breast cancer. PLoS One 2014; 9:e85398; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0085398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guerriero JL, Ditsworth D, Fan Y, Zhao F, Crawford HC, Zong WX. Chemotherapy induces tumor clearance independent of apoptosis. Cancer Res 2008; 68:9595-600; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beneteau M, Zunino B, Jacquin MA, Meynet O, Chiche J, Pradelli LA, Marchetti S, Cornille A, Carles M, Ricci JE. Combination of glycolysis inhibition with chemotherapy results in an antitumor immune response. Proc Natl Acad Sci U S A 2012; 109:20071-6; PMID:; http://dx.doi.org/ 10.1073/pnas.1206360109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov 2013; 12:829-46; PMID:; http://dx.doi.org/ 10.1038/nrd4145 [DOI] [PubMed] [Google Scholar]
- 51. Hoffmann J, Vitale I, Buchmann B, Galluzzi L, Schwede W, Senovilla L, Skuballa W, Vivet S, Lichtner RB, Vicencio JM, et al. . Improved cellular pharmacokinetics and pharmacodynamics underlie the wide anticancer activity of sagopilone. Cancer Res 2008; 68:5301-8; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0237 [DOI] [PubMed] [Google Scholar]
- 52. Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, et al. . An immunosurveillance mechanism controls cancer cell ploidy. Science 2012; 337:1678-84; PMID:; http://dx.doi.org/ 10.1126/science.1224922 [DOI] [PubMed] [Google Scholar]
- 53. Pellicciotta I, Yang CP, Goldberg GL, Shahabi S. Epothilone B enhances Class I HLA and HLA-A2 surface molecule expression in ovarian cancer cells. Gynecol Oncol 2011; 122:625-31; PMID:; http://dx.doi.org/ 10.1016/j.ygyno.2011.05.007 [DOI] [PubMed] [Google Scholar]
- 54. Dutcher JD, Vonsaltza MH, Pansy FE. Septacidin, a new antitumor and antifungal antibiotic produced by streptomyces fibriatus. Antimicrob Agents Chemother (Bethesda) 1963; 161:83-8; PMID: [PubMed] [Google Scholar]
- 55. Sukkurwala AQ, Martins I, Wang Y, Schlemmer F, Ruckenstuhl C, Durchschlag M, Michaud M, Senovilla L, Sistigu A, Ma Y, et al. . Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell Death Differ 2014; 21:59-68; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perez CA, Fu A, Onishko H, Hallahan DE, Geng L. Radiation induces an antitumour immune response to mouse melanoma. Int J Radiat Biol 2009; 85:1126-36; PMID:; http://dx.doi.org/ 10.3109/09553000903242099 [DOI] [PubMed] [Google Scholar]
- 57. Vacchelli E, Vitale I, Tartour E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: anticancer radioimmunotherapy. Oncoimmunology 2013; 2:e25595; PMID:; http://dx.doi.org/ 10.4161/onci.25595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology 2013; 2:e26536; PMID:; http://dx.doi.org/ 10.4161/onci.26536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012; 72:3967-76; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0851 [DOI] [PubMed] [Google Scholar]
- 60. Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, Mariño G, Kepp O, Michaud M, Perfettini JL, et al. . Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ 2014; 21:92-9; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys 2012; 84:879-80; PMID:; http://dx.doi.org/ 10.1016/j.ijrobp.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014; 5:403-16; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schildkopf P, Frey B, Ott OJ, Rubner Y, Multhoff G, Sauer R, Fietkau R, Gaipl US. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol 2011; 101:109-15; PMID:; http://dx.doi.org/ 10.1016/j.radonc.2011.05.056 [DOI] [PubMed] [Google Scholar]
- 64. Gorin JB, Menager J, Gouard S, Maurel C, Guilloux Y, Faivre-Chauvet A, Morgenstern A, Bruchertseifer F, Chérel M, Davodeau F, et al. . Antitumor immunity induced after alpha irradiation. Neoplasia 2014; 16:319-28; PMID:; http://dx.doi.org/ 10.1016/j.neo.2014.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Garg AD, Krysko DV, Vandenabeele P, Agostinis P. Hypericin-based photodynamic therapy induces surface exposure of damage-associated molecular patterns like HSP70 and calreticulin. Cancer Immunol Immunother 2012; 61:215-21; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, et al. . A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 2012; 31:1062-79; PMID:; http://dx.doi.org/ 10.1038/emboj.2011.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Galluzzi L, Kepp O, Kroemer G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J 2012; 31:1055-7; PMID:; http://dx.doi.org/ 10.1038/emboj.2012.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, Mathieu C, Agostinis P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy 2013; 9:1292-307; PMID:; http://dx.doi.org/ 10.4161/auto.25399 [DOI] [PubMed] [Google Scholar]
- 69. Garg AD, Agostinis P. ER stress, autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol Sci 2014; 13:474-87; PMID:; http://dx.doi.org/ 10.1039/c3pp50333j [DOI] [PubMed] [Google Scholar]
- 70. Garg AD, Dudek AM, Agostinis P. Autophagy-dependent suppression of cancer immunogenicity and effector mechanisms of innate and adaptive immunity. Oncoimmunology 2013; 2:e26260; http://dx.doi.org/ 10.4161/onci.26260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res 2005; 65:1018-26; PMID: [PubMed] [Google Scholar]
- 72. Panzarini E, Inguscio V, Dini L. Immunogenic cell death: can it be exploited in PhotoDynamic Therapy for cancer? Biomed Res Int 2013; 2013:482160; PMID:; http://dx.doi.org/ 10.1155/2013/482160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yu P, Fu YX. Targeting tumors with LIGHT to generate metastasis-clearing immunity. Cytokine Growth Factor Rev 2008; 19:285-94; PMID:; http://dx.doi.org/ 10.1016/j.cytogfr.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fucikova J, Moserova I, Truxova I, Hermanova I, Vancurova I, Partlova S, Fialova A, Sojka L, Cartron PF, Houska M, et al. . High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int J Cancer 2014; 135:1165-77; PMID:; http://dx.doi.org/ 10.1002/ijc.28766 [DOI] [PubMed] [Google Scholar]
- 75. Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, Erbs P, Limacher JM, Preville X, Zitvogel L, et al. . Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology 2014; 3:28694; PMID:; http://dx.doi.org/ 10.4161/onci.28694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology 2013; 2:e24612; PMID:; http://dx.doi.org/ 10.4161/onci.24612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Angelova AL, Grekova SP, Heller A, Kuhlmann O, Soyka E, Giese T, Aprahamian M, Bour G, Rüffer S, Cziepluch C, et al. . Complementary induction of immunogenic cell death by oncolytic parvovirus H-1PV and gemcitabine in pancreatic cancer. J Virol 2014; 88:5263-76; PMID:; http://dx.doi.org/ 10.1128/JVI.03688-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Workenhe ST, Mossman KL. Rewiring cancer cell death to enhance oncolytic viro-immunotherapy. Oncoimmunology 2013; 2:e27138; http://dx.doi.org/ 10.4161/onci.27138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Workenhe ST, Pol JG, Lichty BD, Cummings DT, Mossman KL. Combining oncolytic HSV-1 with immunogenic cell death-inducing drug mitoxantrone breaks cancer immune tolerance and improves therapeutic efficacy. Cancer Immunol Res 2013; 1:309-19; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0059-T [DOI] [PubMed] [Google Scholar]
- 80. Bartlett DL, Liu Z, Sathaiah M, Ravindranathan R, Guo Z, He Y, Guo ZS. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer 2013; 12:103; PMID:; http://dx.doi.org/ 10.1186/1476-4598-12-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Guo ZS, Liu Z, Bartlett DL. Oncolytic immunotherapy: dying the right way is a key to eliciting potent antitumor immunity. Front Oncol 2014; 4:74; PMID:; http://dx.doi.org/ 10.3389/fonc.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Huang B, Sikorski R, Kirn DH, Thorne SH. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther 2011; 18:164-72; PMID:; http://dx.doi.org/ 10.1038/gt.2010.121 [DOI] [PubMed] [Google Scholar]
- 83. Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, Hemminki O, Diaconu I, Pesonen S, Koski A, et al. . Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther 2013; 21:1212-23; PMID:; http://dx.doi.org/ 10.1038/mt.2013.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mineharu Y, King GD, Muhammad AK, Bannykh S, Kroeger KM, Liu C, Lowenstein PR, Castro MG. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res 2011; 17:4705-18; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood 2007; 109:4839-45; PMID:; http://dx.doi.org/ 10.1182/blood-2006-10-054221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Demaria S, Santori FR, Ng B, Liebes L, Formenti SC, Vukmanovic S. Select forms of tumor cell apoptosis induce dendritic cell maturation. J Leukoc Biol 2005; 77:361-8; PMID:; http://dx.doi.org/ 10.1189/jlb.0804478 [DOI] [PubMed] [Google Scholar]
- 87. Cirone M, Di Renzo L, Lotti LV, Conte V, Trivedi P, Santarelli R, Gonnella R, Frati L, Faggioni A. Primary effusion lymphoma cell death induced by bortezomib and AG 490 activates dendritic cells through CD91. PLoS One 2012; 7:e31732; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0031732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen HM, Wang PH, Chen SS, Wen CC, Chen YH, Yang WC, Yang NS. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol Immunother 2012; 61:1989-2002; PMID:; http://dx.doi.org/ 10.1007/s00262-012-1258-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Garrido G, Rabasa A, Sanchez B, Lopez MV, Blanco R, Lopez A, Hernández DR, Pérez R, Fernández LE. Induction of immunogenic apoptosis by blockade of epidermal growth factor receptor activation with a specific antibody. J Immunol 2011; 187:4954-66; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1003477 [DOI] [PubMed] [Google Scholar]
- 90. D’Eliseo D, Manzi L, Velotti F. Capsaicin as an inducer of damage-associated molecular patterns (DAMPs) of immunogenic cell death (ICD) in human bladder cancer cells. Cell Stress Chaperones 2013; 18:801-8; http://dx.doi.org/ 10.1007/s12192-013-0422-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gilardini Montani MS, D’Eliseo D, Cirone M, Di Renzo L, Faggioni A, Santoni A, et al. . Capsaicin-mediated apoptosis of human bladder cancer cells activates dendritic cells via CD91. Nutrition 2014; In Press; PMID 25220876; http://dx.doi: 10.1016/j.nut.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 92. Molinari R, D’Eliseo D, Manzi L, Zolla L, Velotti F, Merendino N. The n3-polyunsaturated fatty acid docosahexaenoic acid induces immunogenic cell death in human cancer cell lines via pre-apoptotic calreticulin exposure. Cancer Immunol Immunother 2011; 60:1503-7; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1074-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Emeagi PU, Van Lint S, Goyvaerts C, Maenhout S, Cauwels A, McNeish IA, Bos T, Heirman C, Thielemans K, Aerts JL, et al. . Proinflammatory characteristics of SMACDIABLO-induced cell death in antitumor therapy. Cancer Res 2012; 72:1342-52; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2400 [DOI] [PubMed] [Google Scholar]
- 94. Emeagi PU, Thielemans K, Breckpot K. The role of SMAC mimetics in regulation of tumor cell death and immunity. Oncoimmunology 2012; 1:965-7; PMID:; http://dx.doi.org/ 10.4161/onci.20369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et al. . Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 2012; 4:143ra99; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3003807 [DOI] [PubMed] [Google Scholar]
- 96. Kepp O, Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Sukkurwala AQ, Michaud M, Galluzzi L, Zitvogel L, et al. . Anticancer activity of cardiac glycosides: at the frontier between cell-autonomous and immunological effects. Oncoimmunology 2012; 1:1640-2; PMID:; http://dx.doi.org/ 10.4161/onci.21684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Cardiac glycosides and cancer therapy. Oncoimmunology 2013; 2:e23082; PMID:; http://dx.doi.org/ 10.4161/onci.23082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Riganti C, Castella B, Kopecka J, Campia I, Coscia M, Pescarmona G, Bosia A, Ghigo D, Massaia M. Zoledronic acid restores doxorubicin chemosensitivity and immunogenic cell death in multidrug-resistant human cancer cells. PLoS One 2013; 8:e60975; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0060975 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99. Riganti C, Massaia M. Inhibition of the mevalonate pathway to override chemoresistance and promote the immunogenic demise of cancer cells: Killing two birds with one stone. Oncoimmunology 2013; 2:e25770; http://dx.doi.org/ 10.4161/onci.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liljenfeldt L, Gkirtzimanaki K, Vyrla D, Svensson E, Loskog AS, Eliopoulos AG. Enhanced therapeutic anti-tumor immunity induced by co-administration of 5-fluorouracil and adenovirus expressing CD40 ligand. Cancer Immunol Immunother 2014; 63:273-82; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1507-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Palombo F, Focaccetti C, Barnaba V. Therapeutic implications of immunogenic cell death in human cancer. Front Immunol 2014; 4:503; PMID:; http://dx.doi.org/ 10.3389/fimmu.2013.00503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Olsson L, Ebbesen P. Experimental induction of tumor growth control by immune adjuvants: current status and some theories to be explored. Biomedicine 1978; 28:88-91; PMID: [PubMed] [Google Scholar]
- 103. Matzinger P. The danger model: a renewed sense of self. Science 2002; 296:301-5; PMID:; http://dx.doi.org/ 10.1126/science.1071059 [DOI] [PubMed] [Google Scholar]
- 104. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 105. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11:215-33; PMID:; http://dx.doi.org/ 10.1038/nrd3626 [DOI] [PubMed] [Google Scholar]
- 106. Ghiringhelli F, Apetoh L. The interplay between the immune system and chemotherapy: emerging methods for optimizing therapy. Expert Rev Clin Immunol 2014; 10:19-30; PMID:; http://dx.doi.org/ 10.1586/1744666X.2014.865520 [DOI] [PubMed] [Google Scholar]
- 107. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. . Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:; http://dx.doi.org/ 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 108. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 109. Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, Sautès-Fridman C, Ma Y, Tartour E, Zitvogel L, et al. . Trial watch: prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012; 1:1323-43; PMID:; http://dx.doi.org/ 10.4161/onci.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, et al. . Towards the introduction of the ‘immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232:199-209; PMID:; http://dx.doi.org/ 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, et al. . Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013; 39:782-95; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 112. Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, et al. . Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res 2014; 20:1891-9; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2830 [DOI] [PubMed] [Google Scholar]
- 113. Bindea G, Mlecnik B, Angell HK, Galon J. The immune landscape of human tumors: implications for cancer immunotherapy. Oncoimmunology 2014; 3:e27456; PMID:; http://dx.doi.org/ 10.4161/onci.27456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Paroli M, Bellati F, Videtta M, Focaccetti C, Mancone C, Donato T, Antonilli M, Perniola G, Accapezzato D, Napoletano C, et al. . Discovery of chemotherapy-associated ovarian cancer antigens by interrogating memory T cells. Int J Cancer 2014; 134:1823-34; PMID:; http://dx.doi.org/ 10.1002/ijc.28515 [DOI] [PubMed] [Google Scholar]
- 115. Kohles N, Nagel D, Jungst D, Stieber P, Holdenrieder S. Predictive value of immunogenic cell death biomarkers HMGB1, sRAGE, and DNase in liver cancer patients receiving transarterial chemoembolization therapy. Tumour Biol 2012; 33:2401-9; PMID:; http://dx.doi.org/ 10.1007/s13277-012-0504-2 [DOI] [PubMed] [Google Scholar]
- 116. Stoetzer OJ, Wittwer C, Lehner J, Fahmueller YN, Kohles N, Fersching DM, Leszinski G, Roessner J, Holdenrieder S. Circulating nucleosomes and biomarkers of immunogenic cell death as predictive and prognostic markers in cancer patients undergoing cytotoxic therapy. Expert Opin Biol Ther 2012; 12 Suppl 1:S217-24; PMID:; http://dx.doi.org/ 10.1517/14712598.2012.689280 [DOI] [PubMed] [Google Scholar]
- 117. Wittwer C, Boeck S, Heinemann V, Haas M, Stieber P, Nagel D, Holdenrieder S. Circulating nucleosomes and immunogenic cell death markers HMGB1, sRAGE and DNAse in patients with advanced pancreatic cancer undergoing chemotherapy. Int J Cancer 2013; 133:2619-30; PMID:; http://dx.doi.org/ 10.1002/ijc.28294 [DOI] [PubMed] [Google Scholar]
- 118. Stoll G, Enot D, Mlecnik B, Galon J, Zitvogel L, Kroemer G. Immune-related gene signatures predict the outcome of neoadjuvant chemotherapy. Oncoimmunology 2014; 3:e27884; http://dx.doi.org/ 10.4161/onci.27884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T,Mignot G, Ullrich E, et al. . Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170-8; PMID:; http://dx.doi.org/ 10.1038/nm.2028 [DOI] [PubMed] [Google Scholar]
- 120. Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A, et al. . In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 2011; 224:389-400; PMID:; http://dx.doi.org/ 10.1002/path.2866 [DOI] [PubMed] [Google Scholar]
- 121. Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell 2010; 140:798-804; PMID:; http://dx.doi.org/ 10.1016/j.cell.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 122. Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell Death Dis 2013; 4:e631; PMID:; http://dx.doi.org/ 10.1038/cddis.2013.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Garg AD, Dudek AM, Agostinis P. Cancer immunogenicity, danger signals, and DAMPs: what, when, and how? Biofactors 2013; 39:355-67; PMID:; http://dx.doi.org/ 10.1002/biof.1125 [DOI] [PubMed] [Google Scholar]
- 124. Hou W, Zhang Q, Yan Z, Chen R, Zeh HJ, Iii, Kang R, Lotze MT, Tang D. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis 2013; 4:e966; PMID:; http://dx.doi.org/ 10.1038/cddis.2013.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Garg AD, Martin S, Golab J, Agostinis P. Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ 2014; 21:26-38; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 2010; 1805:53-71; PMID:; http://dx.doi.org/ 10.1016/j.bbcan.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 127. Honeychurch J, Dive C, Illidge TM. Synchronous apoptosis in established tumors leads to the induction of adaptive immunity. Oncoimmunology 2013; 2:e24501; http://dx.doi.org/ 10.4161/onci.24501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Inoue H, Tani K. Multimodal immunogenic cancer cell death as a consequence of anticancer cytotoxic treatments. Cell Death Differ 2014; 21:39-49; http://dx.doi.org/ 10.1038/cdd.2013.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Melis MH, Simpson KL, Dovedi SJ, Welman A, MacFarlane M, Dive C, Honeychurch J, Illidge TM. Sustained tumour eradication after induced caspase-3 activation and synchronous tumour apoptosis requires an intact host immune response. Cell Death Differ 2013; 20:765-73; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Matzinger P. An innate sense of danger. Ann N Y Acad Sci 2002; 961:341-2; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2002.tb03118.x [DOI] [PubMed] [Google Scholar]
- 131. Lotze MT, Deisseroth A, Rubartelli A. Damage associated molecular pattern molecules. Clin Immunol 2007; 124:1-4; PMID:; http://dx.doi.org/ 10.1016/j.clim.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pouwels SD, Heijink IH, ten Hacken NH, Vandenabeele P, Krysko DV, Nawijn MC, van Oosterhout AJ. DAMPs activating innate and adaptive immune responses in COPD. Mucosal Immunol 2014; 7:215-26; PMID:; http://dx.doi.org/ 10.1038/mi.2013.77 [DOI] [PubMed] [Google Scholar]
- 133. Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004; 4:469-78; PMID:; http://dx.doi.org/ 10.1038/nri1372 [DOI] [PubMed] [Google Scholar]
- 134. Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res 2007; 67:7941-4; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-1622 [DOI] [PubMed] [Google Scholar]
- 135. Kepp O, Galluzzi L, Martins I, Schlemmer F, Adjemian S, Michaud M, Sukkurwala AQ, Menger L, Zitvogel L, Kroemer G. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev 2011; 30:61-9; PMID:; http://dx.doi.org/ 10.1007/s10555-011-9273-4 [DOI] [PubMed] [Google Scholar]
- 136. Kepp O, Menger L, Vacchelli E, Locher C, Adjemian S, Yamazaki T, Martins I, Sukkurwala AQ, Michaud M, Senovilla L, et al. . Crosstalk between ER stress and immunogenic cell death. Cytokine Growth Factor Rev 2013; 24:311-8; PMID:; http://dx.doi.org/ 10.1016/j.cytogfr.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 137. Martins I, Tesniere A, Kepp O, Michaud M, Schlemmer F, Senovilla L, Séror C, Métivier D, Perfettini JL, Zitvogel L, et al. . Chemotherapy induces ATP release from tumor cells. Cell Cycle 2009; 8:3723-8; PMID:; http://dx.doi.org/ 10.4161/cc.8.22.10026 [DOI] [PubMed] [Google Scholar]
- 138. Martins I, Michaud M, Sukkurwala AQ, Adjemian S, Ma Y, Shen S, Kepp O, Menger L, Vacchelli E, Galluzzi L, et al. . Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy 2012; 8:413-5; PMID:; http://dx.doi.org/ 10.4161/auto.19009 [DOI] [PubMed] [Google Scholar]
- 139. Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. . Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573-7; PMID:; http://dx.doi.org/ 10.1126/science.1208347 [DOI] [PubMed] [Google Scholar]
- 140. Wang Y, Martins I, Ma Y, Kepp O, Galluzzi L, Kroemer G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 2013; 9:1624-5; PMID:; http://dx.doi.org/ 10.4161/auto.25873 [DOI] [PubMed] [Google Scholar]
- 141. Ma Y, Adjemian S, Yang H, Catani JP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, et al. . ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Oncoimmunology 2013; 2:e24568; PMID:; http://dx.doi.org/ 10.4161/onci.24568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Métivier D, Galluzzi L, Perfettini JL, et al. . Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 2014; 21:79-91; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lavieri R, Piccioli P, Carta S, Delfino L, Castellani P, Rubartelli A. TLR costimulation causes oxidative stress with unbalance of proinflammatory and anti-inflammatory cytokine production. J Immunol 2014; 192:5373-81; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1303480 [DOI] [PubMed] [Google Scholar]
- 144. Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, et al. . HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 2004; 5:825-30; PMID:; http://dx.doi.org/ 10.1038/sj.embor.7400205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Brusa D, Migliore E, Garetto S, Simone M, Matera L. Immunogenicity of 56 degrees C and UVC-treated prostate cancer is associated with release of HSP70 and HMGB1 from necrotic cells. Prostate 2009; 69:1343-52; PMID:; http://dx.doi.org/ 10.1002/pros.20981 [DOI] [PubMed] [Google Scholar]
- 146. Arnold T, Michlmayr A, Baumann S, Burghuber C, Pluschnig U, Bartsch R, Steger G, Gnant M, Bergmann M, Bachleitner-Hofmann T, et al. . Plasma HMGB-1 after the initial dose of epirubicindocetaxel in cancer. Eur J Clin Invest 2013; 43:286-91; PMID:; http://dx.doi.org/ 10.1111/eci.12043 [DOI] [PubMed] [Google Scholar]
- 147. Candolfi M, Yagiz K, Foulad D, Alzadeh GE, Tesarfreund M, Muhammad AK, Puntel M, Kroeger KM, Liu C, Lee S, et al. . Release of HMGB1 in response to proapoptotic glioma killing strategies: efficacy and neurotoxicity. Clin Cancer Res 2009; 15:4401-14; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med 1999; 5:1249-55; PMID:; http://dx.doi.org/ 10.1038/15200 [DOI] [PubMed] [Google Scholar]
- 149. Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol 2001; 13:114-9; PMID:; http://dx.doi.org/ 10.1016/S0952-7915(00)00191-6 [DOI] [PubMed] [Google Scholar]
- 150. Fredly H, Ersvaer E, Gjertsen BT, Bruserud O. Immunogenic apoptosis in human acute myeloid leukemia (AML): primary human AML cells expose calreticulin and release heat shock protein (HSP) 70 and HSP90 during apoptosis. Oncol Rep 2011; 25:1549-56; PMID: [DOI] [PubMed] [Google Scholar]
- 151. Aguilera R, Saffie C, Tittarelli A, Gonzalez FE, Ramirez M, Reyes D, Pereda C, Hevia D, García T, Salazar L, et al. . Heat-shock induction of tumor-derived danger signals mediates rapid monocyte differentiation into clinically effective dendritic cells. Clin Cancer Res 2011; 17:2474-83; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2384 [DOI] [PubMed] [Google Scholar]
- 152. Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012; 287:15874-85; PMID:; http://dx.doi.org/ 10.1074/jbc.M112.340588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Vega VL, Rodriguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, De Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol 2008; 180:4299-307; PMID:; http://dx.doi.org/ 10.4049/jimmunol.180.6.4299 [DOI] [PubMed] [Google Scholar]
- 154. Korbelik M, Banath J, Sun J, Canals D, Hannun YA, Separovic D. Ceramide and sphingosine-1-phosphate act as photodynamic therapy-elicited damage-associated molecular patterns: cell surface exposure. Int Immunopharmacol 2014; 20:359-65; PMID:; http://dx.doi.org/ 10.1016/j.intimp.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 2012; 13:780-8; PMID:; http://dx.doi.org/ 10.1038/nrm3479 [DOI] [PubMed] [Google Scholar]
- 156. Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 2011; 32:157-64; PMID:; http://dx.doi.org/ 10.1016/j.it.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 157. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010; 464:104-7; PMID:; http://dx.doi.org/ 10.1038/nature08780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237-41; PMID:; http://dx.doi.org/ 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 159. Shi Y, Mucsi AD, Ng G. Monosodium urate crystals in inflammation and immunity. Immunol Rev 2010; 233:203-17; PMID:; http://dx.doi.org/ 10.1111/j.0105-2896.2009.00851.x [DOI] [PubMed] [Google Scholar]
- 160. Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241-7; PMID:; http://dx.doi.org/ 10.1038/ni.1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, Fletcher G, Durkin C, Postigo A, Skehel M, et al. . F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity 2012; 36:635-45; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 162. Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 2004; 279:17079-84; PMID:; http://dx.doi.org/ 10.1074/jbc.M310859200 [DOI] [PubMed] [Google Scholar]
- 163. Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 2006; 177:1272-81; PMID:; http://dx.doi.org/ 10.4049/jimmunol.177.2.1272 [DOI] [PubMed] [Google Scholar]
- 164. Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 2008; 20:504-11; PMID:; http://dx.doi.org/ 10.1016/j.coi.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 165. Sonnemann J, Gressmann S, Becker S, Wittig S, Schmudde M, Beck JF. The histone deacetylase inhibitor vorinostat induces calreticulin exposure in childhood brain tumour cells in vitro. Cancer Chemother Pharmacol 2010; 66:611-6; PMID:; http://dx.doi.org/ 10.1007/s00280-010-1302-4 [DOI] [PubMed] [Google Scholar]
- 166. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J, Weiskopf K, Willingham SB, Raveh T, Park CY, et al. . Calreticulin is the dominant pro-phagocytic signal on multiple human cancers and is counterbalanced by CD47. Sci Transl Med 2010; 2:63ra94; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3001375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001; 14:303-13; PMID:; http://dx.doi.org/ 10.1016/S1074-7613(01)00111-X [DOI] [PubMed] [Google Scholar]
- 168. Lauber K, Ernst A, Orth M, Herrmann M, Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol 2012; 2:116; PMID:; http://dx.doi.org/ 10.3389/fonc.2012.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Krysko DV, Vandenabeele P. Clearance of dead cells: mechanisms, immune responses and implication in the development of diseases. Apoptosis 2010; 15:995-7; PMID:; http://dx.doi.org/ 10.1007/s10495-010-0524-6 [DOI] [PubMed] [Google Scholar]
- 170. Krysko DV, Vanden Berghe T, Parthoens E, D’Herde K, Vandenabeele P. Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Methods Enzymol 2008; 442:307-41; PMID:; http://dx.doi.org/ 10.1016/S0076-6879(08)01416-X [DOI] [PubMed] [Google Scholar]
- 171. Krysko DV, Vandenabeele P. From regulation of dying cell engulfment to development of anti-cancer therapy. Cell Death Differ 2008; 15:29-38; PMID:; http://dx.doi.org/ 10.1038/sj.cdd.4402271 [DOI] [PubMed] [Google Scholar]
- 172. Krysko DV, D’Herde K, Vandenabeele P. Clearance of apoptotic and necrotic cells and its immunological consequences. Apoptosis 2006; 11:1709-26; PMID:; http://dx.doi.org/ 10.1007/s10495-006-9527-8 [DOI] [PubMed] [Google Scholar]
- 173. Weiss EM, Frey B, Rodel F, Herrmann M, Schlucker E, Voll RE, Fietkau R, Gaipl US. Ex vivo- and in vivo-induced dead tumor cells as modulators of antitumor responses. Ann N Y Acad Sci 2010; 1209:109-17; PMID:; http://dx.doi.org/ 10.1111/j.1749-6632.2010.05743.x [DOI] [PubMed] [Google Scholar]
- 174. Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. . Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010; 467:863-7; PMID:; http://dx.doi.org/ 10.1038/nature09413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ayna G, Krysko DV, Kaczmarek A, Petrovski G, Vandenabeele P, Fesus L. ATP release from dying autophagic cells and their phagocytosis are crucial for inflammasome activation in macrophages. PLoS One 2012; 7:e40069; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0040069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Garg AD, Krysko DV, Vandenabeele P, Agostinis P. The emergence of phox-ER stress induced immunogenic apoptosis. Oncoimmunology 2012; 1:786-8; PMID:; http://dx.doi.org/ 10.4161/onci.19750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. . Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009; 461:282-6; PMID:; http://dx.doi.org/ 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. . Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729-41; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 179. McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010; 330:362-6; PMID:; http://dx.doi.org/ 10.1126/science.1195491 [DOI] [PubMed] [Google Scholar]
- 180. Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 2010; 70:855-8; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3566 [DOI] [PubMed] [Google Scholar]
- 181. Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 2007; 28:465-72; PMID:; http://dx.doi.org/ 10.1016/j.tips.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 182. Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, et al. . Extracellular ATP is a danger signal activating P2´7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 2010; 182:774-83; PMID:; http://dx.doi.org/ 10.1164/rccm.201003-0359OC [DOI] [PubMed] [Google Scholar]
- 183. Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis 2014; 5:e1102; http://dx.doi.org/ 10.1038/cddis.2014.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Xiang Y, Wang X, Yan C, Gao Q, Li SA, Liu J, Zhou K, Guo X, Lee W, Zhang Y. Adenosine-5′-triphosphate (ATP) protects mice against bacterial infection by activation of the NLRP3 inflammasome. PLoS One 2013; 8:e63759; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0063759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Gombault A, Baron L, Couillin I. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol 2012; 3:414; PMID:; http://dx.doi.org/ 10.3389/fimmu.2012.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis 2012; 3:e403; PMID:; http://dx.doi.org/ 10.1038/cddis.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, et al. . Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A 2009; 106:20388-93; PMID:; http://dx.doi.org/ 10.1073/pnas.0908698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of interleukin-1alpha or interleukin-1beta depends on mechanism of cell death. J Biol Chem 2014; 289:15942-50; PMID:; http://dx.doi.org/ 10.1074/jbc.M114.557561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Steer SA, Scarim AL, Chambers KT, Corbett JA. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med 2006; 3:e17; http://dx.doi.org/ 10.1371/journal.pmed.0030017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology 2012; 1:393-5; PMID:; http://dx.doi.org/ 10.4161/onci.19070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Aliagas E, Vidal A, Texido L, Ponce J, Condom E, Martin-Satue M. High expression of ecto-nucleotidases CD39 and CD73 in human endometrial tumors. Mediators Inflamm 2014; 2014:509027; PMID:; http://dx.doi.org/ 10.1155/2014/509027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013; 110:11091-6; PMID:; http://dx.doi.org/ 10.1073/pnas.1222251110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19:355-67; PMID:; http://dx.doi.org/ 10.1016/j.molmed.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ghiringhelli F, Bruchard M, Chalmin F, Rebe C. Production of adenosine by ectonucleotidases: a key factor in tumor immunoescape. J Biomed Biotechnol 2012; 2012:473712; PMID:; http://dx.doi.org/ 10.1155/2012/473712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1CD39 is a promising therapeutic target in oncology. Oncogene 2013; 32:1743-51; PMID:; http://dx.doi.org/ 10.1038/onc.2012.269 [DOI] [PubMed] [Google Scholar]
- 196. Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 2011; 187:676-83; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1003884 [DOI] [PubMed] [Google Scholar]
- 197. Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, Sykacek P, Frank L, Schramek D, Komnenovic V, et al. . A dual role for autophagy in a murine model of lung cancer. Nat Commun 2014; 5:3056; PMID:; http://dx.doi.org/ 10.1038/ncomms4056 [DOI] [PubMed] [Google Scholar]
- 198. Senovilla L, Aranda F, Galluzzi L, Kroemer G. Impact of myeloid cells on the efficacy of anticancer chemotherapy. Curr Opin Immunol 2014; 30C:24-31; PMID:; http://dx.doi.org/ 10.1016/j.coi.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 199. Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity 2013; 39:211-27; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 200. Li H, Li Y, Jiao J, Hu HM. Alpha-alumina nanoparticles induce efficient autophagy-dependent cross-presentation and potent antitumour response. Nat Nanotechnol 2011; 6:645-50; PMID:; http://dx.doi.org/ 10.1038/nnano.2011.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ 2009; 16:175-83; PMID:; http://dx.doi.org/ 10.1038/cdd.2008.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418:191-5; PMID:; http://dx.doi.org/ 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- 203. Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, et al. . High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 2006; 290:C917-24; PMID:; http://dx.doi.org/ 10.1152/ajpcell.00401.2005 [DOI] [PubMed] [Google Scholar]
- 204. Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 2006; 26:174-9; PMID:; http://dx.doi.org/ 10.1097/01.shk.0000225404.51320.82 [DOI] [PubMed] [Google Scholar]
- 205. Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005; 5:331-42; PMID:; http://dx.doi.org/ 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- 206. Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 2005; 61:1-9; PMID:; http://dx.doi.org/ 10.1111/j.0300-9475.2005.01534.x [DOI] [PubMed] [Google Scholar]
- 207. Dong Xda E, Ito N, Lotze MT, Demarco RA, Popovic P, Shand SH, Watkins S, Winikoff S, Brown CK, Bartlett DL, et al. . High mobility group box I (HMGB1) release from tumor cells after treatment: implications for development of targeted chemoimmunotherapy. J Immunother 2007; 30:596-606; PMID:; http://dx.doi.org/ 10.1097/CJI.0b013e31804efc76 [DOI] [PubMed] [Google Scholar]
- 208. Pathak SK, Skold AE, Mohanram V, Persson C, Johansson U, Spetz AL. Activated apoptotic cells induce dendritic cell maturation via engagement of Toll-like receptor 4 (TLR4), dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN), and beta2 integrins. J Biol Chem 2012; 287:13731-42; PMID:; http://dx.doi.org/ 10.1074/jbc.M111.336545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 2007; 220:35-46; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00574.x [DOI] [PubMed] [Google Scholar]
- 210. Curtin JF, Liu N, Candolfi M, Xiong W, Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, et al. . HMGB1 mediates endogenous TLR2 activation and brain tumor regression. PLoS Med 2009; 6:e10; http://dx.doi.org/ 10.1371/journal.pmed.1000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, et al. . HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 2012; 209:551-63; PMID:; http://dx.doi.org/ 10.1084/jem.20111739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P, Hoppe G, Bianchi ME, Tracey KJ, Zeh HJ 3rd, et al. . Endogenous HMGB1 regulates autophagy. J Cell Biol 2010; 190:881-92; PMID:; http://dx.doi.org/ 10.1083/jcb.200911078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011; 18:571-80; PMID:; http://dx.doi.org/ 10.1038/cdd.2010.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT, Tang D. HMGB1 as an autophagy sensor in oxidative stress. Autophagy 2011; 7:904-6; PMID:; http://dx.doi.org/ 10.4161/auto.7.8.15704 [DOI] [PubMed] [Google Scholar]
- 215. Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ, et al. . Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 2012; 18:250-9; PMID:; http://dx.doi.org/ 10.2119/molmed.2011.00389 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 216. Tang D, Billiar TR, Lotze MT. A Janus tale of two active high mobility group box 1 (HMGB1) redox states. Mol Med 2012; 18:1360-2; PMID:; http://dx.doi.org/ 10.2119/molmed.2012.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, et al. . Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med 2012; 209:1519-28; PMID:; http://dx.doi.org/ 10.1084/jem.20120189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Tang D, Kang R, Cheh CW, Livesey KM, Liang X, Schapiro NE, Benschop R, Sparvero LJ, Amoscato AA, Tracey KJ, et al. . HMGB1 release and redox regulates autophagy and apoptosis in cancer cells. Oncogene 2010; 29:5299-310; PMID:; http://dx.doi.org/ 10.1038/onc.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Kazama H, Ricci JE, Herndon JM, Hoppe G, Green DR, Ferguson TA. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity 2008; 29:21-32; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2008.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol 2007; 28:429-36; PMID:; http://dx.doi.org/ 10.1016/j.it.2007.08.004 [DOI] [PubMed] [Google Scholar]
- 221. Urbonaviciute V, Meister S, Furnrohr BG, Frey B, Guckel E, Schett G, Herrmann M, Voll RE. Oxidation of the alarmin high-mobility group box 1 protein (HMGB1) during apoptosis. Autoimmunity 2009; 42:305-7; PMID:; http://dx.doi.org/ 10.1080/08916930902831803 [DOI] [PubMed] [Google Scholar]
- 222. Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, et al. . Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol 2012; 13:832-42; PMID:; http://dx.doi.org/ 10.1038/ni.2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Tang D, Lotze MT. Tumor immunity times out: TIM-3 and HMGB1. Nat Immunol 2012; 13:808-10; PMID:; http://dx.doi.org/ 10.1038/ni.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Patel J, Bozeman EN, Selvaraj P. Taming dendritic cells with TIM-3: another immunosuppressive strategy used by tumors. Immunotherapy 2012; 4:1795-8; PMID:; http://dx.doi.org/ 10.2217/imt.12.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 2012; 1:894-907; PMID:; http://dx.doi.org/ 10.4161/onci.20931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Vacchelli E, Eggermont A, Sautes-Fridman C, Galon J, Zitvogel L, Kroemer G, lluzzi L. Trial watch: toll-like receptor agonists for cancer therapy. Oncoimmunology 2013; 2:e25238; http://dx.doi.org/ 10.4161/onci.25238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, et al. . Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ 2014; 21:69-78; PMID:; http://dx.doi.org/ 10.1038/cdd.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Aranda F, Vacchelli E, Obrist F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Henrik Ter Meulen J, Zitvogel L, Kroemer G, et al. . Trial Watch: Toll-like receptor agonists in oncological indications. Oncoimmunology 2014; 3:e29179; http://dx.doi.org/ 10.4161/onci.29179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al. . The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007; 220:47-59; PMID:; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00573.x [DOI] [PubMed] [Google Scholar]
- 230. Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, Boucontet L, Apetoh L, Ghiringhelli F, Casares N, et al. . Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491-503; PMID:; http://dx.doi.org/ 10.1084/jem.20100269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. . Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012; 8:445-544; PMID:; http://dx.doi.org/ 10.4161/auto.19496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, et al. . Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology 2014; 3:e28473; http://dx.doi.org/ 10.4161/onci.28473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235-71; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 234. Zirger JM, Puntel M, Bergeron J, Wibowo M, Moridzadeh R, Bondale N, Barcia C, Kroeger KM, Liu C, Castro MG, et al. . Immune-mediated loss of transgene expression from virally transduced brain cells is irreversible, mediated by IFNgamma, perforin, and TNFalpha, and due to the elimination of transduced cells. Mol Ther 2012; 20:808-19; PMID:; http://dx.doi.org/ 10.1038/mt.2011.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Tailler M, Senovilla L, Lainey E, Thepot S, Metivier D, Sebert M, Baud V, Billot K, Fenaux P, Galluzzi L, et al. . Antineoplastic activity of ouabain and pyrithione zinc in acute myeloid leukemia. Oncogene 2012; 31:3536-46; PMID:; http://dx.doi.org/ 10.1038/onc.2011.521 [DOI] [PubMed] [Google Scholar]
- 236. Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G. Cell death assays for drug discovery. Nat Rev Drug Discov 2011; 10:221-37; PMID:; http://dx.doi.org/ 10.1038/nrd3373 [DOI] [PubMed] [Google Scholar]
- 237. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods 1995; 184:39-51; PMID:; http://dx.doi.org/ 10.1016/0022-1759(95)00072-I [DOI] [PubMed] [Google Scholar]
- 238. Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994; 84:1415-20; PMID: [PubMed] [Google Scholar]
- 239. Galluzzi L, Vitale I, Kepp O, Seror C, Hangen E, Perfettini JL, Modjtahedi N, Kroemer G. Methods to dissect mitochondrial membrane permeabilization in the course of apoptosis. Methods Enzymol 2008; 442:355-74; PMID:; http://dx.doi.org/ 10.1016/S0076-6879(08)01418-3 [DOI] [PubMed] [Google Scholar]
- 240. Galluzzi L, Zamzami N, de La Motte Rouge T, Lemaire C, Brenner C, Kroemer G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 2007; 12:803-13; PMID:; http://dx.doi.org/ 10.1007/s10495-007-0720-1 [DOI] [PubMed] [Google Scholar]
- 241. Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere JL, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 1995; 181:1661-72; PMID:; http://dx.doi.org/ 10.1084/jem.181.5.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Mellen MA, de la Rosa EJ, Boya P. Autophagy is not universally required for phosphatidyl-serine exposure and apoptotic cell engulfment during neural development. Autophagy 2009; 5:964-72; PMID:; http://dx.doi.org/ 10.4161/auto.5.7.9292 [DOI] [PubMed] [Google Scholar]
- 243. Mellen MA, de la Rosa EJ, Boya P. The autophagic machinery is necessary for removal of cell corpses from the developing retinal neuroepithelium. Cell Death Differ 2008; 15:1279-90; PMID:; http://dx.doi.org/ 10.1038/cdd.2008.40 [DOI] [PubMed] [Google Scholar]
- 244. Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014; 344:1164-8; PMID:; http://dx.doi.org/ 10.1126/science.1252809 [DOI] [PubMed] [Google Scholar]
- 245. Kenis H, Zandbergen HR, Hofstra L, Petrov AD, Dumont EA, Blankenberg FD, Haider N, Bitsch N, Gijbels M, Verjans JW, et al. . Annexin A5 uptake in ischemic myocardium: demonstration of reversible phosphatidylserine externalization and feasibility of radionuclide imaging. J Nucl Med 2010; 51:259-67; PMID:; http://dx.doi.org/ 10.2967/jnumed.109.068429 [DOI] [PubMed] [Google Scholar]
- 246. Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res 2012; 111:1198-207; PMID:; http://dx.doi.org/ 10.1161/CIRCRESAHA.112.268946 [DOI] [PubMed] [Google Scholar]
- 247. Galluzzi L, Blomgren K, Kroemer G. Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 2009; 10:481-94; PMID:; http://dx.doi.org/ 10.1038/nrn2665 [DOI] [PubMed] [Google Scholar]
- 248. Metivier D, Dallaporta B, Zamzami N, Larochette N, Susin SA, Marzo I, Kroemer G. Cytofluorometric detection of mitochondrial alterations in early CD95FasAPO-1-triggered apoptosis of Jurkat T lymphoma cells. Comparison of seven mitochondrion-specific fluorochromes. Immunol Lett 1998; 61:157-63; PMID:; http://dx.doi.org/ 10.1016/S0165-2478(98)00013-3 [DOI] [PubMed] [Google Scholar]
- 249. Komoriya A, Packard BZ, Brown MJ, Wu ML, Henkart PA. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J Exp Med 2000; 191:1819-28; PMID:; http://dx.doi.org/ 10.1084/jem.191.11.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kepp O, Rajalingam K, Kimmig S, Rudel T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J 2007; 26:825-34; PMID:; http://dx.doi.org/ 10.1038/sj.emboj.7601533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L, Araujo N, Pinna G, Larochette N, Zamzami N, et al. . Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene 2008; 27:4221-32; PMID:; http://dx.doi.org/ 10.1038/onc.2008.63 [DOI] [PubMed] [Google Scholar]
- 252. Pauleau AL, Larochette N, Giordanetto F, Scholz SR, Poncet D, Zamzami N, Goldmacher VS, Kroemer G. Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 2007; 26:7067-80; PMID:; http://dx.doi.org/ 10.1038/sj.onc.1210511 [DOI] [PubMed] [Google Scholar]
- 253. Garg AD, Dudek AM, Agostinis P. Calreticulin surface exposure is abrogated in cells lacking, chaperone-mediated autophagy-essential gene, LAMP2A. Cell Death Dis 2013; 4:e826; http://dx.doi.org/ 10.1038/cddis.2013.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Hetz C, Chevet E, Harding HP. Targeting the unfolded protein response in disease. Nat Rev Drug Discov 2013; 12:703-19; PMID:; http://dx.doi.org/ 10.1038/nrd3976 [DOI] [PubMed] [Google Scholar]
- 255. Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev 2011; 91:1219-43; PMID:; http://dx.doi.org/ 10.1152/physrev.00001.2011 [DOI] [PubMed] [Google Scholar]
- 256. Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 2008; 8:663-74; PMID:; http://dx.doi.org/ 10.1038/nri2359 [DOI] [PubMed] [Google Scholar]
- 257. Omasa T, Chen YG, Mantalaris A, Tsai YC, Wu JH. Molecular cloning and sequencing of the human heme-regulated eukaryotic initiation factor 2 alpha (eIF-2 alpha) kinase from bone marrow culture. DNA Seq 2002; 13:133-7; PMID: [DOI] [PubMed] [Google Scholar]
- 258. Samuel CE. The eIF-2 alpha protein kinases, regulators of translation in eukaryotes from yeasts to humans. J Biol Chem 1993; 268:7603-6; PMID: [PubMed] [Google Scholar]
- 259. Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, Wek RC. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 1998; 18:7499-509; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999; 397:271-4; PMID: http://dx.doi.org/ 10.1038/16729 [DOI] [PubMed] [Google Scholar]
- 261. Yang Y, Li XJ, Chen Z, Zhu XX, Wang J, Zhang LB, Qiang L, Ma YJ, Li ZY, Guo QL, et al. . Wogonin induced calreticulinannexin A1 exposure dictates the immunogenicity of cancer cells in a PERKAKT dependent manner. PLoS One 2012; 7:e50811; http://dx.doi.org/ 10.1371/journal.pone.0050811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. van Schadewijk A, van't Wout EF, Stolk J, Hiemstra PS. A quantitative method for detection of spliced X-box binding protein-1 (XBP1) mRNA as a measure of endoplasmic reticulum (ER) stress. Cell Stress Chaperones 2012; 17:275-9; PMID:; http://dx.doi.org/ 10.1007/s12192-011-0306-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Hayashi A, Kasahara T, Iwamoto K, Ishiwata M, Kametani M, Kakiuchi C, Furuichi T, Kato T. The role of brain-derived neurotrophic factor (BDNF)-induced XBP1 splicing during brain development. J Biol Chem 2007; 282:34525-34; PMID:; http://dx.doi.org/ 10.1074/jbc.M704300200 [DOI] [PubMed] [Google Scholar]
- 264. Prischi F, Nowak PR, Carrara M, Ali MM. Phosphoregulation of Ire1 RNase splicing activity. Nat Commun 2014; 5:3554; PMID:; http://dx.doi.org/ 10.1038/ncomms4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Reineke LC, Dougherty JD, Pierre P, Lloyd RE. Large G3BP-induced granules trigger eIF2alpha phosphorylation. Mol Biol Cell 2012; 23:3499-510; PMID:; http://dx.doi.org/ 10.1091/mbc.E12-05-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Lundin A, Thore A. Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem 1975; 66:47-63; PMID:; http://dx.doi.org/ 10.1016/0003-2697(75)90723-X [DOI] [PubMed] [Google Scholar]
- 267. McElroy WD, DeLuca MA. Firefly and bacterial luminescence: basic science and applications. J Appl Biochem 1983; 5:197-209; PMID: [PubMed] [Google Scholar]
- 268. Sorensen CE, Novak I. Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 2001; 276:32925-32; PMID:; http://dx.doi.org/ 10.1074/jbc.M103313200 [DOI] [PubMed] [Google Scholar]
- 269. Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A 2009; 106:15651-6; PMID:; http://dx.doi.org/ 10.1073/pnas.0904764106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Tasdemir E, Galluzzi L, Maiuri MC, Criollo A, Vitale I, Hangen E, Modjtahedi N, Kroemer G. Methods for assessing autophagy and autophagic cell death. Methods Mol Biol 2008; 445:29-76; PMID:; http://dx.doi.org/ 10.1007/978-1-59745-157-4_3 [DOI] [PubMed] [Google Scholar]
- 271. Klionsky DJ. A human autophagy interaction network. Autophagy 2012; 8:439-41; PMID:; http://dx.doi.org/ 10.4161/auto.19926 [DOI] [PubMed] [Google Scholar]
- 272. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000; 19:5720-8; PMID:; http://dx.doi.org/ 10.1093/emboj/19.21.5720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Klionsky DJ. Autophagy in mammalian systems, part B. Preface. Methods Enzymol 2009; 452:xxi-xxii. [DOI] [PubMed] [Google Scholar]
- 274. Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 2001; 152:657-68; PMID:; http://dx.doi.org/ 10.1083/jcb.152.4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Martins I, Kepp O, Menger L, Michaud M, Adjemian S, Sukkurwala AQ, Vacchelli E, Galluzzi L, Kroemer G. Fluorescent biosensors for the detection of HMGB1 release. Methods Mol Biol 2013; 1004:43-56; PMID:; http://dx.doi.org/ 10.1007/978-1-62703-383-1_4 [DOI] [PubMed] [Google Scholar]
- 276. Antoine DJ, Harris HE, Andersson U, Tracey KJ, Bianchi ME. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med 2014; 20:135-7; PMID:; http://dx.doi.org/ 10.2119/molmed.2014.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Perne A, Muellner MK, Steinrueck M, Craig-Mueller N, Mayerhofer J, Schwarzinger I, Sloane M, Uras IZ, Hoermann G, Nijman SM, et al. . Cardiac glycosides induce cell death in human cells by inhibiting general protein synthesis. PLoS One 2009; 4:e8292; http://dx.doi.org/ 10.1371/journal.pone.0008292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Vatakis DN, Koya RC, Nixon CC, Wei L, Kim SG, Avancena P, Bristol G, Baltimore D, Kohn DB, Ribas A, et al. . Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci U S A 2011; 108:E1408-16; PMID:; http://dx.doi.org/ 10.1073/pnas.1115050108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 2012; 12:786-98; PMID:; http://dx.doi.org/ 10.1038/nri3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Payne KJ, Crooks GM. Immune-cell lineage commitment: translation from mice to humans. Immunity 2007; 26:674-7; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 281. Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL, Saito Y, Marches F, Halene S, Palucka AK, et al. . Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 2014; 32:364-72; PMID:; http://dx.doi.org/ 10.1038/nbt.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Ladoire S, Chaba K, Martins I, Sukkurwala AQ, Adjemian S, Michaud M, Poirier-Colame V, Andreiuolo F, Galluzzi L, White E, et al. . Immunohistochemical detection of cytoplasmic LC3 puncta in human cancer specimens. Autophagy 2012; 8:1175-84; PMID:; http://dx.doi.org/ 10.4161/auto.20353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ladoire S, Hannani D, Vetizou M, Locher C, Aymeric L, Apetoh L, Kepp O, Kroemer G, Ghiringhelli F, Zitvogel L. Cell-death-associated molecular patterns as determinants of cancer immunogenicity. Antioxid Redox Signal 2014; 20:1098-116; PMID:; http://dx.doi.org/ 10.1089/ars.2012.5133 [DOI] [PubMed] [Google Scholar]
- 284. Zitvogel L, Tanchot C, Granier C, Tartour E. Following up tumor-specific regulatory T cells in cancer patients. Oncoimmunology 2013; 2:e25444; http://dx.doi.org/ 10.4161/onci.25444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Popadic D, Anegon I, Baeten D, Eibel H, Giese T, Marits P, Martinez-Caceres E, Mascart F, Nestle F, Pujol-Borrell R, et al. . Predictive immunomonitoring – the COST ENTIRE initiative. Clin Immunol 2013; 147:23-6; PMID:; http://dx.doi.org/ 10.1016/j.clim.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 286. Jager M, Schoberth A, Ruf P, Hess J, Hennig M, Schmalfeldt B, Wimberger P, Ströhlein M, Theissen B, Heiss MM, et al. . Immunomonitoring results of a phase IIIII study of malignant ascites patients treated with the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3). Cancer Res 2012; 72:24-32; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2235 [DOI] [PubMed] [Google Scholar]
- 287. Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 2000; 11:2389-401; PMID:; http://dx.doi.org/ 10.1089/104303400750038499 [DOI] [PubMed] [Google Scholar]
- 288. Fillat C, Carrio M, Cascante A, Sangro B. Suicide gene therapy mediated by the Herpes Simplex virus thymidine kinase geneGanciclovir system: fifteen years of application. Curr Gene Ther 2003; 3:13-26; PMID:; http://dx.doi.org/ 10.2174/1566523033347426 [DOI] [PubMed] [Google Scholar]