Abstract
Whereas macrophages use the scavenger receptor MARCO primarily in antimicrobial immunity by interacting with both exogenous and endogenous environments, in dendritic cells (DCs) MARCO is believed to pleiotropically link innate to adaptive immunity. MARCO exerts a significant modulatory effect on TLR-induced DC activation, thus offering novel avenues in cancer immunotherapy.
Keywords: cancer immunotherapy, dendritic cell activation, immune checkpoint inhibitor, scavenger receptor MARCO, Toll-like receptor
Abbreviations: ICI, immune checkpoint inhibitor; MARCO, macrophage receptor with collagenous structure; SR, scavenger receptor; PAMP, pathogen-associated molecular pattern; TLR, Toll-like receptor; DC, dendritic cell; LPS, lipopolysaccharide; PKA, protein kinase A; SHP-2, Src-homology 2 domain (SH2)-containing
Curative cancer immunotherapy is increasingly becoming a reality. Recent clinical success in this area has excited much promise attracting special interest in novel treatments based upon immune checkpoint inhibitors (ICI). As blockade of cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programmed cell death 1 (PDCD1, better known as PD-1) continue to boost clinical evaluation of immunotherapeutic agents, new ICI family members are under scrutiny. One potential new member, the macrophage receptor with collagenous structure (MARCO), a class A scavenger receptor (SR), is best characterized in macrophages, wherein it is appreciated for its role in sensing and clearing pathogens through the recognition of pathogen-associated molecular patterns (PAMPs). MARCO recognizes ligands that are often polyanionic in nature, including environmental particles, nucleic acids, bacterial lipopolysaccharides, oxidized lipoproteins, and numerous endogenous proteins. Interestingly, many of these ligands are also recognized by and trigger cell signals through Toll-like receptors (TLR). Consequently, recent evidence demonstrates that TLR-signaling is finely tuned by SRs receptors expressed on macrophages.1,2 For example, MARCO and other SRs internalize such ligands to render them accessible to TLR3, TLR7/8 and TLR9 localized in the cytosol.
Recently,3 we investigated the role of MARCO in TLR-induced regulation of gene expression in dendritic cells (DCs) via genetic deletion analysis. Specifically, our work unraveled significant divergences in gene expression profiles of non-activated wild type (WT) and MARCO−/− DCs. These divergent transcriptomes were more prominent following lipopolysaccharide (LPS) challenge. Furthermore, marked differences between WT and MARCO−/− DCs were observed in the expression of pro-inflammatory markers elicited in response to all TLR agonists, suggesting MARCO's contribution to cell signaling might be a critical component that is commonly held by all TLRs expressed on DCs. Of note, MARCO's effects were not merely a reflection of diverse TLR expression, as only TLR-3 expression was found to correlate with MARCO expression. Upon in-depth ontological examination, Ingenuity Pathway Analysis revealed perturbations in many signaling pathways and biological functions. Some of these perturbations, namely leukocyte extravasation, actin cytoskeleton signaling, and clathrin mediated endocytosis, are in line with previously reported roles for MARCO in modulating DC morphology and migration. Other perturbations were found to affect key signaling pathways such as RhoA, protein kinase A (PKA), nuclear factor kB (NF-kB) signaling, and Fc γ receptor (FcγR)-mediated phagocytosis, thus suggesting an important role for MARCO in DC signaling machinery. Notably, effects on transforming growth factor β (TGFβ)/SMAD-dependent gene expression, interleukin (IL)-12β and CD209 expression may suggest a potential involvement of MARCO in DC tolerization, T helper type 1 (Th1) cell differentiation, and human immunodeficiency virus (HIV) and hepatitis C infections. Our data also showed a more pronounced effect of MARCO on microRNA activation in LPS-stimulated DCs. Specifically, MARCO seems to affect the expression of many genes that are regulated by miR-155, a microRNA that is triggered by inflammatory stimuli such as TLR ligation. More importantly, LPS-induced expression of miR-155 in DCs was suggested to affect key genes in antigen presentation or T-cell stimulation.4 Collectively, our findings underscore the importance of MARCO in TLR-induced DC activation and suggest MARCO may play a pivotal modulatory role in the context of cancer immunotherapy, specifically in regards to DC-based vaccines and TLR ligand adjuvanticity.
Accumulating evidence suggests that MARCO's involvement may be an early event in DC hematopoiesis. In fact, enforced MARCO expression in DCs, as well as in other cell types, is sufficient to induce dramatic phenotypic changes without the need for ligand engagement. MARCO-expressing cells develop dendritic plasma membrane processes, including the appearance of large lamellipodia-like structures and long plasma membrane extensions.5,6 It is unclear whether these membrane changes mediate the negative effect of MARCO on DC migration. Nevertheless, melanoma tumor lysate-pulsed DCs do dramatically overexpress MARCO7 and undergo morphologic changes, including the disappearance of dendritic-like processes, following treatment with an anti-MARCO antibody. Consequently, antibody blockade of MARCO enhances DCs migratory capacity, thus augmenting antitumor immunity in a mouse model of melanoma.8 While differing in context, this interesting finding is in line with our previous work, showing that MARCO-deficient pulmonary DC exhibit enhanced migration to mediastinal lymph nodes following allergen exposure, resulting in exacerbated airway inflammation in vivo.9 It is worth mentioning that cyclophosphamide treatment induces MARCO expression in circulating DCs of patients with hematologic malignancies, suggesting MARCO might play a role in shaping the crosstalk between chemo- and immunotherapy.
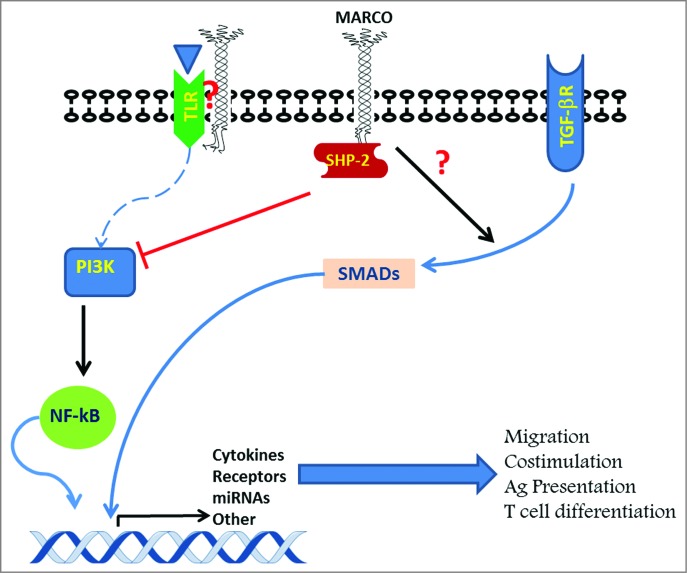
MARCO is equipped with a very short cytoplasmic tail that exhibits no obvious signaling features. Currently, the molecular aspects that govern the functions of DC MARCO remain largely unknown. However, one could put forward a number of hypotheses (Fig. 1) by extrapolation from what is known about macrophages. In this regard, we have previously shown that antibody ligation of MARCO on the surface of macrophages modulates the production of inflammatory mediators, including IL-12, suggesting MARCO's involvement in cell signaling. Additionally, macrophage MARCO is thought to cooperate with TLR4 and FcγRIII in response to E.coli sepsis in a signaling cascade involving phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), NF-κB and recruitment of the tyrosine phosphatase Src-homology 2 domain (SH2)-containing (SHP-2) to MARCO's cytoplasmic domain.10 This mechanism resembles recruitment of SHP-2 (and SHP-1) to the immune checkpoint PD-1 in T cells, thus inhibiting their function and contributing to the onset of immune escape in cancer.
The recent discovery of key immune modulators has enabled the development of more efficacious immunotherapeutic combinations, thus inducing a paradigm shift in clinical oncology. Whether MARCO receptor has acquired enough credentials to deserve membership in the checkpoint inhibitors club remains debatable. Nevertheless, regardless of the mechanism, interfering with MARCO receptor functionality via either antibody blockade or genetic deletion (in mice) clearly results in immune potentiation, including an elevation in tumor-directed immune responses. The undeniable relationship with TLRs may be the driving force behind MARCO's function. Therefore, blocking MARCO in conjunction with DC-based vaccines or vaccine/adjuvant combinations warrants inclusion into the emerging cancer immunotherapy strategies that are altering the landscape of cancer treatment.
Figure 1.
Possible mechanisms underlying modulation of TLR-induced DC activation by MARCO. Alternative scenarios by which MARCO may mediate dendritic cell (DC) activation via Toll-like receptor (TLR) signaling: MARCO receptor interacts with TLRs expressed on the cell surface; MARCO binds ligands and brings them in close proximity to TLRs; MARCO shuttles ligands from the surface to the endosome to elicit TLR3, 7/8 and 9 signaling; MARCO recruits Src-homology 2 domain (SH2)-containing (SHP-2) and induces inhibition of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) and other downstream signaling events; MARCO expression results in modulation of tumor growth factor β) receptor (TGFβ) /SMAD pathway; MARCO-induced effect on gene expression results in morphologic changes that affect cell migration; MARCO-induced signals affect the DC inflammatory signature, subsequently impacting T-cell responses to their cognate antigens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S, Leifer CA, Tryggvason K, Gordon S, Russell DG. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog 2009; 5:e1000474; PMID:; http://dx.doi.org/ 10.1371/journal.ppat.1000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukhopadhyay S, Varin A, Chen Y, Liu B, Tryggvason K, Gordon S. SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood 2011; 117:1319–28; PMID:; http://dx.doi.org/ 10.1182/blood-2010-03-276733 [DOI] [PubMed] [Google Scholar]
- 3.Kissick HT, Dunn LK, Ghosh S, Nechama M, Kobzik L, Arredouani MS. The Scavenger Receptor MARCO Modulates TLR-Induced Responses in Dendritic Cells. PLoS One 2014; 9:e104148; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0104148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao CP, He L, Tsai YC, Peng S, Kang TH, Pang X, Monie A, Hung CF, Wu TC. In vivo microRNA-155 expression influences antigen-specific T cell-mediated immune responses generated by DNA vaccination. Cell Biosci 2011; 1:3; PMID:; http://dx.doi.org/ 10.1186/2045-3701-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granucci F, Petralia F, Urbano M, Citterio S, Di Tota F, Santambrogio L, Ricciardi-Castagnoli P. The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood 2003; 102:2940-7; PMID:; http://dx.doi.org/ 10.1182/blood-2002-12-3651 [DOI] [PubMed] [Google Scholar]
- 6.Pikkarainen T, Brannstrom A, Tryggvason K. Expression of macrophage MARCO receptor induces formation of dendritic plasma membrane processes. J Biol Chem 1999; 274:10975-82; PMID:; http://dx.doi.org/ 10.1074/jbc.274.16.10975 [DOI] [PubMed] [Google Scholar]
- 7.Grolleau A, Misek DE, Kuick R, Hanash S, Mule JJ. Inducible expression of macrophage receptor Marco by dendritic cells following phagocytic uptake of dead cells uncovered by oligonucleotide arrays. J Immunol 2003; 171:2879-88; PMID:; http://dx.doi.org/ 10.4049/jimmunol.171.6.2879 [DOI] [PubMed] [Google Scholar]
- 8.Matsushita N, Komine H, Grolleau-Julius A, Pilon-Thomas S, Mule JJ. Targeting MARCO can lead to enhanced dendritic cell motility and anti-melanoma activity. Cancer Immunol Immunother 2010; 59:875-84; PMID:; http://dx.doi.org/ 10.1007/s00262-009-0813-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arredouani MS, Franco F, Imrich A, Fedulov A, Lu X, Perkins D, Soininen R, Tryggvason K, Shapiro SD, Kobzik L. Scavenger Receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J Immunol 2007; 178:5912-20; PMID:; http://dx.doi.org/ 10.4049/jimmunol.178.9.5912 [DOI] [PubMed] [Google Scholar]
- 10.Pinheiro da Silva F, Aloulou M, Skurnik D, Benhamou M, Andremont A, Velasco IT, Chiamolera M, Verbeek JS, Launay P, Monteiro RC. CD16 promotes Escherichia coli sepsis through an FcR gamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med 2007; 13:1368-74; PMID:; http://dx.doi.org/ 10.1038/nm1665 [DOI] [PubMed] [Google Scholar]