Abstract
Ultrasound imaging has facilitated the reliable measure of the architectural variables fascicle length (LF) and pennation angle (PA), at rest and during static and dynamic contractions in many human skeletal muscles in vivo. Despite its small size and very modest contribution to elbow extension torque, the anconeus muscle has proven a useful model for the study of neuromuscular function in health and disease. Recent single motor unit (MU) studies in the anconeus have reported discrete and identifiable individual trains of MU potentials from intramuscular electromyography (EMG) recordings during dynamic elbow extensions. It is unknown whether the anconeus has unique architectural features related to alterations in LF and PA throughout the elbow joint range of motion that may help explain these high-quality recordings. Previous anatomical studies have investigated this muscle in cadavers and at mainly one elbow joint angle. The purpose of this study was to measure in vivo PA and LF of the anconeus muscle in a relaxed state at different degrees of elbow flexion using ultrasonography. Ultrasound images were collected from 10 healthy males (25 ± 3 years) at 135°, 120°, 90°, 45°, and 0° of elbow flexion. Average values of LF decreased by 6 mm (10%), 6 mm (12%), and 4 mm (9%) from 135–120°, 120–90°, and 90–45° of elbow flexion, respectively, whereas average PA values increased by 1° (9%), 1° (8%), and 2° (14%) from 135–120°, 120–90°, and 45–0°, respectively. The results indicate that anconeus muscle architecture is dynamic, undergoing moderate changes with elbow joint excursion that are similar to other limb muscles reported elsewhere. The data obtained here are more comprehensive and representative of architectural changes at various elbow joint positions than those data reported in cadaveric studies. Furthermore, the results of this study indicate that despite experiencing similar relative changes in muscle architecture to other skeletal muscles about the elbow joint, the minimal absolute changes in LF of the anconeus likely contribute to the clarity of intramuscular EMG previously reported in this muscle.
Keywords: anatomy, elbow extensors, muscle architecture, ultrasound
Introduction
Muscle architecture, together with fiber type composition and distribution, is an important determinant of muscle contractile properties (Edgerton et al. 1975; Lieber & Bodine-Fowler, 1993; Kellis et al. 2012; Gerling & Brown, 2013). Muscle architecture has been classically studied using cadaveric specimens. However, the applicability of gross anatomic measurements obtained from cadavers is often limited to older aged specimens, influenced by structural changes that occur during embalming procedures, and limited to the angle at which the joint is fixed (Narici et al. 1996; Fukunaga et al. 1997). Alternatively, ultrasonography has facilitated the reliable measure of architectural variables at rest, during static and dynamic contractions, and in many human skeletal muscles in vivo (Narici et al. 1996; Fukunaga et al. 1997; Kawakami et al. 1998; Chleboun et al. 2001, 2007; Kwah et al. 2013; Power et al. 2013).
One small and seemingly insignificant muscle of the elbow joint, the anconeus, has been used frequently as a model in neuromuscular and anatomical investigations. The anconeus has been shown to provide high-quality recordings of motor unit (MU) properties during isometric and dynamic elbow extensions (Harwood et al. 2011, 2012; Harwood & Rice, 2012; Stevens et al. 2013), has been used to record surface and intramuscular electromyography (EMG) to study synergistic elbow extensor activity (Le Bozec & Maton, 1982; Davidson & Rice, 2010; Harwood et al. 2013) and is often used clinically in the assessment of neuromuscular transmission disorders (Maselli et al. 1991; Kennett & Fawcett, 1993). One explanation for the high signal clarity of anconeus intramuscular EMG recordings over the full range of dynamic elbow extensions (Harwood et al. 2011, 2012) is that MU number estimates of the anconeus are relatively low compared with other skeletal muscles, which manifests as less electrical interference from adjacent MUs and a less dense signal (Stevens et al. 2013). An alternative or complementary explanation for the high intramuscular EMG clarity of the anconeus may be that minimal physical displacement of the recording electrode during contractile shortening occurs due to smaller changes in architectural features compared with other skeletal muscles. However, this hypothesis has not been substantiated in vivo.
Two architectural features are measured predominantly using ultrasonography: fascicle length (LF) and pennation angle (PA). Fascicle length, which is an estimate of muscle fiber length, is defined as the length of a line coincident with the fascicle between the deep and superficial aponeuroses. Fascicle length indicates the range of lengths over which the muscle is capable of actively producing force, known as the excursion potential (Lieber & Friden, 2000). Pennation angle represents the angle of the muscle fibers that constitute a muscle fascicle relative to the force-generating axis, and directly affects both the force production and the excursion (Gans & de Vree, 1987); larger angles of pennation limiting the excursion potential. It is apparent from ultrasound imaging that these architectural variables are dynamic, changing in response to muscle length changes or to a transition from rest to contraction (including isometric) (Narici et al. 1996; Fukunaga et al. 1997). For example, Chleboun et al. (2001) demonstrated a disordinal interaction between LF and PA of the human biceps femoris muscle in a relaxed state as a function of hip and knee angle. Similarly, in the tibialis anterior it has been shown that LF decreases and PA increases at higher isometric dorsiflexion contractile intensities (Maganaris & Baltzopoulos, 1999; Simoneau et al. 2012). Therefore, alterations in LF and PA are dependent on the shortening or lengthening of sarcomeres, and respond to variations in tendon slack and whole muscle length. As a result, these changes have important functional relevance with respect to force production that is modified by sarcomeric and whole muscle length changes (Duchateau & Enoka, 2008).
Except for one pilot study reported in abstract form (Harwood et al. 2010), the anconeus has not been studied in vivo using ultrasonography. Several cadaveric (Coriolano et al. 2009; Molinier et al. 2011; Ng et al. 2012; Pereira, 2013) and EMG (Basmajian & Griffin, 1972; Le Bozec & Maton, 1982; Bergin et al. 2013) studies have described the gross anatomy of the anconeus and have largely defined its function. From these various independent anatomical and functional studies, the primary functions of the anconeus appear to be active stabilization of the elbow joint (Pereira, 2013; Molinier et al. 2011) and an approximate 15% contribution to maximum elbow extension torque (Basmajian & Griffin, 1972; Le Bozec & Maton, 1982; Zhang & Nuber, 2000). However, the functional anatomy of the anconeus, specifically the changes in FL and PA across the full elbow joint range of motion (ROM) have not been described. Because muscle architectural properties are reported to affect patterns of MU recruitment and discharge characteristics (Pasquet et al. 2006, 2005), and the anconeus has been used recently as a model for the study of MU properties during dynamic contractions, it is important to determine the degree to which the anconeus responds architecturally throughout the ROM to substantiate the value of this muscle for study during actively changing elbow joint angles. Furthermore, a description of FL and PA changes across the full elbow joint ROM will provide insight to whether the absolute changes in muscle architecture of the anconeus contribute to its reported high EMG signal clarity, and whether the anconeus is comparable to other upper limb muscles on a relative basis. Thus, the purpose of this study was to evaluate, using ultrasonography, changes in architectural features (LF and PA) of the anconeus at rest across the full ROM for the elbow joint.
Methods
Participants
Ten young adult male participants (25 ± 3 years, 178 ± 7 cm, 77 ± 10 kg) volunteered for the study. Participants were asked to refrain from unaccustomed and strenuous upper limb exercise for 1 day prior to testing. The participants were recruited from the university population and were considered to be recreationally active but not systematically trained. All participants were free from known neuromuscular or cardiovascular diseases. The study protocol was approved by the local university ethics board and conformed to the Declaration of Helsinki. Informed written consent was obtained prior to testing.
Experimental arrangement
Elbow angle was recorded and ultrasound imaging conducted with the participant seated on a HUMAC NORM dynamometer (CSMi Medical Solutions, Stoughton, MA, USA) (Fig. 1A). The non-dominant arm (left arm for all participants) was secured tightly to a custom forearm dynamometer attachment at the wrist and midpoint of the forearm (∼12 cm proximal to the head of the ulna) using two 5-cm-wide inelastic Velcro restraints, which aligned the medial epicondyle of the humerus with the rotational axis of the dynamometer. Extraneous movements were minimized using inelastic shoulder and waist restraints. Participants sat in an upright position, such that the inertial weight of the left arm was supported in testing position, with the shoulder flexed at 90° and the forearm in a prone position. Ultrasound recordings were obtained at 135°, 120°, 90°, 45°, and 0° of elbow flexion (elbow joint angle of 0° was considered full extension) (Fig. 1B).
Fig. 1.
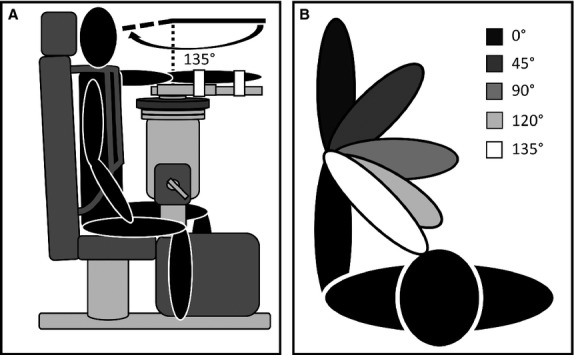
Schematic diagrams of the experimental set up. (A) Participant situated in testing position in a HUMAC NORM dynamometer with shoulder flexed at 90° and forearm in semi-prone position (participant shown at 0° elbow flexion). (B) Ultrasound imaging positions.
Ultrasonography
To investigate the effect of changing elbow joint angle on LF and PA, ultrasound imaging was performed using a linear array probe (GE model M12L, 4.9 mm, 5–13 MHz), attached to a Vivid 7 ultrasound unit (GE Healthcare, Mississauga, ON, Canada). Because of the size and location of the muscle in relation to bony contours and fascial sheaths it was not possible to follow LF and PA in the anconeus during continuous low intensity contractile movements or at static angles during various contractile intensities. Therefore, images were collected at rest for the five angles of elbow flexion. For all measures of LF and PA, the probe was placed directly on the skin overlying the anconeus muscle approximately 3 cm distal to the lateral epicondyle of the humerus, and olecranon process of the ulna. The probe was positioned parallel to the direction of the aponeurosis to allow the fascicles to be displayed as a banded pattern. Once a suitable recording position was obtained (minimum of one distinct muscle fascicles per image; Fig. 2A), the location was marked with indelible ink on the skin surface. Due to the limitations of the anconeus outlined above, images which yielded a better quality and number of fascicles generally were obtained from the more distal aspects of the anconeus. Anconeus muscle thickness was determined at 135° and 0° of elbow flexion with the probe positioned perpendicular to the aponeurosis. The probe was moved distally from the lateral epicondyle of the humerus toward the ulna, identifying the deepest border of the anconeus muscle, from which the measurement was made (Fig. 2B). Imaging was repeated for a given elbow angle if the operator deemed the previous image unsatisfactory, and was repeated until a useful image was obtained. The probe was held firmly in place by the same operator for all tests and standard ultrasound gel was used as the coupling agent.
Fig. 2.
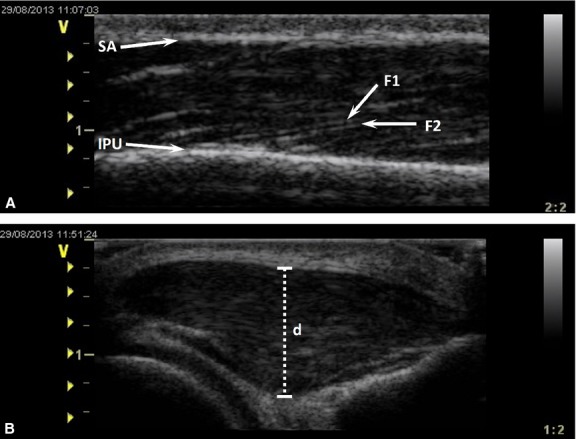
Ultrasound images of the anconeus from a representative participant. (A) Longitudinal section visualizing two distinct fascicles (F1 and F2) for 120° of elbow flexion at rest. SA, superficial aponeurosis; IPU, location of fascicle insertion on the posterior face of the ulna. (B) Cross-section showing muscle thickness measurement (d) for 0° of elbow flexion at rest.
Ultrasound data reduction and analysis
All ultrasound images captured during testing were transferred to a desktop computer for offline analysis using echopac software (v.7.0.1, GE Vingmed Ultrasound, Horton, Norway), which allowed the LF and PA calculation to be calculated. Pennation angle was defined as the angle created by the fascicle at its insertion point along the force-generating axis on the posterior face of the ulna (An et al. 1981). Fascicle length was defined as the length of a line coincident with the fascicle, between the insertion point of the fascicle onto the ulna and the superficial aponeurosis. Images were selected so that fascicles were visible near the point of insertion onto the ulna. However, the fascicle was often not visible in its entirety, in which case its intercept with the aponeurosis was extrapolated (Reeves & Narici, 2003) (see Fig. 3).
Fig. 3.
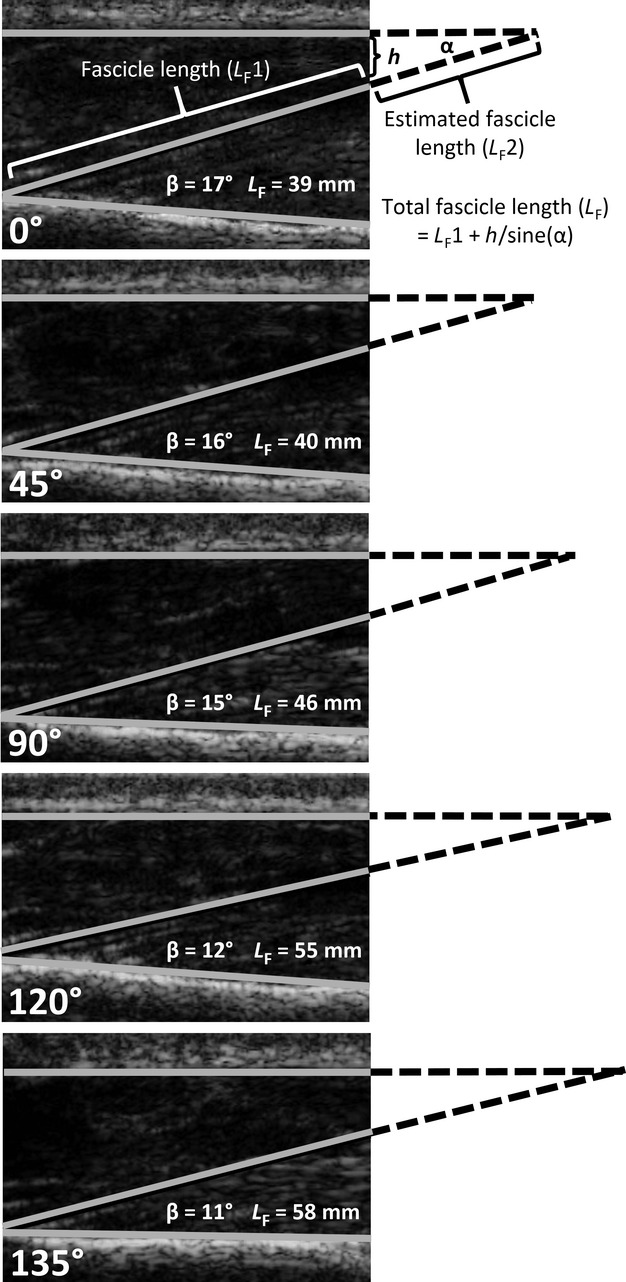
Ultrasound images of the anconeus from a representative participant showing fascicle length (LF) and pennation angle (PA) measurement at rest. The solid lines represent the aponeurosis and posterior face of the ulna. Pennation angle (denoted as β) is the angle at which the fascicle leaves the posterior face of the ulna and intersects with the theoretical aponeurosis indicated with an extrapolated broken line. Fascicle length was calculated as the sum of the measured fascicle length (LF1) and the estimated (LF2) fascicle length [h/sine(χ)].
Repeatability of ultrasound measurements
The repeatability of the measurements for LF and PA was tested by performing eight separate ultrasound scans of the anconeus in one participant. Repeatability scans were performed over the course of a day, with two distinct muscle fascicles imaged per session.
Validation of ultrasound measurements
The accuracy of the ultrasound technique used in this study was assessed in a human cadaver by comparing ultrasound-determined muscle architecture with direct anatomical measurements. The anconeus muscle of a 74-year-old embalmed cadaver was imaged with an angle of 120° of elbow flexion using ultrasonography as described above. Following imaging, the skin, subcutaneous fat, and fascia were removed and the corresponding ultrasound plane was delineated by careful dissection along the insertion of the anconeus on the posterior face of the ulna. A large flap mainly of the posterior portion of the anconeus was exposed, corresponding to the image axis through the muscle, so that the pennation angles and fiber lengths of the fascicles could be measured manually in situ. On the exposed muscle, measurements of LF and PA were made using a millimeter ruler and protractor. These measures were compared with the images taken with ultrasound before dissection.
Statistical analysis
Data were analyzed with SPSS statistical software (version 16, SPSS Inc., Chicago, IL, USA). Separate one-factor (elbow joint angle) repeated measures univariate analyses of variance (anovas) were performed with an a priori repeated contrast, to compare the dependent variables, average LF and average PA, for each angle of elbow flexion to the subsequent elbow joint angle (135° elbow flexion representing baseline). A paired t-test was used to compare anconeus muscle thickness at 0° and 135° of elbow flexion. Repeated measures anovas were also performed to assess the repeatability of LF and PA measures for the eight ultrasound scans. When a main effect was observed, a post hoc analysis using paired t-tests was performed with a modified Bonferroni correction factor to determine where significant differences existed among visits. Average values of LF and PA obtained using ultrasonography were compared with those obtained directly from the exposed muscle using unpaired t-tests. Lastly, Cohen's d effect sizes were determined for each pairwise comparison. The level of significance was set at P < 0.05. Data are presented as mean ± standard deviation (SD).
Results
Despite the challenges of applying ultrasound to a small muscle that is enveloped by a relatively thick layer of fascia and surrounded by bony contours at various elbow angles, useful images were obtained for all participants and at all joint angles. On average, 3.9 ± 0.5 images were obtained per elbow joint angle, and each image yielded 1.7 ± 0.2 fascicles per participant per elbow joint angle.
In all 10 participants, LF decreased and PA increased from 135° to 0° of elbow flexion. The overall or maximum change throughout the entire ROM in LF and PA was 18 mm (32%) and 5° (45%), respectively. Average values of LF decreased by ∼12% from 135–120° and 120–90°, and ∼11% from 90–45° (P < 0.05; d = 0.74, 0.67, 0.47, respectively; Table 1, Fig. 4A). Average values of PA were increased from 135–120°, 120–90°, and 45–0° (P < 0.05; d = 0.63, 0.39, 0.78, respectively; Table 1, Fig. 4B). Percent increase for PA between each elbow joint angle (135–120°, 120–90°, and 45–0°) was determined to be ∼12%. The thickness of the muscle ranged from 8 to 12 mm at 135°, and increased by 9% between 135° and 0° of elbow flexion (P < 0.05; d = 0.50; Table 1).
Table 1.
Muscle architecture measurements.
| Angle of elbow flexion (°) | 135 | 120 | 90 | 45 | 0 |
|---|---|---|---|---|---|
| Fascicle length (mm) | 56 ± 7* | 50 ± 9* | 44 ± 9* | 40 ± 8 | 38 ± 7 |
| Pennation angle (°) | 11 ± 1* | 12 ± 2* | 13 ± 3 | 14 ± 2* | 16 ± 3 |
| Thickness (mm) | 10 ± 2† | – | – | – | 11 ± 2 |
Measurements were obtained from 10 healthy young males at rest. Values are mean ± SD.
Difference compared with the subsequent degree of elbow flexion.
Difference between 135° and 0° of elbow flexion.
Fig. 4.
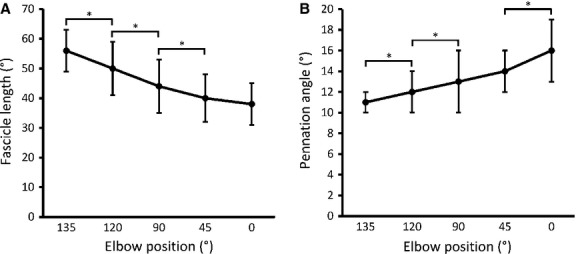
(A) Mean fascicle length (mm) at five angles of elbow flexion (°). (B) Mean pennation angle (°) at five angles of elbow flexion (°). Data are presented as means ± SD. *Difference between angles of elbow flexion (P < 0.05).
No main effect was found for the analysis of LF and PA for the eight repeated ultrasound scans obtained in one participant and the coefficient of variation was 0.10 for LF (3.7 mm) and 0.13 for PA (1.69°). Although the images obtained from the cadaver were not as clear as those obtained in vivo, we were able to establish good agreement between ultrasound-determined muscle architecture and direct anatomical measurement for both LF [37 ± 6 mm vs. 32 ± 3 mm (P > 0.05), respectively] and PA [17 ± 2° and 15 ± 2° (P > 0.05), respectively].
Discussion
This study examined the architectural features, LF and PA, of the human anconeus muscle in vivo during rest at five elbow joint angles. A few studies have described anconeus muscle architecture from cadavers at a single, often unspecified, fixed joint angle (Coriolano et al. 2009; Ng et al. 2012; Pereira, 2013), and one study estimated LF and PA over a 120° ROM using computer software (Pereira, 2013); here, however, we investigated these key architectural features using ultrasonography in vivo over the full range of elbow joint excursion. The results indicated that anconeus LF and PA substantially decreased and increased, respectively, as the elbow joint angle approached full extension from a flexed position. The ultrasound technique used for the measurement of these architectural parameters was determined to be reliable over several scans and valid based on good agreement with cadaveric measurements.
Cadaveric studies have described the anatomy of the human anconeus muscle, including muscle architectural measures LF and PA, yet most have not reported a specific elbow joint angle. For muscle thickness, ultrasound-determined values in this study (10–11 mm) agree with those reported by Coriolano et al. (2009) (12 ± 2 mm) obtained by measuring the width of the anconeus at the proximal end of its insertion onto the posterior face of the ulna. A moderate discrepancy between previous reports (Coriolano et al. 2009; Pereira, 2013) of LF in cadavers (30 mm) and that reported in the current study (LF, 46 ± 10 mm), is likely the result of comparing: (i) an average LF derived from multiple joint angles to a single LF recorded at one often unspecified, angle; and (ii) in vivo measurements obtained from a healthy, young population to in situ preparations from elderly cadavers. Skeletal muscle architecture of human cadaver muscle has been found to differ greatly from age-matched in vivo ultrasonographic measurements, where PA and FL differed ∼13–180% and ∼4–21%, respectively, depending on the muscle under investigation (Martin et al. 2001). Martin et al. (2001) attributed these differences to shortened cadaveric fiber bundle length, suggesting that cadaveric muscle exists architecturally in a state of partial contraction. It is likely that this presumed state of partial muscle contraction also attributed to the large disparity between the cadaveric value (71 ± 12°) reported by Ng et al. (2012) and the in vivo PA values reported here at 90° of elbow flexion (13 ± 3°), as pennation has been shown to increase relative to muscle length during shortening contractions (Narici et al. 1996; Fukunaga et al. 1997; Kawakami et al. 1993; Maganaris & Baltzopoulos, 1999; Simoneau et al. 2012). However, the difference in measurement procedure is likely to be a more prominent contributor to the significant differences found in one cadaveric study (Ng et al. 2012) and in the present findings. In cadavers, the average PA (71 ± 12°) was determined as the angle at which the fascicle intersects 90° quarterly intervals along the long axis of the muscle (see Ng et al. 2012; Fig. 3). In the present study, PA was measured as the angle at which the fascicle emerges from its insertion on the force-generating axis along the posterior face of the ulna. This is significant, as it has been shown that PAs are systematically smaller at the insertion of the muscle onto the tendon compared with those imaged from more central locations of the muscle (Blazevich et al. 2006), as was done by Ng et al. (2012). Furthermore, anconeus compartmentalization may explain variations in PA (Bergin et al. 2013), as measures made at a more distal location would yield a less oblique angle. Finally, a PA of 70° does not seem functionally useful and such extreme angles are not reported for other limb muscles (Kwah et al. 2013). Therefore, it is likely not valid to compare PA values directly from the current study with the Ng et al. (2012) study in cadavers.
More important for the purpose of the present study is that the anconeus studies cited above only described the muscle architecture at a single elbow joint angle (position of fixation). One study (Pereira, 2013) attempted to measure changes in anconeus muscle fiber length over multiple elbow joint angles (ranging from 0° to 120° of elbow flexion) using a 2-D kinematic model. That study reported that muscle fiber lengths differed over the ROM tested, with the greatest change recorded at 90° elbow flexion. However, the investigation was limited by a small sample of human cadavers (comprised of 8 elderly men) and a simple 2-D kinematic model, which the authors admitted did not fully represent physiological in vivo conditions. Thus, the use of ultrasonography here was necessary to obtain a more accurate representation of changes in LF and PA in vivo in relation to elbow joint angle.
The relative change in LF and PA reported for the anconeus in the current study closely resembles that derived using ultrasonography for other muscles in vivo, under passive conditions, relative to the ROM tested at their respective joints. For example, LF and PA measured in the biceps femoris at three knee angles, covering a 90° ROM (0°, 45°, 90° flexion), were reported to decrease 27% and increase 27%, respectively (Chleboun et al. 2001). Similarly, in the vastus lateralis, LF decreased 27% and PA increased 29% when knee angle changed from 110° to 0° of flexion (Fukunaga et al. 1997). Moreover, Kawakami et al. (1998) measured percent change in LF and PA in the relaxed medial gastrocnemius (MG), lateral gastrocnemius (LG), and soleus (SOL) across an ankle joint ROM of 45° (−15° to 30° extension) and observed a 21, 23, and 30% decrease in LF for the MG, LG, and SOL, respectively, whereas PA increased 32, 42, and 47% in these same muscles. In another study of the MG, Narici et al. (1996) measured LF and PA changes over a slightly larger ROM (60°) and observed a 40% decrease in LF and a 75% increase in PA. With the exception of one study of the MG (Narici et al. (1996), the percent changes in these muscle groups across a full or nearly full ROM and those reported here for the anconeus (LF, decreased 32%; PA, increased 45%) are comparable. Thus, although the anconeus is a short stabilizing muscle it undergoes architectural changes during extension as the elbow joint moves throughout a large ROM.
Absolute values of PA reported previously for the three heads of the triceps brachii (TB) are also similar to those determined for the anconeus in the present study. Although resting LF and PA values for the three heads of the TB across the full ROM have not been assessed, different studies have examined the muscle architecture (PA) of the TB at different elbow joint angles. Using ultrasound, Blazevich & Giorgi (2001) found PA for the relaxed lateral head of the TB, in men similar in age to those in the present study, to be 12 ± 2° when the elbow was flexed 90°. At 0° of elbow flexion, PAs of 15 ± 6° (Kawakami et al. 1993) and 20 ± 3° (Kubo et al. 2003) were reported for the TB long head, and 11 ± 5° for the medial head (Kawakami et al. 1993). As mentioned above, these values do not differ with those reported in the present study (13 ± 3° and 16 ± 3° at 90° and 0° of elbow flexion, respectively). These results indicate that the anconeus experiences similar absolute and relative changes in PA as the TB during elbow extension movements (Basmajian & Griffin, 1972), and therefore PA cannot explain the differences in EMG signal clarity between the anconeus and TB.
Alternatively, it is plausible that low absolute changes in LF relative to elbow joint ROM contribute partially to high EMG signal clarity. Although the average LF of the anconeus relative to total muscle length in this study were similar to those reported in the TB, the average absolute change in LF is ∼55% less than that of the TB (Murray et al. 1995; Pereira, 2013). One important factor contributing to poor intramuscular EMG signal clarity is electrode displacement (Merletti & Farina, 2009); less absolute change in LF facilitates better quality recordings. Therefore, it is likely that less interference from neighboring MUs due to the low number of estimated MUs in the anconeus (Stevens et al. 2013) and small absolute changes in LF relative to elbow joint range of motion collectively produce a scenario in which high quality recordings are more probable. It is these unique features of the anconeus together with its similarity on a relative basis to other upper limb muscles that make it an attractive model to investigate MU properties in situations where larger muscles are not appropriate.
Concluding remarks
In summary, LF and PA of the anconeus muscle at rest were observed to change as a function of elbow joint angle. The values obtained here, using ultrasonography, differed slightly from those reported previously in cadaveric studies (Coriolano et al. 2009; Pereira, 2013; Molinier et al. 2011; Ng et al. 2012) with respect to LF, but were significantly different for PA (Ng et al. 2012), which was predominately attributed to a difference in measurement procedure and the limitation of comparing in vivo measures to cadaveric. The relative change in LF and PA for the anconeus was consistent with that of other muscles measured using the same technique (Chleboun et al. 2001; Kawakami et al. 1998; Fukunaga et al. 1997), and absolute values of PA observed for the anconeus were very similar to those reported in the TB (Kubo et al. 2003; Blazevich & Giorgi, 2001; Kawakami et al. 1993), which shares the same innervation and function as the anconeus. These similarities in muscle architectural changes indicate that the anconeus behaves like other upper limb muscles. However, the absolute changes in FL over a full joint ROM are small compared with other muscles about the elbow joint. Therefore, the high intramuscular EMG signal clarity reported for this muscle during functional contractions does not appear to be related to changes in PA but may rely partially on the small absolute changes in FL experienced by the anconeus. Collectively, these findings indicate that the anconeus is a valuable muscle model for study in neuromuscular physiology and functional anatomy.
Acknowledgments
We are indebted to Dr. Gary S. Chelboun School of Rehabilitation and Communication Sciences – PT at Ohio University, Athens, OH, for helping us with the original pilot data. This study has been financially supported by NSERC.
Conflict of interest
The authors have no conflicts of interest to report.
Author contributions
All authors made substantive intellectual contributions to this study, in conception and design (D.S., C.S., B.H., C.R.), analysis and interpretation of data (D.S., C.S.), and drafting and critically revising the manuscript (D.S., C.S., B.H., C.R.).
References
- An KN, Hui FC, Morrey BF, et al. Muscles across the elbow joint: a biomechanical analysis. J Biomech. 1981;14:663–669. doi: 10.1016/0021-9290(81)90048-8. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Griffin WR. Function of anconeus muscle. An electromyographic study. J Bone Joint Surg Am. 1972;54:1712–1714. [PubMed] [Google Scholar]
- Bergin MJ, Vicenzino B, Hodges PW. Functional differences between anatomical regions of the anconeus muscle in humans. J Electromyogr Kinesiol. 2013;23:1391–1397. doi: 10.1016/j.jelekin.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Blazevich AJ, Giorgi A. Effect of testosterone administration and eight training on muscle architecture. Med Sci Sports Exerc. 2001;33:1688–1693. doi: 10.1097/00005768-200110000-00012. [DOI] [PubMed] [Google Scholar]
- Blazevich AJ, Gill ND, Zhou S. Intra- and intermuscular variation in human quadriceps femoris architecture assessed in vivo. J Anat. 2006;209:289–310. doi: 10.1111/j.1469-7580.2006.00619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chleboun GS, France AR, Crill MT, et al. In vivo measurement of fascicle length and pennation angle of the human biceps femoris muscle. Cells Tissues Organs. 2001;169:401–409. doi: 10.1159/000047908. [DOI] [PubMed] [Google Scholar]
- Chleboun GS, Busic AB, Graham KK, et al. Fascicle length change of the human tibialis anterior and vastus lateralis during walking. J Orthop Sports Phys Ther. 2007;37:372–379. doi: 10.2519/jospt.2007.2440. [DOI] [PubMed] [Google Scholar]
- Coriolano MGWS, Lins OG, Amorim MJAAL, et al. Anatomy and functional architecture of the anconeus muscle. Int J Morph. 2009;27:1009–1012. [Google Scholar]
- Davidson AW, Rice CL. Effect of shoulder angle on the activation pattern of the elbow extensors during a submaximal isometric fatiguing contraction. Muscle Nerve. 2010;42:514–521. doi: 10.1002/mus.21717. [DOI] [PubMed] [Google Scholar]
- Duchateau J, Enoka RM. Neural control of shortening and lengthening contractions: influence of task constraints. J Physiol. 2008;586(pt 24):5853–5864. doi: 10.1113/jphysiol.2008.160747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton V, Smith J, Simpson D. Muscle fiber type populations of human leg muscles. J Histochem Cytochem. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Fukunaga T, Ichinose Y, Ito M, et al. Determination of fascicle length and pennation in a contracting human muscle in vivo. J Appl Physiol. 1997;82:354–358. doi: 10.1152/jappl.1997.82.1.354. [DOI] [PubMed] [Google Scholar]
- Gans C, de Vree F. Functional bases of fiber length and angulation in muscle. J Morphol. 1987;192:63–85. doi: 10.1002/jmor.1051920106. [DOI] [PubMed] [Google Scholar]
- Gerling ME, Brown SH. Architectural analysis and predicted functional capability of the human latissimus dorsi muscle. J Anat. 2013;223:112–122. doi: 10.1111/joa.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood B, Rice CL. Changes in motor unit recruitment thresholds of the human anconeus muscle during torque development preceding shortening elbow extensions. J Neurophysiol. 2012;107:2876–2884. doi: 10.1152/jn.00902.2011. [DOI] [PubMed] [Google Scholar]
- Harwood B, Hamberg CM, Chleboun GS, et al. Effect of elbow joint angle on anconeus fascicle length and motor unit firing rates. [Abstract] Med Sci Sports Exerc. 2010;42:584–585. [Google Scholar]
- Harwood B, Davidson AW, Rice CL. Motor unit discharge rates of the anconeus muscle during high-velocity elbow extensions. Exp Brain Res. 2011;208:103–113. doi: 10.1007/s00221-010-2463-4. [DOI] [PubMed] [Google Scholar]
- Harwood B, Choi IH, Rice CL. Reduced motor unit discharge rates of maximal velocity dynamic contractions in response to a submaximal dynamic fatigue protocol. J Appl Physiol. 2012;113:1821–1830. doi: 10.1152/japplphysiol.00879.2012. [DOI] [PubMed] [Google Scholar]
- Harwood B, Dalton BH, Power GA, et al. Motor unit properties from three synergistic muscles during ramp isometric elbow extensions. Exp Brain Res. 2013;231:501–510. doi: 10.1007/s00221-013-3714-y. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–2744. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol. 1998;85:398–404. doi: 10.1152/jappl.1998.85.2.398. [DOI] [PubMed] [Google Scholar]
- Kellis E, Galanis N, Kapetanos G, et al. Architectural difference between the hamstring muscles. J Electromyogr Kinesiol. 2012;22:520–526. doi: 10.1016/j.jelekin.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Kennett RP, Fawcett PR. Repetitive nerve stimulation of anconeus in the assessment of neuromuscular transmission disorders. Electroencephalogr Clin Neurophysiol. 1993;89:170–176. doi: 10.1016/0168-5597(93)90130-h. [DOI] [PubMed] [Google Scholar]
- Kubo K, Kanehisa H, Azuma K, et al. Muscle architectural characteristics in young and elderly men and women. Int J Sports Med. 2003;24:125–130. doi: 10.1055/s-2003-38204. [DOI] [PubMed] [Google Scholar]
- Kwah LK, Pinto RZ, Diong J, et al. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: a systematic review. J Appl Physiol. 2013;114:761–769. doi: 10.1152/japplphysiol.01430.2011. [DOI] [PubMed] [Google Scholar]
- Le Bozec S, Maton B. The activity of anconeus during voluntary elbow extension: the effect of lidocaine blocking of the muscle. Electromyogr Clin Neurophysiol. 1982;22:265–275. [PubMed] [Google Scholar]
- Lieber RL, Bodine-Fowler SC. Skeletal muscle mechanics: implications for rehabilitation. Phys Ther. 1993;73:844–856. doi: 10.1093/ptj/73.12.844. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–1666. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Maganaris C, Baltzopoulos V. Predictability of in vivo changes in pennation angle of human tibialis anterior muscle from rest to maximum isometric dorsiflexion. Eur J Appl Physiol Occup Physiol. 1999;79:294–297. doi: 10.1007/s004210050510. [DOI] [PubMed] [Google Scholar]
- Martin DC, Medri MK, Chow RS, et al. Comparing human skeletal muscle architectural parameters of cadavers with in vivo ultrasonographic measurements. J Anat. 2001;199(pt. 4):429–434. doi: 10.1046/j.1469-7580.2001.19940429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselli RA, Mass DP, Distad BJ, et al. Anconeus muscle: a human muscle preparation suitable for in-vitro microelectrode studies. Muscle Nerve. 1991;14:1189–1192. doi: 10.1002/mus.880141208. [DOI] [PubMed] [Google Scholar]
- Merletti R, Farina D. Analysis of intramuscular electromyogram signals. Philos Trans A Math Phys Eng Sci. 2009;367:357–368. doi: 10.1098/rsta.2008.0235. [DOI] [PubMed] [Google Scholar]
- Molinier F, Laffosse JM, Bouali O, et al. The anconeus, an active lateral ligament of the elbow: new anatomical arguments. Surg Radiol Anat. 2011;33:617–621. doi: 10.1007/s00276-010-0767-5. [DOI] [PubMed] [Google Scholar]
- Murray WM, Delp SL, Buchanan TS. Variation of muscle moment arms with elbow and forearm position. J Biomech. 1995;28:513–525. doi: 10.1016/0021-9290(94)00114-j. [DOI] [PubMed] [Google Scholar]
- Narici MV, Binzoni T, Hiltbrand E, et al. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496:287–297. doi: 10.1113/jphysiol.1996.sp021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng ZY, Lee SW, Mitchell JH, et al. Functional anconeus free flap for thenar reconstruction: a cadaveric study. Hand. 2012;7:286–292. doi: 10.1007/s11552-012-9412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J. Change in muscle fascicle length influences the recruitment and discharge rate of motor units during isometric contractions. J Neurophysiol. 2005;94:3126–3133. doi: 10.1152/jn.00537.2005. [DOI] [PubMed] [Google Scholar]
- Pasquet B, Carpentier A, Duchateau J. Specific modulation of motor unit discharge for a similar change in fascicle length during shortening and lengthening contractions in humans. J Physiol. 2006;577:753–765. doi: 10.1113/jphysiol.2006.117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BP. Revisiting the anatomy and biomechanics of the anconeus muscle and its role in elbow stability. Ann Anat. 2013;195:365–370. doi: 10.1016/j.aanat.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Power GA, Makrakos DP, Rice CL, et al. Enhanced force production in old age is not a far stretch: an investigation of residual force enhancement and muscle architecture. Physiol Rep. 2013;1:e00004. doi: 10.1002/phy2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves ND, Narici MV. Behaviour of human muscle fascicles during shortening and lengthening contractions in vivo. J Appl Physiol. 2003;95:1090–1096. doi: 10.1152/japplphysiol.01046.2002. [DOI] [PubMed] [Google Scholar]
- Simoneau EM, Longo S, Seyennes OR, et al. Human muscle fascicle behaviour in agonist and antagonist isometric contractions. Muscle Nerve. 2012;45:92–99. doi: 10.1002/mus.22257. [DOI] [PubMed] [Google Scholar]
- Stevens DE, Harwood B, Power GA, et al. Anconeus motor unit number estimates using decomposition-based quantitative electromyography. Muscle Nerve. 2013;50:52–59. doi: 10.1002/mus.24092. [DOI] [PubMed] [Google Scholar]
- Zhang LQ, Nuber GW. Moment distribution among human elbow extensor muscles during isometric and submaximal extension. J Biomech. 2000;33:145–154. doi: 10.1016/s0021-9290(99)00157-8. [DOI] [PubMed] [Google Scholar]


