Abstract
Liver cancer has a poor prognosis. Our recent study demonstrates that hyper-IL-15, composed of IL-15 and the sushi domain of IL-15 receptor α chain, provides an effective therapy against well-established metastatic and autochthonous liver cancers in mouse models by triggering activation and expansion of hepatic CD8+ T cells.
Keywords: CD8+ T cell, hyper-IL-15, IFNγ, immunotherapy, liver cancer
Hepatocellular carcinoma (HCC) is one of the most deadly cancers worldwide, which is the fifth most common cancer worldwide and the third leading cause of death from cancer. Most HCCs are diagnosed at an advanced stage for which there is no curative option.1 The current treatment efficacy is limited by low response rates, severe toxicity, and high recurrence rates, resulting in mean survival time of only 6 mo. Thus, new strategies are urgently needed to improve the therapy of HCCs.
The liver is an immunologically unique organ, which is constantly exposed to various antigens such as microbial products from intestinal bacteria. There are numerous immunosuppressive mechanisms to maintain a tolerogenic liver microenvironment. Nonetheless, the liver organ still possesses the necessary capabilities for the development of effective immune responses.2 Works in recent years have demonstrated that local and systemic immunity can be harnessed to fight against hepatic neoplastic development, and a variety of evidence points toward immunotherapy as a promising strategy against HCCs. Liver contains high frequency of NK and CD8+ T cells, which are the most important effector cells of antitumor immunity. Thus, boosting NK and/or CD8+ T cell responses may be an effective immunotherapy for liver cancers.
Interleukin-15 (IL-15) is a cytokine for efficiently triggering the activation and proliferation of NK and CD8+ T cells. It shares the use of two receptor subunits (IL-2Rβ and IL-2Rγc)and similar intracellular signaling events with IL-2.3 However, IL-15 is presented in trans to the IL-2Rβγc complex on effector cells by binding to IL-15 receptor α (IL-15Rα) expressed on antigen-presenting cells.4 Unlike IL-2, IL-15 does not induce activation-induced T-cell death or expansion of T regulatory cells. In addition, IL-15 provides anti-apoptotic signals to effector CD8+ T cells. The biological activity of IL-15 is greatly improved by pre-associating IL-15 with a fusion protein IL-15Rα−Fc or by direct fusion with the sushi domain of IL-15Rα (hyper-IL-15) to mimic trans-presentation of IL-15 by cell-associated IL-15Rα.5,6 IL-15, either administrated alone or as a complex with IL-15Rα, exhibits potent antitumor activities in animal models.7,8
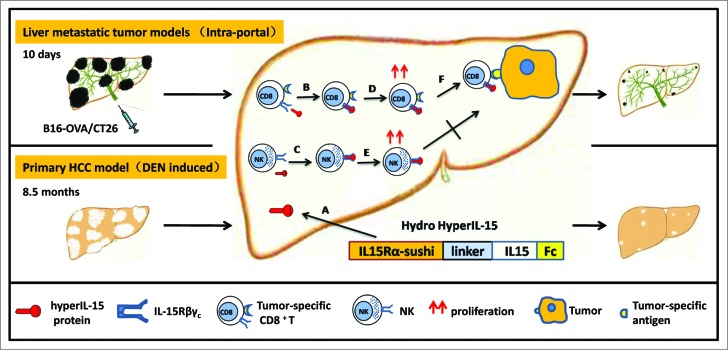
Because the liver is heavily populated by NK and CD8+ T lymphocytes, we hypothesized that activation of these hepatic lymphocytes by complex of IL-15 and IL-15Rα might offer an effective therapy against HCCs. Thus, we studied the therapeutic effect of hyper-IL-15 in both the well-established metastatic and autochthonous liver cancer models9 (Fig. 1). The liver metastatic tumor models were established by intraportally injecting CT26 colon carcinoma cells or B16-OVA melanoma cells into syngeneic BALB/c or C57BL/6 mice. The hyper-IL-15 or Fc control plasmid was transferred to the liver by hydrodynamic injection, which achieved a high and sustained concentration of the protein and a clear effect on expansion of NK and CD8+ T cells. The tumor-bearing mice were treated by one hydrodynamic injection of hyper-IL-15 plasmid 10 d after tumor inoculation when the disseminated metastatic tumors in the liver were palpable. Our results showed impressive therapeutic effect of hyper-IL-15 on well-established metastatic tumors in the liver. It is important that hyper-IL-15 also dramatically inhibited the growth of DEN-induced primary HCC when the treatment was given after HCC had been developed. To our knowledge, this is the first such therapy that shows significant therapeutic effect on DEN-induced primary HCC.
Figure 1.
The immunotherapeutic treatment of liver cancer by liver delivery of hyper-IL-15. Liver metastatic tumor models were established by intraportally injecting mice with murine syngeneic CT26 colon carcinoma cells or B16-OVA melanoma cells. Primary hepatocellular carcinoma (HCC) was induced by diethylnitrosamine (DEN). (A) A hydrodynamic-based gene delivery method was used to achieve sustained hyper-IL-15 expression in the liver. (B and C) Hyper-IL-15 protein binds to IL-15Rβγc complex expressed on CD8+ T cells and NK cells (D and E), and triggers proliferation of CD8+ T cells and NK cells. (F) The therapeutic effect of hyper-IL-15 is predominantly mediated by CD8+ T cells, not NK cells.
Since hyper-IL-15 treatment increased both CD8+ T cells and NK cells in the liver, we tried to define the contribution of each subset to the therapeutic effect of hyper-IL-15. Systemic depletion of CD8+ T cells but not NK cells abrogated the therapeutic effect of hyper-IL-15 on liver metastatic tumors. Depletion of CD8+ T cells during treatment also dramatically diminished the therapeutic effect of hyper-IL-15 on well-developed DEN-induced primary HCC. Thus hyper-IL-15 induced CD8+ T cells predominantly mediated the therapeutic effect. It is well documented that tumor-specific T cells are usually rendered tolerant during tumor development. Indeed, pre-transferred OVA-specific OT-1 T cells from the spleen at 14 d after B16-OVA tumor was inoculated in the liver were not responsive to the in vitro stimulation of OVA peptide. In contrast, in hyper-IL-15-treated mice with liver metastatic B16-OVA tumors, the tumor-specific CD8+ T cells were preferentially expanded and their effector functions (IFNγ synthesis and cytotoxic activity) were significantly enhanced. This result is consistent with the previous study that IL-15 can rescue the functions of the tolerant tumor-specific T cells.8
These findings are clinically relevant as they show that activation and expansion of hepatic CD8+T cells by liver delivery of hyper-IL-15 not only exhibit potent antitumor activity against well-established liver metastatic tumors, but also show dramatic therapeutic effect on DEN-induced primary HCC. They provide strong evidence to develop hyper-IL-15 as a therapeutic for treating metastatic and spontaneous liver cancers in humans.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69-90; PMID:; http://dx.doi.org/ 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2. Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol 2009; 27:147-63; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.021908.132629 [DOI] [PubMed] [Google Scholar]
- 3. Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006; 6:595-601; PMID:; http://dx.doi.org/ 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- 4. Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 2002; 17:537-47; PMID:; http://dx.doi.org/ 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- 5. Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci U S A 2006; 103:9166-71; PMID:; http://dx.doi.org/ 10.1073/pnas.0600240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R betagamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem 2006; 281:1612-9; PMID:; http://dx.doi.org/ 10.1074/jbc.M508624200 [DOI] [PubMed] [Google Scholar]
- 7. Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. CancerRres 2008; 68:2972-83; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-0045 [DOI] [PubMed] [Google Scholar]
- 8. Teague RM, Sather BD, Sacks JA, Huang MZ, Dossett ML, Morimoto J, Tan X, Sutton SE, Cooke MP, Ohlén C, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med 2006; 12:335-41; PMID:; http://dx.doi.org/ 10.1038/nm1359 [DOI] [PubMed] [Google Scholar]
- 9. Cheng L, Du X, Wang Z, Ju J, Jia M, Huang Q, Xing Q, Xu M, Tan Y, Liu M, et al. Hyper-IL-15 suppresses metastatic and autochthonous liver cancers by promoting tumor-specific CD8+ T cell responses. J Hepatol 2014; pii: S0168–8278(14)00472-3; PMID:; http://dx.doi.org/ 10.4161/21624011.2014.963409 [DOI] [PMC free article] [PubMed] [Google Scholar]