Abstract
Background
The aim of this study was to design a self-retaining rat contact lens to simplify intravitreous injection in rats.
Material/Methods
A self-retaining, plane-concave prism contact lens customized for rats was designed. Forty diabetic rats were randomly divided into 2 groups and received the intravitreous injection of 10 μl of a cell suspension containing bone marrow-derived stroma cell (BMSC). Group A: used a microsyringe and a rat contact lens (n=20). Group B: used the same microsyringe and a traditional cover-slip (n=20). The duration of the intravitreous injection course and the success rate of intravitreous injection were observed.
Results
With the use of a self-retaining rat contact lens, a clear and stable view of the rat fundus was provided and the intravitreous injection course of rats quickly achieved, averaging 4.65±0.53 min in Group A and 12.33±2.79 min in Group B. The difference was statistically significant, and the time saved averaged 7.68 min. None of the Group A rats had retinal bleeding or lens injury; whereas 2 of 20 Group B rats had bleeding and 1 of 20 had lens injury. There was no significant difference between the rats in Group A and Group B.
Conclusions
A self-retaining rat contact lens is a potentially powerful instrument that allows high-quality observation of the rat fundus and simplifies the course of intravitreal injection.
MeSH Keywords: Contact Lenses; Intravitreal Injections; Models, Animal
Background
Intravitreous injection of rat is a widely used technique in visual sciences research [1]. It has been applied to treat animal models with ocular diseases, including diabetic retinopathy [2] and choroid neovascularization [3]. Good performance of intravitreous injection has 3 aspects: 1) the injection site should not disrupt retina structure, 2) bleeding should be avoided to reduce the risk of infection, and 3) the lens should be untouched to avoid traumatic cataract [1].
As a typical microsurgery, intravitreous injection has to be performed with a surgical microscope. Since the refractive power for an adult rat’s eye is close to +70 D, it is impossible to capture the images of fundus structures directly by the microscope unless performing rat funduscopy. Typically, images of the ocular fundus of rats can be obtained by using a modified fundus camera, indirect ophthalmoscopy, confocal scanning laser ophthalmoscopy, or optical coherence tomography, all of which are noncontact methods that relay the fundus image several centimeters in front of the cornea. A contact fundus camera based on Rol’s GRIN lenses or topical endoscopy especially designed to examine rats was introduced recently [4]. However, these instruments are costly, bulky, and difficult to align with the surgical microscope in the course of intravitreous injection of rats.
At present, a more economical and general solution of observing rat fundus is to planish the cornea with a transparent disc or a cover-glass until the retina, vitreous, and lens can be discerned [2,3]. Then the operator should manipulate a microsyringe to puncture the eyeball and then inject cells or drugs slowly. The entire injection procedure often lasts for at least 10 min per eye and complications such as lens injury and retinal vascular injury have been reported.
Use of corneal contact lenses during clinical vitreous surgery is popular because they afford excellent visualization of the fundus. In 2004, Chalam et al. manufactured an acrylic, self-containing corneal contact lens for improving the efficiency of vitrectomy [5]. The contact surface curvature almost equals the curvature of the anterior corneal surface, eliminating the refractive power of the cornea. This does not change the image formation but eliminates the reflection on the inferior surface of the lens and the cornea, which increases the image resolution and stereopsis.
Similarly, we designed an acrylic contact lens fit for rats. Its geometric profile and material property enabled it to maintain position steadily on the cornea by negative suction and present a clear view with no need to change the shape of the cornea during the operation. In this paper, we demonstrate its utility in the course of intravitreous injection in rats.
Material and Methods
Features of the rat corneal contact lens
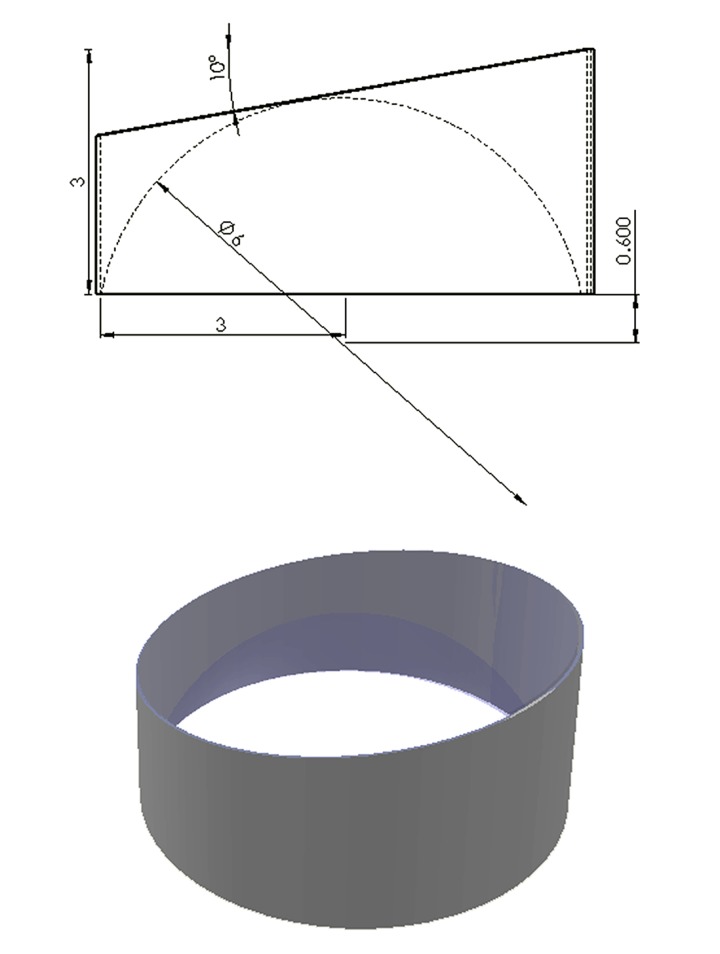
The contour of the rat corneal contact lens was designed using SolidWorks 2010 software (Dassault Systèmes SolidWorks Corp., MA, USA) (Figure 1). The lens is made of PMMA (polymethylmethacrylate, also known as acrylic) with a refractive index of 1.5 at 550 nm and weight of 0.05 g. In size, the lens is 3.0 mm in length; 6.0 mm in diameter, the superior prism surface of the lens is 10°, and the radius of the curvature of the inferior concave surface is 3.0 mm.
Figure 1.
Schematic representation of rat contact lens.
Intravitreal injection experiments
To evaluate the efficiency of this lens for intraocular observation and surgery in rats, an intravitreal injection experiment was performed. According to previous methods reported [2], 40 male Sprague-Dawley (SD) rats were induced to be diabetic with streptozotocin (STZ, Sigma-Aldrich, St. Louis, MO) and then used in this study. Forty diabetic rats were randomly divided into 2 groups and received the intravitreous injection of 10 μl of a cell suspension containing bone marrow-derived stroma cells (BMSC). In Group A we used a microsyringe and a self-retaining rat contact lens (n=20). In Group B we used the same microsyringe and a traditional cover-slip (n=20). All procedures were performed by the same operator. The animals were cared for in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All experimental procedures used aseptic techniques and were approved by the Animal Care and Use Committee of He University.
The diabetic rats to be anesthesia were placed in a box ventilated with mixed gas containing 5 MAC (maximum alveolar concentration) isoflurane (Beijing Pharmaceutical Co., Ltd., China), and 0.5 L/min O2 by a Matrx VIP 3000 anesthesia machine (Midmark Co., USA). After sedation, the diabetic rat was transferred out of the box and isoflurane was continuously supplied at the rate of 1–1.5 MAC through a mask made of the protective sleeve of a disposable 10-ml syringe.
We then performed intravitreal injections. In group A, a topical anaesthetic (0.5% tetracaine hydrochloride; Santen, Inc., Osaka, Japan) was administered and the pupil was dilated with 1% tropicamide (1% Mydriacyl; Alcon Laboratories, Hempstead, UK). With a drop of viscoelastic material (Healon; Pharmacia and Upjohn, Uppsala, Sweden), the contact lens was kept in place on the cornea by negative suction (Figure 2). Upon visualizing the fundus with a surgical microscope, a sclerotomy was made at 1 mm peripherally adjacent to the limbus. Next, a 27-gauge needle (Hamilton, Reno, NV, USA) was inserted into the vitreous cavity, and 10 μl of the cell suspension containing 1.1×107 bone marrow-derived stroma cells (BMSC) was injected. The needle had to be left in the vitreous cavity for at least 1 min after injection to reduce reflux. Finally, 0.5% topical Tobra Dexointment (Alcon Laboratories, Inc., Fort Worth, TX, USA) was applied to prevent infection and the inhalant anesthesia of isoflurane was stopped.
Figure 2.
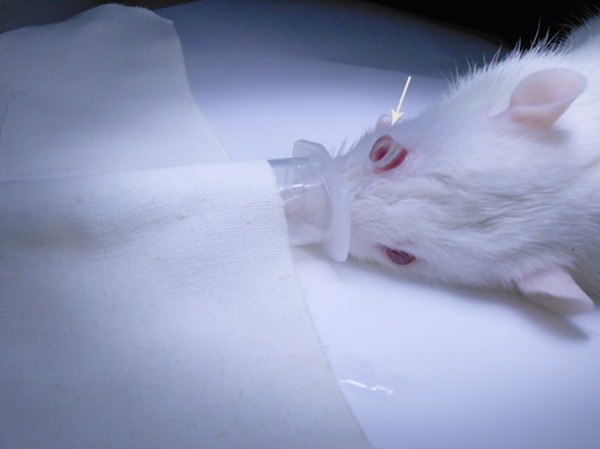
A self-containing contact lens placed on the eye of a diabetic rat maintained anesthesia in respirator mask before the intraocular operation; white arrow indicates that the contact lens attaches onto the rat cornea firmly by the aid of viscoelastic material.
In matched Group B, a coverslip (Boxinda Co. Ltd., Tianjin, China) was used as control. One exception was the hand-held coverslip used in observation of the ocular fundus, but anesthesia, animal eye preparation, and injection procedures in Group B were similar to that of Group A.
Data analysis
The duration of the intravitreous injection course and the success rate of intravitreous injection were observed. Operative faults included retinal bleeding and lens injury. Operation time was analyzed using the 2 independent samples t test, and success rate was analyzed using the Fisher’s exact test. A level of p<0.05 was accepted as statistically significant.
Results
After inhalation inducing anesthesia, all diabetic rats fell into sleep placidly and slept during the whole experiment, and no animal showed stress reactions such as squealing or struggling. In Group A, typical fundus images of the rats including, crystalline lens, vitreous body, and retina, were instantly shown through the self-retaining contact lens, and then the operator used 2-hand technique to puncture the rat eyeball and inject the cell suspension. Finally, the cells adhering to the posterior capsule as oil droplets could be observed after the BMSC liquid was smoothly injected into the vitreous cavity (Figure 3). In Group B, the operator had to use single-hand technique to puncture the rat eyeball and inject cell suspension and another hand was needed to planish the cornea with a cover-slip.
Figure 3.
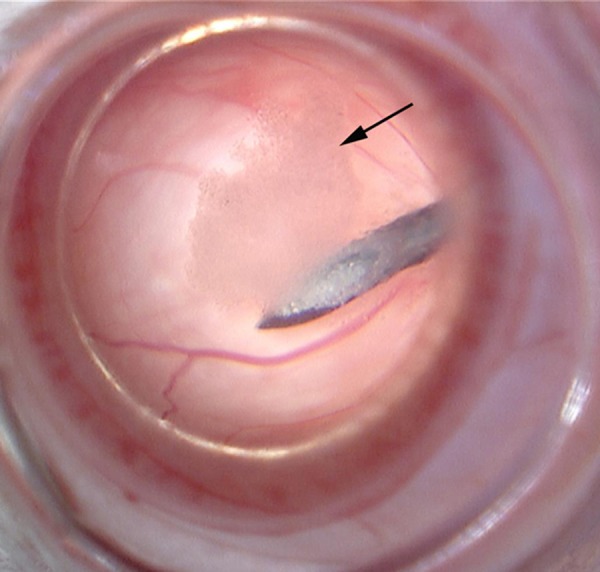
The course of BMSC injection; black arrow indicates the cells adhering to the posterior capsule.
The average time of intravitreous injection was 4.65±0.53 min in Group A and 12.33±2.79 min in Group B. The difference was statistically significant (P<0.01) between Group A and Group B. Compared with the traditional cover-slip used in group B, the using of the newly-designed self-containing rat lens reduced operation time by an average of 7.68 min.
Moreover, there was no lens injury or retinal vascular injury in Group A and the success rate was 100% (20/20), whereas 2 of 20 Group B rats had retinal bleeding, 1 of 20 had lens injury, and the success rate was 85% (17/20). Between the Groups A and B, the difference was no statistically significant (P>0.05).
Discussion
Because the inferior surface curvature approximately matches the curvature of the anterior corneal surface of rats, the refractive power of the cornea would be compensated without planishing the cornea and then the image resolution and stereopsis would be also improved, using the same principle as that used in the Chalam KV’s prism lens [5]. In terms of stability, the new-designed lens is made of a high refractive acrylic material instead of quartz or optical crown glass, which gives the lens a weight advantage as it is lighter than 0.05 g, allowing the self-containing lens to attach to the cornea firmly with the aid of the viscoelastic material. As we shown in this paper, the results in the experimental group achieved the criterion of good intravitreous injection [1], and this lens might provide a friendly-used instrument with good versatility and stability for researchers. Compared to previous rat funduscopies [4], the newly designed rat lens could conveniently and inexpensively match the surgical microscope in the course of intraocular operation.
As one of the commonest experimental techniques in ophthalmic research [1], the repeated frequency of intravitreal injection of rats usually exceeds 60 times [2,3]. In this study, the data suggest that using the self-containing rat contact lens could reduce the operation time and minimize complications. Although the time saved is 7 min per time, but total time saving of multiple operates will by several hours, which will obviously increase the coefficient of performance. Additionally, the lower complication rate in the rat will improve animal welfare.
Conclusions
In conclusion, the self-retaining rat contact lens is a potentially powerful instrument that allows high-quality observation of the rat fundus and simplifies the course of intravitreal injection, with important benefits for researchers.
Footnotes
Statement
None of the authors has any proprietary interest.
Source of support: Self financing
Reference
- 1.Chiu K, Chang RC, So KF. Intravitreous injection for establishing ocular diseases model. J Vis Exp. 2007;(8):313. doi: 10.3791/313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu WC, Lai CC, Chen SL, et al. GDNF gene therapy attenuates retinal ischemic injuries in rats. Mol Vis. 2004;10:93–102. [PubMed] [Google Scholar]
- 3.Murata T, He S, Hangai M, et al. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000;41(8):2309–17. [PubMed] [Google Scholar]
- 4.Hernandez V, Albini T, Lee W, et al. A portable, contact animal fundus imaging system based on Rol’s GRIN lenses. Vet Ophthalmol. 2012;15(3):141–44. doi: 10.1111/j.1463-5224.2011.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalam KV, Shah VA. Self retaining contact lens system for vitreous surgery. Indian J Ophthalmol. 2004;52(1):67–71. [PubMed] [Google Scholar]