Abstract
20-Hydroxy-5, 8, 11, 14-eicosatetraenoic acid (20-HETE) is a cytochrome P450 (CYP)–derived omega-hydroxylation metabolite of arachidonic acid. 20-HETE has been shown to play a complex role in blood pressure regulation. In the kidney tubules, 20-HETE inhibits sodium reabsorption and promotes natriuresis, thus, contributing to antihypertensive mechanisms. In contrast, in the microvasculature, 20-HETE has been shown to play a pressor role by sensitizing smooth muscle cells to constrictor stimuli and increasing myogenic tone, and by acting on the endothelium to further promote endothelial dysfunction and endothelial activation. In addition, 20-HETE induces endothelial angiotensin-converting enzyme, thus, setting forth a potential feed forward prohypertensive mechanism by stimulating the renin–angiotensin–aldosterone system. With the advancement of gene sequencing technology, numerous polymorphisms in the regulatory coding and noncoding regions of 20-HETE–producing enzymes, CYP4A11 and CYP4F2, have been associated with hypertension. This in-depth review article discusses the biosynthesis and function of 20-HETE in the cardiovascular system, the pharmacological agents that affect 20-HETE action, and polymorphisms of CYP enzymes that produce 20-HETE and are associated with systemic hypertension in humans.
Keywords: 20-HETE, hypertension, CYP4F2, CYP4A11, androgen, angiotensin-converting enzyme
20-Hydroxy-5, 8, 11, 14-eicosatetraenoic acid (20-HETE) is the omega (ω)-hydroxylation metabolite of arachidonic acid formed by cytochrome P450 (CYP) monooxygenases of the CYP4 gene family. 20-HETE has been shown to play a complex role in blood pressure regulation, having effects on vascular tone and water and salt balance. The report by Sacerdoti et al1 showing that depletion of CYP450 normalizes blood pressure in the spontaneously hypertensive rat (SHR) was the first to implicate 20-HETE in the pathogenesis of hypertension. Consequently, numerous studies in animal models and humans have provided ample evidence that either a deficiency in tubular 20-HETE biosynthesis or increased vascular 20-HETE contributes to hypertension.2 Moreover, single nucleotide polymorphisms (SNPs) in CYP4A11 and CYP4F2, enzymes that produce 20-HETE, have been linked to a risk of developing hypertension. This in-depth review article discusses the biosynthesis and function of 20-HETE in the cardiovascular system, the pharmacological agents that affect 20-HETE action, and polymorphisms of CYP enzymes that produce 20-HETE and associate with hypertension in humans.
20-HETE BIOSYNTHESIS, METABOLISM, AND RELEASE
20-HETE is a bioactive eicosanoid derived from the ω-hydroxylation of arachidonic acid by enzymes of the CYP4 gene family. The CYP4 gene family comprises 30 proteins that are divided into 3 subfamilies (CYP4A, CYP4B, and CYP4F), all of which catalyze the hydroxylation of the terminal carbon and the (ω-1) carbon of saturated and unsaturated fatty acids. CYP4 proteins share high sequence similarity and common catalytic properties; however, their expression pattern in the tissue and their hormonal regulation differ. Arachidonic acid metabolism by CYP4A and CYP4F occurs in multiple organs: liver, kidney, heart, lung, brain, and the vasculature.3–12 Both the CYP4A and CYP4F subfamilies produce 20-HETE in animals and humans to varying degrees.13,14 The major 20-HETE–producing CYP4 isoforms are CYP4F2 and CYP4A11 in humans15,16; CYP4A1, CYP4A2/3, CYP4A8, CYP4F1, and CYP4F4 in rats17,18; and cyp4a12 in mice.19,20 The expression pattern of CYP4F is species-specific; it is found in the mouse glomerulus, rat kidney, rabbit aortic vascular smooth muscle cells, and human kidney and liver.17,21–24 Furthermore, different species have different isoforms: 4 CYP4F isoforms in the rat (4F1, 4F4, 4F5, and 4F6) and 2 in humans (4F2 and 4F3), which can produce 20-HETE.24 In the mouse, the catalytic activity of the 5 Cyp4f isoforms (4f13, 4f14, 4f15, 4f16, and 4f18) for producing 20-HETE is unknown.21 CYP4F2 is the major 20-HETE–producing enzyme in the human kidney. Studies by Lasker et al15 suggest that up to 70% of the 20-HETE found in human kidney microsomes comes from CYP4F2-catalyzed metabolism of arachidonic acid.
The CYP4A isoforms are also found in multiple species, including humans, rats, mice, and rabbits. Different species have different isoforms of the CYP4A enzyme. In the mouse, the 3 Cyp4a isoforms (Cyp4a10, Cyp4a12, and Cyp4a14) have the ability to convert arachidonic acid to 20-HETE. However, among those proteins, only Cyp4a12 shows a significant 20-HETE synthase activity.19 There are 2 Cyp4a12 genes that express Cyp4a12: Cyp4a12a and Cyp4a12b. Both genes are the product of a tandem 100-kb duplication within the Cyp4abx cluster.25 The expression pattern of Cyp4a10, Cyp4a12, and Cyp4a14 exhibits sex differences. Cyp4a10 is expressed in both males and females, Cyp4a12a is male-specific and androgen-regulated, and Cyp4a14 is highly expressed in females.19,20,26 As indicated above, Cyp4a12a is the predominant 20-HETE synthase.19,27 With respect to the rat counterparts, Cyp4a10, 4a12, and 4a14 are murine homologues of rat CYP4A1, 4A8, and 4A2/4A3.28 In the rat kidney, CYP4A1, CYP4A3, and CYP4A8 are highly expressed in the S1, S2, S3 segments of the proximal tubules; CYP4A2 is expressed in the outer-medullary thick ascending limb and renal vasculature.3,29 Different isoforms have distinct catalytic activities.18,24,30 Rat CYP4A1 and CYP4A8 specifically catalyze ω/ω-1-hydroxylation of arachidonic acid, whereas CYP4A2 and CYP4A3, in addition to ω/ω-1-hydroxylation, catalyze 11,12-epoxidation of arachidonic acid.18,31 Furthermore, CYP4A proteins are believed to be the predominant arachidonic acid ω/ω-hydroxylases in both rats and mice, although CYP4F proteins are also present.32
In humans, the predominant CYP4A isoforms are CYP4A11 and CYP4A22. CYP4A11 is mostly present in the S2 and S3 segments of the proximal tubules.15,33 The localization of 20-HETE synthesis along the nephron parallels the expression of the CYP4 proteins. CYP4A22 mRNA has also been detected in the human kidney; however, work by Gainer et al16 suggests that it is a nonfunctional protein. In the rat vasculature, CYP4A proteins show a distinct distribution along the vascular tree where their expression increases with decreased vascular diameter.29 Likewise, the rate of 20-HETE biosynthesis is inversely proportional to blood vessel diameter.29 20-HETE is not detected in large conduit vessels,29 therefore, it is largely believed that 20-HETE is an eicosanoid of the microcirculation.
The synthesis and release of 20-HETE is regulated by numerous autacoids including angiotensin II (Ang II), endothe-lin-1, serotonin [5-hydroxytryptamine (5-HT)], and other growth factors.10,34 Clinically, patients with renovascular disease have an increase in the expression of the renin–angiotensin system and elevated levels of Ang II, which parallels an increase in plasma 20-HETE.35 Inhibition of 20-HETE synthesis attenuates Ang II–mediated hypertension by 40% in rats.36 Endothelin-1, the most potent vasoconstrictor, also promotes the release of 20-HETE from the rat kidney, whereas inhibition of CYP-dependent 20-HETE production attenuates the renal vascular response to endothelin-1.37 20-HETE has been shown to negatively regulate endothelin-1, and the ability of endothelin-1 to stimulate the secretion of atrial natriuretic peptide from the heart is blocked by an inhibitor of 20-HETE synthesis.38 Cerebral vessels during subarachnoid hemorrhage have increased 20-HETE synthesis through 5-HT–mediated activation of the 5-HT1B receptor. This increase in vascular 20-HETE contributes to the vasoconstrictor response to 5-HT.39 In addition, both parathyroid hormone and epidermal growth factor have been shown to stimulate 20-HETE production in the kidneys of SHRs.5,40 More recently, multiple studies have shown that androgen increases CYP4A expression and 20-HETE production in renal interlobar arteries.32,41,42 20-HETE synthesis in the renal and cerebral arteries is inhibited by nitric oxide (NO), carbon monoxide, and superoxide radicals.12,43–49
Exogenous factors can also affect the production and release of 20-HETE. Lipid-lowering agents such as fibrates induce CYP4A1 and CYP4A3 expression in the liver and kidney.50 Treatment of Dahl salt-sensitive (SS) hypertensive rats with clofibrate increases levels of 20-HETE and prevents the development of SS hypertension.50 Similar effects are observed upon treatment with fenofibrate, another hypolipidemic drug.51 Interestingly, CYP4A expression in the blood vessels that do not express peroxisome proliferator–activated receptor-α (PPARα) is not induced by fibrates.52,53 Dietary consumption also alters the synthesis of 20-HETE. A high-fat diet reduces CYP4A activity and decreases the production of 20-HETE in male rats. Induction of 20-HETE by clofibrate also attenuates high-fat diet–induced hypertension in rats.54 Diets high in sodium increase the expression of multiple CYP4A isoforms, including CYP4A2, CYP4A3, and CYP4A8 in mesenteric arteries from normotensive rat, whereas diets low in sodium upregulate CYP4A3.55 Further clinical studies also suggest that plant lignans can modulate levels of 20-HETE. Patients receiving sesame supplementation in the form of sesamin have lower plasma 20-HETE and urinary 20-HETE levels. Furthermore, sesamin inhibits the activity of CYP4A11 and CYP4F2 in renal and liver microsomes.56 Age and sex have also been shown to affect 20-HETE levels.42 The age-dependent increase in 20-HETE synthesis has been shown in cortical microsomes from SHR, normotensive rats, and Dahl rats.5,57
Metabolism of 20-HETE occurs through many of the similar pathways that metabolize arachidonic acid, including cyclooxygenases (COX), lipoxygenases, and CYP monooxygenases. 20-HETE can also be metabolized by alcohol dehydrogenases and β-oxidation.58 20-Hydroxy-prostaglandins are COX-derived metabolites of 20-HETE that have been shown to add to the 20-HETE–mediated constrictor response of rat aortic rings. Inhibition of COX decreased 20-HETE–mediated constriction in a dose-dependent manner.59 This phenomenon is not seen in all vascular beds. 20-HETE–induced vaso-constriction in rat afferent arterioles is COX-independent.3 20-HETE is also metabolized by alcohol dehydrogenases to 20-carboxy-arachi-donic acid. Studies have shown that 20-carboxy-arachidonic acid is a potent vasodilator in porcine coronary microvessels.58 It also acts as an activator of PPARα/γ in COS-7 cells.60 20-HETE undergoes β-oxidation to 18-, 16-, and 14β-oxidation products.58 In platelets and polymorphonuclear leukocytes, 20-HETE can be metabolized by lipoxygenases and COX to inactive products.61,62 CYP epoxygenases, such as CYP2C23, metabolize 20-HETE to 20-hydroxy-epoxyeico-satrienoic acid (EETs) in rat kidney, which have been shown to act as PPARα activators63 and contribute to the anti-inflammatory and protective properties of lipid-lowering drugs.64 In the liver and kidney, 20-HETE is esterified into phospholipids and stored.34
The aforementioned studies clearly indicate that (1) 20-HETE biosynthesis occurrs in many cell types and tissues; (2) it is formed by CYP4A and 4F enzymes; (3) its synthesis is regulated by hormones, vasoactive autacoids and by diet and age; (4) once formed, it is subjected to metabolism by other eicosanoid-producing enzymes and to esterification, glucoronate conjugation, and ω-oxidation; and (5) its levels are readily detected in biological fluids, including blood and urine.
PHARMACOLOGIC AGENTS AFFECTING 20-HETE BIOSYNTHESIS AND ACTION
Over the years, many pharmacological inhibitors, both nonseective and selective, have been developed to inhibit 20-HETE synthesis.24,65 One-aminobenzotriazole is a CYP inhibitor that requires the catalytic formation of benzyne with selectivity toward the CYP4 family.66 17-Octadecynoic acid is a suicide inhibitor of CYP, which inhibits the formation of both 20-HETE and epoxyeicosatrienoic acids.67 N-methylsulfonyl-12,12-dibromododec-11-enamide and 12-dibromodibenzo-p-dioxin (DBDD) are other structure-based inhibitors of CYP that are effective in inhibiting 20-HETE synthesis.68 N-(3-Chloro-4-morphorlin-4-yl)Phenyl-N′-hydroxyimido formamide (TS011) has been shown to inhibit 20-HETE synthesis in a dual hemorrhage model of subarachnoid hemorrhage in dogs.69 One of the more recently identified inhibitors is N-hydroxy-N′-(4-butyl-2-methylphenyl) formamidine (HET0016), which is the most potent inhibitor of 20-HETE synthesis in vivo and is highly specific for blocking the CYP4-catalyzed ω-hydroxylation.70,71 Recent studies in our laboratory have shown that hydralazine can inhibit 20-HETE synthesis in renal microsomes from normotensive rats without affecting the synthesis of other CYP-derived arachidonic acid metabolites, such as epoxyeicosatrienoic acids (unpublished data).
Falck and others36,72 designed and synthesized, in addition to inhibitors, numerous 20-HETE analogues, which are now commonly used to mimic or inhibit 20-HETE-mediated signaling. 20-Hydroxyeicosa-6(Z), 15(Z)-dienoic acid (20-6,15-HEDE) and 20-hydroxyeicosa-6(Z), 15(Z)-dienoylglycine (20-6,15-HEDGE) are both stable analogues of 20-HETE that can block the vasocon-strictor actions of 20-HETE.36,72 In contrast, 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid (5, 14-20-HEDE) and N-20-hydroxyeicosa-5(Z), 14(Z)-dienoyl]glycine (5,14-20 HEDGE) are 20-HETE mimetics.72–74 These compounds serve as excellent tools to discern the role of 20-HETE in health and disease. Major efforts are currently being undertaken to improve the bioavailability of these compounds and to design additional compounds that will aid in identifying the cellular target(s) of 20-HETE to further explore whether a cellular receptor for 20-HETE exists.
TUBULAR EFFECTS OF 20-HETE
In the kidney nephron, the synthesis of 20-HETE has been localized to the proximal tubule (S1, S2, S3),5,75 medullary thick ascending limb of Henle (TALH),76 and glomerulus.4 20-HETE mediates sodium and water transport through the regulation of various channels and cotransporters dependent on the localization within tubules. In the proximal tubules, 20-HETE inhibits Na+-K+-ATPase activity via protein kinase C (PKC)–dependent phosphorylation of the Na+-K+-ATPase α subunit at serine 23 residue77 and mediates the inhibitory effects of parathyroid hormone,78–81 dopamine,77 endo-thelin,82 and Ang II34,83 on Na+-K+-ATPase activity in the proximal tubule. In the medullary TALH, 20-HETE prevents K+ efflux and Na+ reabsorption by inhibiting the large conductance 70 pS K+ channel and the Na+-K+-2Cl− cotransporter,84,85 and decreasing transepithelial potential and chloride transport.4 Administration of inhibitors of 20-HETE blocks tubuloglomerular feedback.86,87 The inhibitory effects of Ang II,76,88 bradykinin,89 and elevation in intracellular Ca2+,90–92 on Na+ transport in the TALH are also dependent on the formation of 20-HETE.
The kidney plays an important role in the regulation of arterial pressure via the pressure–natriuretic response,93 which is associated with elevations in renal medullary blood flow,94–96 renal interstitial hydrostatic pressure,97–101 and inhibition of Na+ transport in the proximal tubule.102–104 20-HETE has been shown to play an important role in the regulation of the pressure–natriuretic response. An increase in renal perfusion pressure contributes to increased 20-HETE levels in renal cortex. Inhibitors of 20-HETE synthesis maintain Na+-K+-ATPase activity and prevent the internalization of sodium-hydrogen exchanger 3 protein in the proximal tubule and impair the pressure–natriuretic response of rats by 50%.98,105 The benefit of 20-HETE synthesis in the tubules has been suggested and demonstrated in various studies. Inhibition of 20-HETE synthesis in the medullary TALH106 and outer medullary regions107 has been shown to increase blood pressure and sodium reabsorption in Lewis rats. Stimulation of the renal formation of 20-HETE with clofibrate, a PPAR agonist that upregulates CYP4A proteins, lowers blood pressure in Dahl SS,50 deoxycorticosterone acetate-salt108 and high-fat–induced hypertensive rats.53 The importance of reduction in outer medullary renal 20-HETE to SS hypertension was further demonstrated by Stec et al,109 who crossed the Dahl SS rats, which exhibit decreased CYP4A2 protein levels in the outer medulla, with the Lewis strain rats. The resulting F2 progeny demonstrated that the CYP4A2 allele cosegregates with the development of salt-induced hypertension.109 Williams et al110 also showed that the transfer of chromosome 5 containing the CYP4A genes from Brown Norway rats on the Dahl SS genetic background increases the renal expression of CYP4A protein and the production of 20-HETE and that 20-HETE contributes to the antihypertensive and renoprotective effects seen in the SS.5 (Brown Norway) consomic strain. Also, in humans a deficiency in tubular 20-HETE synthesis and/or action measured as decreased urinary excretion in response to salt loading has been linked to SS hypertension.111 Polymorphisms in the CYP4A11 gene that reduce its catalytic activity by more than 50% are associated with a decrease in the urinary excretion of 20-HETE and the development of SS hypertension in humans.112,113
The glomerulus is another site of 20-HETE synthesis and action.4 Transforming growth factor-beta (TGF-β) production is upregulated in the glomeruli in Dahl SS rats114 and is associated with the elevation of glomerular capillary pressure115 and permeability of the glomerulus to albumin (Palb).116 TGF-β is found to directly increase Palb in isolated glomeruli and inhibits the formation of 20-HETE. Administration of 20-HETE prevents the effects of TFG-β on Palb.116 Thus, in the glomeruli 20-HETE is critical in maintaining a glomerular filtration barrier to albumin, and inhibition of 20-HETE synthesis may contribute to TGF-β–mediated glomerular injury in hypertension. In all, the abovementioned studies provide strong evidence that tubular synthesis and actions of 20-HETE are renal protective and antihypertensive.
VASCULAR EFFECTS OF 20-HETE
As indicated above, 20-HETE has been recognized as an eicosanoid of the microcirculation with renal, cerebral, cardiac, and mesenteric arteries having been shown to be rich sources of 20-HETE.24,29,117 Within the vascular wall, 20-HETE biosynthesis is primarily localized to the smooth muscle cells.32 The ability of the vascular endothelium to produce 20-HETE has only been demonstrated in the pulmonary circulation.118 20-HETE’s effects on vascular function are multifaceted and include stimulation of smooth muscle contractility, migration, and proliferation, and activation of endothelial cell dysfunction, angiogenesis, and inflammation (listed below). Such effects could have significant implications with regard to the development of hypertension and its cardiovascular complications.
20-HETE and Smooth Muscle Contractility
20-HETE participates in the regulation of vascular tone by sensitizing vascular smooth muscle cells to constrictor stimuli such as Ang II, phenylephrine, and endothelin,119 and by contributing to the myogenic response.11,24,120–122 Its synthesis and/or release is induced by Ang II,10,123,124 endothelin,124,125 and serotonin39 and is also increased after treatment with nitric oxide synthase (NOS) inhibitors.46 In 1989, Escalante et al126 first documented the vasoconstrictive effects of 20-HETE, which in the rat aorta was determined to be COX-dependent. Interestingly, these findings were different in the microcirculation as studies showed that the constrictor activity of 20-HETE is largely COX-independent.11,127 20-HETE can also activate PKC, mitogen-activated protein kinase (MAPK), and src-type tyrosine kinase. All of the aforementioned kinases can phosphorylate and inhibit the conductance Ca2+-activated K+ channels, leading to depolarization and elevation in cytosolic [Ca]2+. This leads to an increase in opening and Ca2+ entry through the L-type Ca2+ channels.24 20-HETE alone also increases the conductance of L-type Ca2+ channels through activation of PKC. Recent work by Inoue et al128 showed that 20-HETE enhances the conductance of inward nonselective cations and amplifies myogenic tone through synergistic activation of vascular transient receptor potential canonical 6. 20-HETE can also act through Rho kinase to maintain the phosphorylated status of myosin light chain 20 and increase the contractile apparatus to Ca2+.129 Modulation of CYP4A protein levels in small arteries and arterioles affects vascular reactivity and myogenic tone: overexpression of CYP4A leads to increased vascular reactivity and myogenic tone, whereas downregulation attenuates vascular reactivity and myogenic tone.130–132 These effects may contribute to a 20-HETE–mediated increase in blood pressure and the development of hypertension in experimental models.32,41,133–135 Other notable effects of 20-HETE on smooth muscle function are its ability to increase migration and stimulate proliferation; 2 actions that may contribute to remodeling in hypertension.136,137
20-HETE and Endothelial Dysfunction
Although the capacity of the vascular endothelium to produce 20-HETE is questionable in most circulatory districts, its ability to respond to 20-HETE has been extensively studied. The vascular endothelium is important in the regulation of vascular tone, vessel diameter, and blood flow. It is also the first layer of defense against noxious stimuli.138 The integrity of the endothelium is dependent on many factors, including NO, which is generated from L-arginine by endothelial nitric oxide synthase (eNOS).139 Endothelial dysfunction is a term that describes the loss of NO bioavailability due to the diminished production or increased metabolism/degradation of NO and/ or an imbalance in the relative contribution of endothelium-derived relaxing and contracting factors. Several studies have shown that endothelial dysfunction is a feature of hypertension and an early risk factor for cardiovascular disease.140,141 Frisbee et al142 first suggested that 20-HETE plays a role in NO homeostasis. In their study, they showed that 20-HETE attenuated the effect of acetylcholine-induced relaxation in cremasteric arterioles. Studies from our laboratory provided ample evidence to support a causative relationship between the CYP4A–20-HETE pathway and endothelial dysfunction, both in vitro and in vivo. Rats transduced with adenovirus expressing the rat CYP4A2 cDNA demonstrated increased expression of CYP4A2 protein and production of 20-HETE in renal arteries and were hypertensive. Renal interlobar arteries from rats transduced with the CYP4A2 cDNA displayed endothelial dysfunction of renal interlobar arteries, which displayed reduced vasodilator responses to acetylcholine, reduced levels of NO and cyclic guanosine monophosphate, and increased levels of superoxide anion.143 Targeted vascular endothelial overexpression of CYP4A2 in normotensive rats also leads to hypertension and endothelial dysfunction.135,144 Similar results were seen in the androgen-induced hypertension rat model in which the vascular expression of CYP4A8 and production of 20-HETE were upregulated and were accompanied by a decrease in acetylcholine-mediated vasodilation.32,145 In all models, inhibition of 20-HETE synthesis abrogated the respective vascular dysfunction and hypertension. Further analysis revealed that 20-HETE interferes with the NO-dependent component of acetycholine-induced relaxation without affecting the NO-independent component of the relaxing response to acetylcholine. This suggests that 20-HETE interferes with NO synthesis and/or bioavailability.143 The link between 20-HETE levels and endothelial dysfunction is also seen in hypertensive individuals.113 It should be noted that in the pulmonary circulation, 20-HETE is produced by the vascular endothelium and has been shown to increase relaxation by activating eNOS.146,147
A thorough examination of the relationship between 20-HETE and the eNOS-NO pathway was assessed using in vitro models. In cultured endothelial cells, 20-HETE uncouples eNOS by inhibiting the association of HSP90 with eNOS, reducing NO production and bioavailability.148 Additional studies indicated that 20-HETE–mediated eNOS uncoupling and endothelial dysfunction are endothelial growth factor receptor, MAPK-, and IκB kinase (IKK)-dependent.149 The interaction between 20-HETE and eNOS was also studied in endothelial cells of other vascular beds. Ward et al150 further showed that in human umbilical vein endothelial cells, chronic activation of activated protein kinase inhibited 20-HETE–mediated dissociation of eNOS from HSP90. Studies using several animal models32,135,143,151 have implicated 20-HETE as an important determinant of endothelial dysfunction in the microcirculation, adding to the mechanisms underlying the prohypertensive effect of 20-HETE.
20-HETE, Endothelial Activation, and Vascular Inflammation
Vascular wall inflammation contributes to the pathogenesis of various diseases, including atherosclerosis, cardiovascular disease, and hypertension. Recent studies suggest that inflammation-mediated vascular remodeling contributes to increased vascular resistance. Indeed, increases in wall thickness and wall/lumen ratio of resistance arteries have been associated with increases in blood pressure,152,153 implicating inflammation in the pathophysiology of hypertension.154 Proinflammatory changes in endothelial phenotype, termed as endothelial activation, lead to an increase in cellular adhesion molecules, endothelial–leukocyte interaction, and permeability.155–157 The release of cytokines and chemokines, including monocyte chemoattractant protein-1, by activated endothelial cells further contribute to the migration/adhesion of monocytes through the chemokine receptor type 2.158,159 20-HETE has been shown to induce proinflammatory changes in endothelial cells, including stimulation of adhesion molecule expression and cytokine release.160 Downregulation of these inflammatory molecules abrogates inflammation and the associated vascular dysfunction and activation in an Ang II–induced hypertensive model.161,162
Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) is a proinflammatory transcriptional activator.163,164 There is increasing support showing that NF-κB activation and reactive oxygen species (ROS) play a key role in endothelial activation and subsequent inflammation in cardiovascular diseases.157,163,165–170 NF-κB activation induces the transcription of a large number of genes related to vascular inflammation. Inhibition of NF-κB attenuates endothelial activation and ameliorates atherosclerosis, reduces Ang II–induced hypertension, and prevents Ang II–induced end-organ damage.154,171,172 NF-κB is activated by numerous mediators, many of which act through ROS-dependent pathways.157,163 An increase in the level of vascular 20-HETE is associated with increased expression of the NADPH oxidase system and increased vascular superoxide anion production.32,143 Further studies in vitro showed that treatment of endothelial cells with 20-HETE led to increased ROS and NF-κB activity. This resulted in endothelial activation characterized by increased expression of intracellular adhesion molecule and interleukin-8 levels.160 Incubation of human endothelial cells with inhibitors of NF-κB activation or MAPK prevented the 20-HETE–mediated increase in levels of adhesion molecules (ie, intracellular adhesion molecule-1).160 This suggests that 20-HETE plays a role in endothelial activation and inflammation, thus contributing to hypertension. Importantly, in the rat model of androgen-induced and 20-HETE–dependent hypertension, administration of parthenolide (an IKK inhibitor) prevented endothelial dysfunction and lowered blood pressure, suggesting that IKK activation is a key signaling step for 20-HETE–induced endothelial dysfunction.145
20-HETE and Oxidative Stress
20-HETE has been shown to increase oxidative stress by stimulating the production of superoxide and other ROS.173 Renal inter-lobar arteries from androgen-treated rats that overexpress CYP4A and produce increased amounts of 20-HETE have two- to threefold increases in protein expression of NADPH oxidase subunits, p47phox and gp91phox. This is accompanied by an increase in vascular superoxide levels.32 Similar findings were seen in arteries from rats transduced with the CYP4A2 adenovirus.135,143 These results are of clinical significance as hypertensive patients have been shown to exhibit oxidative stress (measured via F2-isoprostane levels), which was closely correlated with an increase in urinary 20-HETE excretion.174 Studies using endothelial cells demonstrated that 20-HETE stimulates superoxide production by mechanisms that include eNOS uncoupling and activation of NADPH oxidase–dependent and –independent pathways.148,175,176
20-HETE AND THE RENIN–ANGIOTENSIN SYSTEM
The renin–angiotensin system (RAS) is a key regulator of blood pressure and body fluid homeostasis. Components of the RAS include renin, angiotensin-converting enzyme (ACE), and angiotensin type 1 receptor (AT1R), which are generally expressed throughout the body in tissues that impact blood pressure modulation. Ang II, the product of sequential degradation of angiotensinogen by renin and ACE, and the final effector of the RAS system, increases blood pressure by: (1) vasoconstriction via AT1R activation, increased sympathetic tone and the release of arginine vasopressin; and (2) modulation of renal sodium and water reabsorption by stimulating renal AT1R, the production and release of aldosterone or the sensation of thirst in the central nervous system. AT1R blockers or ACE inhibitors are vastly used for the treatment of hypertension. In animal models, mice null for angiotensinogen, renin, ACE, and AT1RA (the closest murine homologue to the single human AT1R gene) exhibit a marked reduction in blood pressure, indicating the role of RAS in normal blood pressure homeostasis.177,178 The interactions between 20-HETE and the RAS occur at several levels. On the one hand, Ang II has been shown to stimulate the release of 20-HETE in isolated preglomerular vessels10 and the renal synthesis of 20-HETE.179 The stimulatory effect of Ang II on 20-HETE synthesis in preglomerular arteries was Ang II type 2 (AT2) receptor-dependent. AT2-dependent increase in 20-HETE was also observed in human platelets and neutrophils treated with Ang II.180 20-HETE has been shown to mediate responses to Ang II. Hence, increased production of 20-HETE in the peripheral vasculature contributes to the acute vasoconstrictor response to Ang II,181 whereas acute182 and chronic183 inhibition of 20-HETE synthesis attenuates the renal pressor response to Ang II and the development of Ang II–dependent hypertension, respectively. In cultured aortic vascular smooth muscle cells, 20-HETE mediates Ang II–induced mitogenic effects. This may contribute to the vascular injury, hypertrophy, and hypertension caused by Ang II in rats.136,184,185 To this end, experimental models of hypertension that show increased vascular 20-HETE production, such as the SHR32,151 and androgen-induced hypertension in rats and mice,19,20,32,135 are also RAS-mediated.
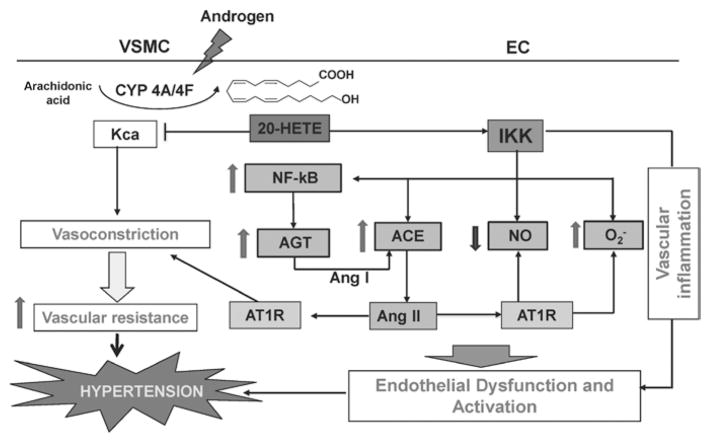
On the other hand, recent studies from our laboratory have shown that 20-HETE activates the RAS via induction of vascular ACE. In endothelial cells, 20-HETE is a potent inducer of ACE expression. Treatment of human microvascular endothelial cells with exogenous 20-HETE induces ACE mRNA, increases ACE protein levels and ACE activity, all of which can be abolished in the presence of 20-HETE.186 The mechanisms by which 20-HETE induces ACE include activation of endothelial growth factor receptor, phosphorylation of MAPK, and activation of the IKK complex with subsequent nuclear translocation of NF-kB.186 Increased expression and synthesis of 20-HETE, as in the CYP4A2-transduced rats144 or the androgen-treated rats (data unpublished), is associated with increased expression of renal and vascular ACE, which is abolished by treatment with 20-HETE synthesis inhibitors. In the model of 20-HETE–dependent hypertension (rats overexpressing the CYP4A2 in the vascular endothelium), blood pressure is normalized by ACE inhibition or AT1R blockade.144 This suggests the presence of a feed-forward amplification of 20-HETE–induced vascular dysfunction by the RAS. Thus, ACE induction by 20-HETE brings about increases in Ang II levels and its actions through the AT1R in vascular smooth muscle and endothelial cells. Such interactions may constitute, at least in part, the mechanism by which 20-HETE causes hypertension (Fig. 1).
FIGURE 1.
Postulated mechanisms underlying the prohypertensive actions of 20-hydroxy-5, 8, 11, 14-eicosatetraenoic acid (20-HETE). ACE indicates angiotensin-converting enzyme; AGT, angiotensinogen; Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; CYP, cytochrome P450; EC, endothelial cell; IKK, IκB kinase; Kca, calcium-activated potassium channel; NF-κB, nuclear factor-kappa B; NO, nitric oxide; O2−, superoxide; VSMC, vascular smooth muscle cell.
ANDROGEN-INDUCED HYPERTENSION AND 20-HETE
The possible role of 20-HETE in androgen-induced hypertension was first introduced by investigators who undertook a genetic approach to substantiate a causal relationship between CYP4, 20-HETE, and blood pressure.20,41 These investigators found that targeted disruption of the Cyp4a14 gene (the mouse homologue of the rat CYP4A2) resulted in spontaneous hypertension that was androgen-dependent.20 The Cyp4a14 null mice displayed increased plasma androgens, elevated expression of Cyp4a12, and increased urinary excretion of 20-HETE. Castration of male Cyp4a14 null mice normalized blood pressure, whereas administration of 5αdihydrotestosterone (DHT) to normotensive animals increased blood pressure.20,32 Additional studies suggested that hypertension in the Cyp4a14 null mice is the consequence of both androgen-driven increase in salt reabsorption at the level of the proximal tubules, a major site of 20-HETE synthesis in the kidney, and a 20-HETE–dependent increase in renal and peripheral vascular resistance.187–189
The correlation between androgen and increased Cyp4a12 expression is also seen in wild-type mice. Muller et al19 compared the level of Cyp4a12a expression in male and female wild-type mice from NMRI, FVB/N, 129 Sv/J, Balb/C, and C57BL/6 backgrounds. In general, levels of Cyp4a12 were higher in males compared with that of females (45-fold for NMRI, 88-fold for FVB/N, 40-fold for 129 Sv/J, 7-fold for Balb/c, and 48-fold for C57BL/6). These results correlated with 20-HETE production in mouse renal microsomes in which males had higher levels than female mice. Along with sex differences, exogenous implantation of a pellet containing 5α-DHT (5 mg/d pellet) led to further increases in Cyp4a12 expression and microsomal 20-HETE production. In addition to this mouse model, exogenous androgen increased 20-HETE production and blood pressure in rats. Both male and female Sprague Dawley rats treated with DHT were found to have elevated blood pressure and increased 20-HETE production in renal microvessels.32,42 This effect was mediated through the androgen receptor.2 RNA analysis from whole kidney and renal interlobar arteries showed that DHT elevated the expression of CYP4A8 (the rat homologue to the mice Cyp4a12).32 Further, inhibition of 20-HETE synthesis with HET0016 attenuated androgen-mediated blood pressure elevation, showing that androgen-dependent hypertension is 20-HETE–mediated.32 The CYP4F2 transgenic mice in which the human CYP4F2 expression is driven by the kidney androgen-regulated protein promoter are also hypertensive and their blood pressure correlates with levels of urinary 20-HETE.190 Administration of exogenous androgen to CYP4F2 transgenic mice aggravates hypertension, which can be attenuated by treatment with HET0016.191
ASSOCIATION BETWEEN CYP4 FAMILY POLYMORPHISMS AND HYPERTENSION IN HUMANS
To date, many different polymorphisms have been identified in the CYP4 family. The contribution of these polymorphisms toward manifestation of hypertension has not yet been clearly defined.192 The CYP4A11 gene is a highly polymorphic gene (Table 1), of which the most important genetic variant is T8590C. The T8590C variant has a thymidine to cytosine substitution at nucleotide 8590 in the CYP4A11, resulting in a phenylalanine to serine substitution at amino acid 434. This missense variant results in a significant reduction in the catalytic activity of CYP4A11 in vitro16; however, its role in blood pressure modulation is yet to be elucidated due to conflicting results from various cohort studies. In the Tennessee cohort,16 the 8590C allele was associated with an increased risk of hypertension in whites; however, it had no effect in African Americans. These findings were also observed in subjects from the Framingham Heart Study.16 In the African American Study of Kidney Disease in which 732 African Americans with hypertensive renal disease were screened, men with 8590CC genotype were associated with higher systolic blood and pulse pressures.193 Other studies of different populations, such as the Swedes194 and the Germans in the MONICA study,195 have also shown that the carriers of the C allele had higher blood pressures and prevalence of hypertension. In a study of 32 hypertensive subjects, the 8590C allele was found to be associated with a higher aldosterone:renin ratio, higher waist:hip ratio, and lower urinary 20-HETE in response to salt loading.112 Williams et al196 also found the 8590C allele to be associated with hypertension. They also showed that CC genotype was associated with SS hypertension. This could be partially related to their observation that the CC genotype was associated with a lesser increase in renal blood flow on salt-loading when compared with TC/TT genotypes. Interestingly, this observation is similar to findings in the Dahl SS rat model of hypertension.50
TABLE 1.
CYP4A11 Gene Polymorphisms
| SNP | Gene Region | Function | Population Studied | Effect of Variant |
|---|---|---|---|---|
| -845 G/A | Regulatory | Promoter activity of variant -845GG was significantly lower than wild-type -845AA200 | Japanese (Sugimoto et al200) | Associated with higher blood pressure |
| rs1126742/T8590C Thymidine to cytosine substitution at nucleotide 8590 resulting in phenyalanine to serine substitution at amino acid 434 |
Intron | Associated with significantly reduced arachidonic acid metabolizing capacity in vitro16 and a reduction in 20-HETE excretion in urine in humans112,113 | White (Gainer et al16) African American (Xu et al17) African American (Gainer et al193) Swedish (Fava et al194) German (Mayer et al195) Mixed population of whites and blacks (Laffer et al112) Mixed (Williams et al196) Japanese (Fu et al197) Australian (Ward et al113) Japanese (Sugimoto et al200) |
Associated with hypertension No association with hypertension Higher systolic BP and pulse pressure in men; no effect seen in women Higher systolic BP in S434S homozygotes Higher systolic and diastolic blood pressures in individuals with CC genotype Higher diastolic blood pressures and diminished 20-HETE excretion in response to salt loading Associated with higher blood pressures. Salt sensitivity of blood pressure among hypertensive individuals Associated with hypertension No difference in systolic or diastolic blood pressures No association with blood pressure |
| rs4660980 | Intron | Unknown | Chinese (Zhang et al199) | Higher systolic and diastolic blood pressure in men. Also, this SNP was in tight LD with rs1127742 (T8590C). Possible that the real association with blood pressure was due to rs1126742 |
20-HETE indicates 20-hydroxy-5, 8, 11, 14-eicosatetraenoic acid; BP, blood pressure; LD, linkage disequilibrium; SNP, single nucleotide polymorphism.
Studies by other investigators have shown contrasting results. Ward et al113 found no difference in systolic or diastolic blood pressure between the TC/CC genotype and the TT genotype in the CYP4A11 gene. On the contrary, the TC and TT genotypes were significantly higher in hypertensive patients than in control subjects in a Japanese population.197 Interethnic studies examining variations of CYP4A11 polymorphism were performed by Lino Cardenas et al.198 The investigators compared the frequency of the T8590C allele between healthy and unrelated subjects from 5 different ethnic groups: French whites, Gabonese African, Senegalese Africans, Peruvians, and Tunisians. They found Tunisians to have the highest allele frequency, followed by Senegalese, Gabonese, and Peruvian, with the lowest being in the French population. Given that 20-HETE has opposing effects in the tubules versus the vasculature, the distribution of CYP4A11 expression within different ethnic groups may play a role in determining blood pressure regulation. In addition to the T8950C polymorphism, SNPs in the intron region of CYP4A11 have been shown to be associated with hypertension. In a study of a Chinese population, Zhang et al199 found the SNP rs4660980 to be positively correlated to both systolic and diastolic blood pressure. Also, there was tight linkage disequilibrium between rs4660980 and rs1126742 (T8590C polymorphism). Functional variants in the promoter region of CYP4A11 have been found to also play a role in blood pressure regulation. Sugimoto et al200 observed that the wild-type -845GG genotype was associated with lower promoter activity when compared with -845AA genotype. Further, the -845G variant was positively correlated to hypertension in a Japanese population comprising 1501 subjects. Interestingly, no association between the 8590C allele and hypertension was observed in the sample population. More studies in different populations are required to further identify the contribution of novel CYP4A11 SNPs such as -845G/A and rs4660980 to hypertension.
Recent studies have also shown that in addition to CYP4A11, polymorphisms in CYP4F2, both in the regulatory region and in the coding region, are associated with hypertension (Table 2). Studies by Liu et al201,202 showed that G421C (c. -48G→C, rs3093100) in the regulatory region of the CYP4F2 gene is correlated with essential hypertension. Further investigation identified 6 additional variants in the regulatory region of CYP4F2: c. -91T→C (rs3093098), c. -77T→C (rs3093099), c. -43C→T (rs3093101), c. -23G→A (rs3093102), c. -13T→C (rs3093103), and c. +34T→G (Trp12Gly, rs3093105) within intron 1 and exon 2. These polymorphisms commonly made up 2 haplotypes, Hap 1 (c. -91T/c.-48G/c.-13T/c.+34T) and Hap II (c.-91C/c.-48C/c.-13C/c.+34G). Hap I was associated with an increased transcriptional activity of CYP4F2 via increased NF-κB binding to the c.-91T→C variant, resulting in elevated urinary 20-HETE levels. This increase in urinary 20-HETE was associated with hypertension in both the case-control and family-based studies. Interestingly, levels of urinary 20-HETE were slightly higher in men than in women.202
TABLE 2.
CYP4F2 Gene Polymorphisms
| SNP | Gene Region | Function | Population Studied | Effect of Variant |
|---|---|---|---|---|
| rs3093100/ G421C (c. -48 G→C) | Regulatory | 421G variant was associated with lower promoter activity201 | Chinese (Liu et al201) | Associated with hypertension |
| c.-91T→C, c.-77T→C, c.-43C→T, c.-23G→A, c.-13T→C and c.+34T→G, which make up 2 haplotypes Hap I (c. -91T/c.-48G/c.-13T/ c.+34T) and Hap II (c.-91C/c.-48C/c.-13C/ c.+34G) | Regulatory | Increased transcriptional activity via increased NF-kappaB binding affinity of c.-91T→C variant in Haplotype I and elevated urinary 20-HETE202 | Chinese (Liu et al202) | Associated with hypertension |
| rs2108622/c.1347G→A Guanine to adenosine missense mutation at nucleotide 1347 resulting in valine to methionine substitution at amino acid 433 |
Exon | Reduced arachidonic acid metabolizing capacity and decreased 20-HETE production in vitro203; however, increased 20-HETE urinary excretion has been noted in multiple human studies113,205 | Australian (Ward et al113) Swedish (Fava et al194) Indians (Munshi et al204) Chinese (Hu et al205) Japanese (Fu et al207) |
Higher blood pressure in GA and AA genotype Higher systolic and diastolic blood pressures among male, but not female M433 carriers Associated with hypertension Greater peripheral and central augmentation indices and faster pulse rate No association with hypertension |
| rs1558139 | Intron | Unknown | Japanese (Fu et al207) | CC genotype of the variant was associated with essential hypertension when compared with CT/TT genotypes in males |
20-HETE indicates 20-hydroxy-5, 8, 11, 14-eicosatetraenoic acid; NF-κB, nuclear factor-kappa B; SNP, single nucleotide polymorphism.
Stec et al203 further identified 2 nonsynonymous SNPs that led to amino acid changes at position 12 (W12G) and 433 (V433M) in African and European Americans (n = 24 and n = 23, respectively). The valine-to-methionine amino acid substitution at 433 was the result of a guanine-to-adenine missense transition at nucleotide 1347 (rs2108622). The M433 amino acid change was associated with a reduced catalytic activity; the recombinant M433 mutated CYP4F2 protein demonstrated a 56–66% decrease in its ability to convert arachidonic acid to 20-HETE.203 However, other studies that are discussed below have shown associations between V433M polymorphism and increased urinary 20-HETE and hypertension.
Ward et al113 performed a study on 235 individuals and found that in the CYP4F2 coding region, 50% were homozygous for the G allele (GG), 40% were heterozygous with a single A transition (GA), and 9% were homozygous for the A allele (AA). Individuals with either GA or AA had significantly higher blood pressure than those with the GG alleles. Further, patients with the GA or AA alleles also had the highest levels of urinary 20-HETE. Similar findings were confirmed by Fava et al194 in a cohort of the Malmö Diet and Cancer Study where it was found that the V433M polymorphism in CYP4F2 is associated with elevated blood pressure and an increase in hypertension prevalence in the male urban population–based sample of middle-aged Swedes. A case-control study in an Indian population by Munshi et al204 also found that the GA or AA genotype was associated with hypertension. Recent studies by Hu et al205 showed that patients with either the M433M or V433M polymorphism had elevated urinary 20-HETE concentrations compared with that of V433V homozygotes. Also, M433 allele carriers had a significantly greater peripheral augmentation index in men versus that in V433V homozygotes, suggesting that 20-HETE may play a role in modulating arterial stiffness, contributing to hypertension. Fava et al206 further illustrated that M433M and V433M resulted in increased susceptibility to hypertension and/or stroke in an androgen-dependent and a sexual dimorphic manner. Although V433M has a decreased catalytic activity, multiple studies have shown that patients with this mutation present with elevated urinary 20-HETE and blood pressure. This phenomenon may be similar to observations seen in the Cyp4a14 knockout mice in which a decreased Cyp4a14 expression and function led to an increase in Cyp4a12 expression and 20-HETE production.20 It is possible to conceive that a decrease in CYP4F2 function in the V433M variant may lead to an increase in expression of an alternative 20-HETE-producing ω-hydroxylase.
Fu et al207 showed that along with SNPs found in the coding region, CC genotype of the SNP rs155139 found in the intron region of CYP4F2 is associated with hypertension when compared with the CT/TT genotypes. Interestingly, no significant difference in prevalence of hypertension was seen in GG or GA/AA genotypes in V433M polymorphisms.207 Given the complex nature of CYP4 family polymorphisms and hypertension, more population-based studies are needed to identify the role of CYP4A11 and CYP4F2, the 2 major 20-HETE–producing enzymes, in the regulation of blood pressure in humans.
Several of the association studies indicated sex-specific effects of genetic variants on 20-HETE levels and/or blood pressure, suggesting that these variants, at least in part, contribute to sexual dimorphism in blood pressure. Sex-specific differences in arterial blood pressure have been well documented in epidemiological, clinical, and experimental studies. Men younger than 60 years have a higher systolic blood pressure than premenopausal women of the same age.208–210 These sex-specific differences in blood pressure are also seen in animal models where males displayed higher blood pressure than females.20,211–217 Genetic polymorphism may be one of the determinants of these sex-specific differences, and androgen, which has been shown to cause hypertension in animal models32,41,42 and is correlated with higher blood pressure in men, postmenopausal women, abusers of anabolic-androgenic steroids, and women with polycystic ovary syndrome, as reviewed by Wu et al.2 Thus, genetic polymorphism in enzymes that produce 20-HETE, coupled with the fact that androgen induces expression and synthesis of 20-HETE together with 20-HETE biological activities, may create an interplay that contributes to male-specific increases in blood pressure.
CONCLUSIONS
The role of 20-HETE in blood pressure regulation is quite complex. On the one hand, it promotes constriction and vascular dysfunction, and on the other hand, it inhibits salt reabsorption in the kidney. Hence, the specific cellular localization of overproduced or downregulated 20-HETE (eg, vascular vs renal tubular structures) significantly impact on the final outcome with regard to hypertension. Other factors that may impact on the contribution of 20-HETE to blood pressure regulation include diet-, age-, and sex-specific alterations in the expression of CYP enzymes that produce 20-HETE. Recent advancements in gene sequencing have identified multiple SNPs in both CYP4A and CYP4F families that are associated with blood pressure regulation. Further studies are needed to: (1) identify mechanisms underlying the antihypertensive/prohypertensive effects of 20-HETE; (2) determine the cellular localization of specific human CYP4A and CYP4F families; and (3) understand the role of various polymorphisms to assist in designing potential therapeutic agents that can amplify the antihypertensive effects of 20-HETE in the tubules or reverse the prohypertensive effects of 20-HETE in the microvasculature for blood pressure regulation.
Acknowledgments
Supported by NIH grants HL34300 (M.L.S.), F30 HL097402 (C.-C.W.), and NHLBI Diversity Supplement to HL034300 (V.G.).
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Sacerdoti D, Escalante B, Abraham NG, et al. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 2.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito O, Alonso-Galicia M, Hopp KA, et al. Localization of cytochrome P-450 4A isoforms along the rat nephron. Am J Physiol. 1998;274(2 pt 2):F395–F404. doi: 10.1152/ajprenal.1998.274.2.F395. [DOI] [PubMed] [Google Scholar]
- 4.Ito O, Roman RJ. Regulation of P-450 4A activity in the glomerulus of the rat. Am J Physiol. 1999;276(6 pt 2):R1749–R1757. doi: 10.1152/ajpregu.1999.276.6.R1749. [DOI] [PubMed] [Google Scholar]
- 5.Omata K, Abraham NG, Schwartzman ML. Renal cytochrome P-450-arachidonic acid metabolism: localization and hormonal regulation in SHR. Am J Physiol. 1992;262(4 pt 2):F591–F599. doi: 10.1152/ajprenal.1992.262.4.F591. [DOI] [PubMed] [Google Scholar]
- 6.Carroll MA, Sala A, Dunn CE, et al. Structural identification of cytochrome P450-dependent arachidonate metabolites formed by rabbit medullary thick ascending limb cells. J Biol Chem. 1991;266:12306–12312. [PubMed] [Google Scholar]
- 7.Escalante B, Erlij D, Falck JR, et al. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802. doi: 10.1126/science.1846705. [DOI] [PubMed] [Google Scholar]
- 8.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33(1 pt 2):419–423. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 9.Carroll MA, Kemp R, Cheng MK, et al. Regulation of preglomerular micro-vascular 20-hydroxyeicosatetraenoic acid levels by salt depletion. Med Sci Monit. 2001;7:567–572. [PubMed] [Google Scholar]
- 10.Croft KD, McGiff JC, Sanchez-Mendoza A, et al. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 11.Imig JD, Zou AP, Stec DE, et al. Formation and actions of 20-hydroxye-icosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270(1 pt 2):R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 12.Sun CW, Alonso-Galicia M, Taheri MR, et al. Nitric oxide-20-hydroxyeicosa-tetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ Res. 1998;83:1069–1079. doi: 10.1161/01.res.83.11.1069. [DOI] [PubMed] [Google Scholar]
- 13.Kalsotra A, Strobel HW. Cytochrome P450 4F subfamily: at the crossroads of eicosanoid and drug metabolism. Pharmacol Ther. 2006;112:589–611. doi: 10.1016/j.pharmthera.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Lasker JM, Chen WB, Wolf I, et al. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J Biol Chem. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 16.Gainer JV, Bellamine A, Dawson EP, et al. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 17.Xu F, Falck JR, Ortiz de Montellano PR, et al. Catalytic activity and isoform-specific inhibition of rat cytochrome p450 4F enzymes. J Pharmacol Exp Ther. 2004;308:887–895. doi: 10.1124/jpet.103.059626. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen X, Wang MH, Reddy KM, et al. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol. 1999;276(6 pt 2):R1691–R1700. doi: 10.1152/ajpregu.1999.276.6.R1691. [DOI] [PubMed] [Google Scholar]
- 19.Muller DN, Schmidt C, Barbosa-Sicard E, et al. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holla VR, Adas F, Imig JD, et al. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci U S A. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stec DE, Flasch A, Roman RJ, et al. Distribution of cytochrome P-450 4A and 4F isoforms along the nephron in mice. Am J Physiol Renal Physiol. 2003;284:F95–102. doi: 10.1152/ajprenal.00132.2002. [DOI] [PubMed] [Google Scholar]
- 22.Parmentier JH, Lavrentyev EN, Falck JR, et al. Evaluation of cytochrome P450 4 family as mediator of phospholipase D activation in aortic vascular smooth muscle cells. Life Sci. 2005;77:1015–1029. doi: 10.1016/j.lfs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Ito O, Nakamura Y, Tan L, et al. Expression of cytochrome P-450 4 enzymes in the kidney and liver: regulation by PPAR and species-difference between rat and human. Mol Cell Biochem. 2006;284:141–148. doi: 10.1007/s11010-005-9038-x. [DOI] [PubMed] [Google Scholar]
- 24.Williams JM, Murphy S, Burke M, et al. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson DR, Zeldin DC, Hoffman SM, et al. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Heng YM, Kuo CS, Jones PS, et al. A novel murine P-450 gene, Cyp4a14, is part of a cluster of Cyp4a and Cyp4b, but not of CYP4F, genes in mouse and humans. Biochem J. 1997;325(pt 3):741–749. doi: 10.1042/bj3250741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakagawa K, Holla VR, Wei Y, et al. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. J Clin Invest. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helvig C, Dishman E, Capdevila JH. Molecular, enzymatic, and regulatory characterization of rat kidney cytochromes P450 4A2 and 4A3. Biochemistry. 1998;37:12546–12558. doi: 10.1021/bi981048g. [DOI] [PubMed] [Google Scholar]
- 29.Marji JS, Wang MH, Laniado-Schwartzman M. Cytochrome P-450 4A isoform expression and 20-HETE synthesis in renal preglomerular arteries. Am J Physiol Renal Physiol. 2002;283:F60–F67. doi: 10.1152/ajprenal.00265.2001. [DOI] [PubMed] [Google Scholar]
- 30.Muerhoff AS, Williams DE, Leithauser MT, et al. Regulation of the induction of a cytochrome P-450 prostaglandin omega-hydroxylase by pregnancy in rabbit lung. Proc Natl Acad Sci U S A. 1987;84:7911–7914. doi: 10.1073/pnas.84.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capdevila JH, Karara A, Waxman DJ, et al. Cytochrome P-450 enzyme-specific control of the regio- and enantiofacial selectivity of the microsomal arachidonic acid epoxygenase. J Biol Chem. 1990;265:10865–10871. [PubMed] [Google Scholar]
- 32.Singh H, Cheng J, Deng H, et al. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 2007;50:123–129. doi: 10.1161/HYPERTENSIONAHA.107.089599. [DOI] [PubMed] [Google Scholar]
- 33.Bellamine A, Wang Y, Waterman MR, et al. Characterization of the CYP4A11 gene, a second CYP4A gene in humans. Arch Biochem Biophys. 2003;409:221–227. doi: 10.1016/s0003-9861(02)00545-3. [DOI] [PubMed] [Google Scholar]
- 34.Carroll MA, Balazy M, Huang DD, et al. Cytochrome P450-derived renal HETEs: storage and release. Kidney Int. 1997;51:1696–1702. doi: 10.1038/ki.1997.234. [DOI] [PubMed] [Google Scholar]
- 35.Minuz P, Jiang H, Fava C, et al. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension. 2008;51:1379–1385. doi: 10.1161/HYPERTENSIONAHA.107.105395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso-Galicia M, Falck JR, Reddy KM, et al. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277(5 pt 2):F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 37.Hercule HC, Oyekan AO. Cytochrome P450 omega/omega-1 hydroxylase-derived eicosanoids contribute to endothelin(A) and endothelin(B) receptor-mediated vasoconstriction to endothelin-1 in the rat preglomerular arteriole. J Pharmacol Exp Ther. 2000;292:1153–1160. [PubMed] [Google Scholar]
- 38.Lee SJ, Landon CS, Nazian SJ, et al. Cytochrome P-450 metabolites in endothelin-stimulated cardiac hormone secretion. Am J Physiol Regul Integr Comp Physiol. 2004;286:R888–R893. doi: 10.1152/ajpregu.00482.2003. [DOI] [PubMed] [Google Scholar]
- 39.Cambj-Sapunar L, Yu M, Harder DR, et al. Contribution of 5-hydroxytryp-tamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 40.Lin F, Rios A, Falck JR, et al. 20-Hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubule. Am J Physiol. 1995;269(6 pt 2):F806–F816. doi: 10.1152/ajprenal.1995.269.6.F806. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa K, Marji JS, Schwartzman ML, et al. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1055–R1062. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 42.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep. 2008;60:29–37. [PubMed] [Google Scholar]
- 43.Alonso-Galicia M, Drummond HA, Reddy KK, et al. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29(1 pt 2):320–325. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 44.Alonso-Galicia M, Hudetz AG, Shen H, et al. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke. 1999;30:2727–2734. doi: 10.1161/01.str.30.12.2727. discussion 2734. [DOI] [PubMed] [Google Scholar]
- 45.López B, Moreno C, Salom MG, et al. Role of guanylyl cyclase and cytochrome P-450 on renal response to nitric oxide. Am J Physiol Renal Physiol. 2001;281:F420–F427. doi: 10.1152/ajprenal.2001.281.3.F420. [DOI] [PubMed] [Google Scholar]
- 46.Oyekan AO, McGiff JC. Functional response of the rat kidney to inhibition of nitric oxide synthesis: role of cytochrome p450-derived arachidonate metabolites. Br J Pharmacol. 1998;125:1065–1073. doi: 10.1038/sj.bjp.0702171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Botros FT, Laniado-Schwartzman M, Abraham NG. Regulation of cyclooxy-genase- and cytochrome p450-derived eicosanoids by heme oxygenase in the rat kidney. Hypertension. 2002;39(2 pt 2):639–644. doi: 10.1161/hy0202.103420. [DOI] [PubMed] [Google Scholar]
- 48.Coceani F, Kelsey L, Seidlitz E, et al. Inhibition of the contraction of the ductus arteriosus to oxygen by 1-aminobenzotriazole, a mechanism-based inactivator of cytochrome P450. Br J Pharmacol. 1996;117:1586–1592. doi: 10.1111/j.1476-5381.1996.tb15325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoagland KM, Maier KG, Roman RJ. Contributions of 20-HETE to the antihypertensive effects of Tempol in Dahl salt-sensitive rats. Hypertension. 2003;41(3 pt 2):697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- 50.Roman RJ, Ma YH, Frohlich B, et al. Clofibrate prevents the development of hypertension in Dahl salt-sensitive rats. Hypertension. 1993;21(6 pt 2):985–988. doi: 10.1161/01.hyp.21.6.985. [DOI] [PubMed] [Google Scholar]
- 51.Wilson TW, Alonso-Galicia M, Roman RJ. Effects of lipid-lowering agents in the Dahl salt-sensitive rat. Hypertension. 1998;31(1 pt 2):225–231. doi: 10.1161/01.hyp.31.1.225. [DOI] [PubMed] [Google Scholar]
- 52.Vera T, Taylor M, Bohman Q, et al. Fenofibrate prevents the development of angiotensin II-dependent hypertension in mice. Hypertension. 2005;45:730–735. doi: 10.1161/01.HYP.0000153317.06072.2e. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Y, Huang H, Chang HH, et al. Induction of renal 20-hydroxyeicosatet-raenoic acid by clofibrate attenuates high-fat diet-induced hypertension in rats. J Pharmacol Exp Ther. 2006;317:11–18. doi: 10.1124/jpet.105.095356. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Y, Lin S, Chang HH, et al. Gender differences of renal CYP-derived eicosanoid synthesis in rats fed a high-fat diet. Am J Hypertens. 2005;18(4 pt 1):530–537. doi: 10.1016/j.amjhyper.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Roman RJ, Falck JR, et al. Effects of high-salt diet on CYP450-4A omega-hydroxylase expression and active tone in mesenteric resistance arteries. Am J Physiol Heart Circ Physiol. 2005;288:H1557–H1565. doi: 10.1152/ajpheart.00755.2004. [DOI] [PubMed] [Google Scholar]
- 56.Wu JH, Hodgson JM, Clarke MW, et al. Inhibition of 20-hydroxyeicosatetrae-noic acid synthesis using specific plant lignans: in vitro and human studies. Hypertension. 2009;54:1151–1158. doi: 10.1161/HYPERTENSIONAHA.109.139352. [DOI] [PubMed] [Google Scholar]
- 57.Omata K, Tsutsumi E, Sheu HL, et al. Effect of aging on renal cytochrome P450-dependent arachidonic acid metabolism in Dahl rats. J Lipid Mediat. 1993;6:369–373. [PubMed] [Google Scholar]
- 58.Kaduce TL, Fang X, Harmon SD, et al. 20-hydroxyeicosatetraenoic acid (20-HETE) metabolism in coronary endothelial cells. J Biol Chem. 2004;279:2648–2656. doi: 10.1074/jbc.M306849200. [DOI] [PubMed] [Google Scholar]
- 59.Schwartzman ML, Falck JR, Yadagiri P, et al. Metabolism of 20-hydroxye-icosatetraenoic acid by cyclooxygenase. Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J Biol Chem. 1989;264:11658–11662. [PubMed] [Google Scholar]
- 60.Fang X, Dillon JS, Hu S, et al. 20-carboxy-arachidonic acid is a dual activator of peroxisome proliferator-activated receptors alpha and gamma. Prostaglandins Other Lipid Mediat. 2007;82:175–184. doi: 10.1016/j.prostaglandins.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Hill E, Fitzpatrick F, Murphy RC. Biological activity and metabolism of 20-hydroxyeicosatetraenoic acid in the human platelet. Br J Pharmacol. 1992;106:267–274. doi: 10.1111/j.1476-5381.1992.tb14327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosolowsky M, Falck JR, Campbell WB. Metabolism of arachidonic acid by canine polymorphonuclear leukocytes synthesis of lipoxygenase and omega-oxidized metabolites. Biochim Biophys Acta. 1996;1300:143–150. doi: 10.1016/0005-2760(95)00238-3. [DOI] [PubMed] [Google Scholar]
- 63.Cowart LA, Wei S, Hsu MH, et al. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activated receptor ligands. J Biol Chem. 2002;277:35105–35112. doi: 10.1074/jbc.M201575200. [DOI] [PubMed] [Google Scholar]
- 64.Muller DN, Theuer J, Shagdarsuren E, et al. A peroxisome proliferator-activated receptor-alpha activator induces renal CYP2C23 activity and protects from angiotensin II-induced renal injury. Am J Pathol. 2004;164:521–532. doi: 10.1016/s0002-9440(10)63142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kroetz DL, Xu F. Regulation and inhibition of arachidonic acid omega-hydroxylases and 20-HETE formation. Annu Rev Pharmacol Toxicol. 2005;45:413–438. doi: 10.1146/annurev.pharmtox.45.120403.100045. [DOI] [PubMed] [Google Scholar]
- 66.Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol. 1998;275(2 pt 2):R426–R438. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- 67.Muerhoff AS, Williams DE, Reich NO, et al. Prostaglandin and fatty acid omega- and (omega-1)-oxidation in rabbit lung. Acetylenic fatty acid mechanism-based inactivators as specific inhibitors. J Biol Chem. 1989;264:749–756. [PubMed] [Google Scholar]
- 68.Wang MH, Brand-Schieber E, Zand BA, et al. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney: characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 69.Hacein-Bey L, Harder DR, Meier HT, et al. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2006;27:1350–1354. [PMC free article] [PubMed] [Google Scholar]
- 70.Seki T, Wang MH, Miyata N, et al. Cytochrome P450 4A isoform inhibitory profile of N-hydroxy-N′-(4-butyl-2-methylphenyl)-formamidine (HET0016), a selective inhibitor of 20-HETE synthesis. Biol Pharm Bull. 2005;28:1651–1654. doi: 10.1248/bpb.28.1651. [DOI] [PubMed] [Google Scholar]
- 71.Miyata N, Taniguchi K, Seki T, et al. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu M, Cambj-Sapunar L, Kehl F, et al. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Tunctan B, Korkmaz B, Buharalioglu CK, et al. A 20-hydroxyeicosatetraenoic acid agonist, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock. 2008;30:329–335. doi: 10.1097/SHK.0b013e31816471c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regner KR, Zuk A, Van Why SK, et al. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009;75:511–517. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laniado-Schwartzman M, Abraham NG. The renal cytochrome P-450 arachidonic acid system. Pediatr Nephrol. 1992;6:490–498. doi: 10.1007/BF00874022. [DOI] [PubMed] [Google Scholar]
- 76.Lu M, Zhu Y, Balazy M, et al. Effect of angiotensin II on the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol. 1996;108:537–547. doi: 10.1085/jgp.108.6.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowicki S, Chen SL, Aizman O, et al. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+, K+-ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Satoh T, Cohen HT, Katz AI. Intracellular signaling in the regulation of renal Na-K-ATPase. I. Role of cyclic AMP and phospholipase A2. J Clin Invest. 1992;89:1496–1500. doi: 10.1172/JCI115740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ominato M, Satoh T, Katz AI. Regulation of Na-K-ATPase activity in the proximal tubule: role of the protein kinase C pathway and of eicosanoids. J Membr Biol. 1996;152:235–243. doi: 10.1007/s002329900101. [DOI] [PubMed] [Google Scholar]
- 80.Ribeiro CM, Dubay GR, Falck JR, et al. Parathyroid hormone inhibits Na(+)-K(+)-ATPase through a cytochrome P-450 pathway. Am J Physiol. 1994;266(3 pt 2):F497–F505. doi: 10.1152/ajprenal.1994.266.3.F497. [DOI] [PubMed] [Google Scholar]
- 81.Silverstein DM, Barac-Nieto M, Falck JR, et al. 20-HETE mediates the effect of parathyroid hormone and protein kinase C on renal phosphate transport. Prostaglandins Leukot Essent Fatty Acids. 1998;58:209–213. doi: 10.1016/s0952-3278(98)90116-8. [DOI] [PubMed] [Google Scholar]
- 82.Escalante BA, McGiff JC, Oyekan AO. Role of cytochrome P-450 arachidonate metabolites in endothelin signaling in rat proximal tubule. Am J Physiol Renal Physiol. 2002;282:F144–F150. doi: 10.1152/ajprenal.0064.2001. [DOI] [PubMed] [Google Scholar]
- 83.Sánchez-Mendoza A, López-Sánchez P, Vázquez-Cruz B, et al. Angiotensin II modulates ion transport in rat proximal tubules through CYP metabolites. Biochem Biophys Res Commun. 2000;272:423–430. doi: 10.1006/bbrc.2000.2807. [DOI] [PubMed] [Google Scholar]
- 84.Escalante B, Erlij D, Falck JR, et al. Cytochrome P-450 arachidonate metabolites affect ion fluxes in rabbit medullary thick ascending limb. Am J Physiol. 1994;266(6 pt 1):C1775–C1782. doi: 10.1152/ajpcell.1994.266.6.C1775. [DOI] [PubMed] [Google Scholar]
- 85.Wang W, Lu M. Effect of arachidonic acid on activity of the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol. 1995;106:727–743. doi: 10.1085/jgp.106.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zou AP, Imig JD, Ortiz de Montellano PR, et al. Effect of P-450 omega-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol. 1994;266(6 pt 2):F934–F941. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 87.Zhao X, Inscho EW, Bondlela M, et al. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H2089–H2096. doi: 10.1152/ajpheart.2001.281.5.H2089. [DOI] [PubMed] [Google Scholar]
- 88.Amlal H, LeGoff C, Vernimmen C, et al. ANG II controls Na(+)-K+(NH4+)-2Cl- cotransport via 20-HETE and PKC in medullary thick ascending limb. Am J Physiol. 1998;274(4 pt 1):C1047–C1056. doi: 10.1152/ajpcell.1998.274.4.C1047. [DOI] [PubMed] [Google Scholar]
- 89.Grider JS, Falcone JC, Kilpatrick EL, et al. P450 arachidonate metabolites mediate bradykinin-dependent inhibition of NaCl transport in the rat thick ascending limb. Can J Physiol Pharmacol. 1997;75:91–96. [PubMed] [Google Scholar]
- 90.Amlal H, Legoff C, Vernimmen C, et al. Na(+)-K+(NH4+)-2Cl- cotransport in medullary thick ascending limb: control by PKA, PKC, and 20-HETE. Am J Physiol. 1996;271(2 pt 1):C455–C463. doi: 10.1152/ajpcell.1996.271.2.C455. [DOI] [PubMed] [Google Scholar]
- 91.Wang W, Lu M, Balazy M, et al. Phospholipase A2 is involved in mediating the effect of extracellular Ca2+ on apical K+ channels in rat TAL. Am J Physiol. 1997;273(3 pt 2):F421–F429. doi: 10.1152/ajprenal.1997.273.3.F421. [DOI] [PubMed] [Google Scholar]
- 92.Wang WH, Lu M, Hebert SC. Cytochrome P-450 metabolites mediate extra-cellular Ca(2+)-induced inhibition of apical K+ channels in the TAL. Am J Physiol. 1996;271(1 pt 1):C103–C111. doi: 10.1152/ajpcell.1996.271.1.C103. [DOI] [PubMed] [Google Scholar]
- 93.Cowley AW, Jr, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581–1589. [PubMed] [Google Scholar]
- 94.Roman RJ, Kaldunski M. Pressure natriuresis and cortical and papillary blood flow in inbred Dahl rats. Am J Physiol. 1991;261(3 pt 2):R595–R602. doi: 10.1152/ajpregu.1991.261.3.R595. [DOI] [PubMed] [Google Scholar]
- 95.Roman RJ, Cowley AW, Jr, Garcia-Estañ J, et al. Pressure-diuresis in volume-expanded rats. Cortical and medullary hemodynamics. Hypertension. 1988;12:168–176. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- 96.Mattson DL, Lu S, Roman RJ, et al. Relationship between renal perfusion pressure and blood flow in different regions of the kidney. Am J Physiol. 1993;264(3 pt 2):R578–R583. doi: 10.1152/ajpregu.1993.264.3.R578. [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Estañ J, Roman RJ. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am J Physiol. 1989;256(1 pt 2):F63–F70. doi: 10.1152/ajprenal.1989.256.1.F63. [DOI] [PubMed] [Google Scholar]
- 98.Williams JM, Sarkis A, Lopez B, et al. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 99.Granger JP. Pressure natriuresis. Role of renal interstitial hydrostatic pressure. Hypertension. 1992;19(1 suppl):I9–I17. doi: 10.1161/01.hyp.19.1_suppl.i9. [DOI] [PubMed] [Google Scholar]
- 100.Khraibi AA, Knox FG. Effect of renal decapsulation on renal interstitial hydrostatic pressure and natriuresis. Am J Physiol. 1989;257(1 pt 2):R44–R48. doi: 10.1152/ajpregu.1989.257.1.R44. [DOI] [PubMed] [Google Scholar]
- 101.Khraibi AA, Haas JA, Knox FG. Effect of renal perfusion pressure on renal interstitial hydrostatic pressure in rats. Am J Physiol. 1989;256(1 pt 2):F165–F170. doi: 10.1152/ajprenal.1989.256.1.F165. [DOI] [PubMed] [Google Scholar]
- 102.Haas JA, Granger JP, Knox FG. Effect of renal perfusion pressure on sodium reabsorption from proximal tubules of superficial and deep nephrons. Am J Physiol. 1986;250(3 pt 2):F425–F429. doi: 10.1152/ajprenal.1986.250.3.F425. [DOI] [PubMed] [Google Scholar]
- 103.Kinoshita Y, Knox FG. Role of prostaglandins in proximal tubule sodium reabsorption: response to elevated renal interstitial hydrostatic pressure. Circ Res. 1989;64:1013–1018. doi: 10.1161/01.res.64.5.1013. [DOI] [PubMed] [Google Scholar]
- 104.Roman RJ. Pressure-diuresis in volume-expanded rats. Tubular reabsorption in superficial and deep nephrons. Hypertension. 1988;12:177–183. doi: 10.1161/01.hyp.12.2.177. [DOI] [PubMed] [Google Scholar]
- 105.Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, et al. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R58–R68. doi: 10.1152/ajpregu.00713.2003. [DOI] [PubMed] [Google Scholar]
- 106.Zou AP, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension. 1996;27(3 pt 2):631–635. doi: 10.1161/01.hyp.27.3.631. [DOI] [PubMed] [Google Scholar]
- 107.Stec DE, Mattson DL, Roman RJ. Inhibition of renal outer medullary 20-HETE production produces hypertension in Lewis rats. Hypertension. 1997;29(1 pt 2):315–319. doi: 10.1161/01.hyp.29.1.315. [DOI] [PubMed] [Google Scholar]
- 108.Zhou Y, Luo P, Chang HH, et al. Colfibrate attenuates blood pressure and sodium retention in DOCA-salt hypertension. Kidney Int. 2008;74:1040–1048. doi: 10.1038/ki.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stec DE, Deng AY, Rapp JP, et al. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension. 1996;27(3 pt 2):564–568. doi: 10.1161/01.hyp.27.3.564. [DOI] [PubMed] [Google Scholar]
- 110.Williams JM, Fan F, Murphy S, et al. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1209–R1218. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Laffer CL, Laniado-Schwartzman M, Wang MH, et al. Differential regulation of natriuresis by 20-hydroxyeicosatetraenoic Acid in human salt-sensitive versus salt-resistant hypertension. Circulation. 2003;107:574–578. doi: 10.1161/01.cir.0000046269.52392.14. [DOI] [PubMed] [Google Scholar]
- 112.Laffer CL, Gainer JV, Waterman MR, et al. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ward NC, Tsai IJ, Barden A, et al. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;51:1393–1398. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 114.Dahly AJ, Hoagland KM, Flasch AK, et al. Antihypertensive effects of chronic anti-TGF-beta antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R757–R767. doi: 10.1152/ajpregu.00098.2002. [DOI] [PubMed] [Google Scholar]
- 115.Williams JM, Sarkis A, Hoagland KM, et al. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol. 2008;295:F1764–F1777. doi: 10.1152/ajprenal.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dahly-Vernon AJ, Sharma M, McCarthy ET, et al. Transforming growth factor-beta, 20-HETE interaction, and glomerular injury in Dahl salt-sensitive rats. Hypertension. 2005;45:643–648. doi: 10.1161/01.HYP.0000153791.89776.43. [DOI] [PubMed] [Google Scholar]
- 117.Harder DR, Gebremedhin D, Narayanan J, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266(5 pt 2):H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 118.Zhu D, Zhang C, Medhora M, et al. CYP4A mRNA, protein, and product in rat lungs: novel localization in vascular endothelium. J Appl Physiol. 2002;93:330–337. doi: 10.1152/japplphysiol.01159.2001. [DOI] [PubMed] [Google Scholar]
- 119.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 120.Amaral SL, Maier KG, Schippers DN, et al. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2003;284:H1528–H1535. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- 121.Jiang M, Mezentsev A, Kemp R, et al. Smooth muscle–specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circ Res. 2004;94:167–174. doi: 10.1161/01.RES.0000111523.12842.FC. [DOI] [PubMed] [Google Scholar]
- 122.Muthalif MM, Benter IF, Karzoun N, et al. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1998;95:12701–12706. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carroll MA, Balazy M, Margiotta P, et al. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am J Physiol. 1996;271(4 pt 2):R863–R869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- 124.Oyekan A, Balazy M, McGiff JC. Renal oxygenases: differential contribution to vasoconstriction induced by ET-1 and ANG II. Am J Physiol. 1997;273(1 pt 2):R293–R300. doi: 10.1152/ajpregu.1997.273.1.R293. [DOI] [PubMed] [Google Scholar]
- 125.Oyekan AO, McGiff JC. Cytochrome P-450-derived eicosanoids participate in the renal functional effects of ET-1 in the anesthetized rat. Am J Physiol. 1998;274(1 pt 2):R52–R61. doi: 10.1152/ajpregu.1998.274.1.R52. [DOI] [PubMed] [Google Scholar]
- 126.Escalante B, Sessa WC, Falck JR, et al. Vasoactivity of 20-hydroxyeicosatet-raenoic acid is dependent on metabolism by cyclooxygenase. J Pharmacol Exp Ther. 1989;248:229–232. [PubMed] [Google Scholar]
- 127.Ma YH, Gebremedhin D, Schwartzman ML, et al. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 128.Inoue R, Jensen LJ, Jian Z, et al. Synergistic activation of vascular TRPC6 channel by receptor and mechanical stimulation via phospholipase C/diacyl-glycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res. 2009;104:1399–1409. doi: 10.1161/CIRCRESAHA.108.193227. [DOI] [PubMed] [Google Scholar]
- 129.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41(3 pt 2):801–806. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 130.Kaide J, Wang MH, Wang JS, et al. Transfection of CYP4A1 cDNA increases vascular reactivity in renal interlobar arteries. Am J Physiol Renal Physiol. 2003;284:F51–F56. doi: 10.1152/ajprenal.00249.2002. [DOI] [PubMed] [Google Scholar]
- 131.Wang MH, Zhang F, Marji J, et al. CYP4A1 antisense oligonucleotide reduces mesenteric vascular reactivity and blood pressure in SHR. Am J Physiol Regul Integr Comp Physiol. 2001;280:R255–R261. doi: 10.1152/ajpregu.2001.280.1.R255. [DOI] [PubMed] [Google Scholar]
- 132.Zhang F, Wang MH, Wang JS, et al. Transfection of CYP4A1 cDNA decreases diameter and increases responsiveness of gracilis muscle arterioles to constrictor stimuli. Am J Physiol Heart Circ Physiol. 2004;287:H1089–H1095. doi: 10.1152/ajpheart.00627.2003. [DOI] [PubMed] [Google Scholar]
- 133.Zhang F, Wang MH, Krishna UM, et al. Modulation by 20-HETE of phenyl-ephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;38:1311–1315. doi: 10.1161/hy1201.096116. [DOI] [PubMed] [Google Scholar]
- 134.Imig JD, Falck JR, Gebremedhin D, et al. Elevated renovascular tone in young spontaneously hypertensive rats. Role of cytochrome P-450. Hypertension. 1993;22:357–364. doi: 10.1161/01.hyp.22.3.357. [DOI] [PubMed] [Google Scholar]
- 135.Inoue K, Sodhi K, Puri N, et al. Endothelial-specific CYP4A2 overexpression leads to renal injury and hypertension via increased production of 20-HETE. Am J Physiol Renal Physiol. 2009;297:F875–F884. doi: 10.1152/ajprenal.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yaghini FA, Zhang C, Parmentier JH, et al. Contribution of arachidonic acid metabolites derived via cytochrome P4504A to angiotensin II-induced neo-intimal growth. Hypertension. 2005;45:1182–1187. doi: 10.1161/01.HYP.0000168051.04275.ea. [DOI] [PubMed] [Google Scholar]
- 137.Stec DE, Gannon KP, Beaird JS, et al. 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cells. Cell Physiol Biochem. 2007;19:121–128. doi: 10.1159/000099200. [DOI] [PubMed] [Google Scholar]
- 138.Schulz E, Jansen T, Wenzel P, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 139.Ignarro LJ. Biosynthesis and metabolism of endothelium-derived nitric oxide. Annu Rev Pharmacol Toxicol. 1990;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- 140.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 142.Frisbee JC, Falck JR, Lombard JH. Contribution of cytochrome P-450 omega-hydroxylase to altered arteriolar reactivity with high-salt diet and hypertension. Am J Physiol Heart Circ Physiol. 2000;278:H1517–H1526. doi: 10.1152/ajpheart.2000.278.5.H1517. [DOI] [PubMed] [Google Scholar]
- 143.Wang JS, Singh H, Zhang F, et al. Endothelial dysfunction and hypertension in rats transduced with CYP4A2 adenovirus. Circ Res. 2006;98:962–969. doi: 10.1161/01.RES.0000217283.98806.a6. [DOI] [PubMed] [Google Scholar]
- 144.Sodhi K, Wu CC, Cheng J, et al. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wu CC, Cheng J, Zhang FF, et al. Androgen-dependent hypertension is mediated by 20-hydroxy-5,8,11,14-eicosatetraenoic acid-induced vascular dysfunction: role of inhibitor of kappaB Kinase. Hypertension. 2011;57:788–794. doi: 10.1161/HYPERTENSIONAHA.110.161570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu M, McAndrew RP, Al-Saghir R, et al. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J Appl Physiol. 2002;93:1391–1399. doi: 10.1152/japplphysiol.00247.2002. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y, Medhora M, Falck JR, et al. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L378–L385. doi: 10.1152/ajplung.00424.2005. [DOI] [PubMed] [Google Scholar]
- 148.Cheng J, Ou JS, Singh H, et al. 20-hydroxyeicosatetraenoic acid causes endo-thelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–H1026. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 149.Cheng J, Wu CC, Gotlinger KH, et al. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J Pharmacol Exp Ther. 2010;332:57–65. doi: 10.1124/jpet.109.159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ward NC, Chen K, Li C, et al. Chronic activation of AMP-activated protein kinase prevents 20-hydroxyeicosatetraenoic acid-induced endothelial dysfunction. Clin Exp Pharmacol Physiol. 2011;38:328–333. doi: 10.1111/j.1440-1681.2011.05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dunn KM, Renic M, Flasch AK, et al. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295:H2455–H2465. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Izzard AS, Rizzoni D, Agabiti-Rosei E, et al. Small artery structure and hypertension: adaptive changes and target organ damage. J Hypertens. 2005;23:247–250. doi: 10.1097/00004872-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 153.Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17(12 pt 1):1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 154.Marchesi C, Paradis P, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 155.Szmitko PE, Wang CH, Weisel RD, et al. New markers of inflammation and endothelial cell activation: part I. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 156.Szmitko PE, Wang CH, Weisel RD, et al. Biomarkers of vascular disease linking inflammation to endothelial activation: part II. Circulation. 2003;108:2041–2048. doi: 10.1161/01.CIR.0000089093.75585.98. [DOI] [PubMed] [Google Scholar]
- 157.Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- 158.Schober A, Zernecke A. Chemokines in vascular remodeling. Thromb Haemost. 2007;97:730–737. [PubMed] [Google Scholar]
- 159.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ishizuka T, Cheng J, Singh H, et al. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324:103–110. doi: 10.1124/jpet.107.130336. [DOI] [PubMed] [Google Scholar]
- 161.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liao TD, Yang XP, Liu YH, et al. Role of inflammation in the development of renal damage and dysfunction in angiotensin II-induced hypertension. Hypertension. 2008;52:256–263. doi: 10.1161/HYPERTENSIONAHA.108.112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Csiszar A, Wang M, Lakatta EG, et al. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 165.Ungvari Z, Orosz Z, Labinskyy N, et al. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. Am J Physiol Heart Circ Physiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 166.Sung B, Park S, Yu BP, et al. Amelioration of age-related inflammation and oxidative stress by PPARgamma activator: suppression of NF-kappaB by 2,4-thiazolidinedione. Exp Gerontol. 2006;41:590–599. doi: 10.1016/j.exger.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 167.Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 168.Chung HY, Sung B, Jung KJ, et al. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 169.de Winther MP, Kanters E, Kraal G, et al. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–914. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 170.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- 171.Theuer J, Dechend R, Muller DN, et al. Angiotensin II induced inflammation in the kidney and in the heart of double transgenic rats. BMC Cardiovasc Disord. 2002;2:3. doi: 10.1186/1471-2261-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Henke N, Schmidt-Ullrich R, Dechend R, et al. Vascular endothelial cell-specific NF-kappaB suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 173.Guo AM, Arbab AS, Falck JR, et al. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosa-tetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321:18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- 174.Ward NC, Puddey IB, Hodgson JM, et al. Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic Biol Med. 2005;38:1032–1036. doi: 10.1016/j.freeradbiomed.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 175.Guo AM, Scicli G, Sheng J, et al. 20-HETE can act as a nonhypoxic regulator of HIF-1alpha in human microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H602–H613. doi: 10.1152/ajpheart.00874.2008. [DOI] [PubMed] [Google Scholar]
- 176.Medhora M, Chen Y, Gruenloh S, et al. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L902–L911. doi: 10.1152/ajplung.00278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Le TH, Coffman TM. Targeting genes in the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2008;17:57–63. doi: 10.1097/MNH.0b013e3282f2fd39. [DOI] [PubMed] [Google Scholar]
- 178.Cvetkovic B, Sigmund CD. Understanding hypertension through genetic manipulation in mice. Kidney Int. 2000;57:863–874. doi: 10.1046/j.1523-1755.2000.057003863.x. [DOI] [PubMed] [Google Scholar]
- 179.Alonso-Galicia M, Maier KG, Greene AS, et al. Role of 20-hydroxyeicosatet-raenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 180.Tsai IJ, Croft KD, Puddey IB, et al. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothe-lin-1. Am J Physiol Heart Circ Physiol. 2011;300:H1194–H1200. doi: 10.1152/ajpheart.00733.2010. [DOI] [PubMed] [Google Scholar]
- 181.Joly E, Seqqat R, Flamion B, et al. Increased renal vascular reactivity to ANG II after unilateral nephrectomy in the rat involves 20-HETE. Am J Physiol Regul Integr Comp Physiol. 2006;291:R977–R986. doi: 10.1152/ajpregu.00401.2005. [DOI] [PubMed] [Google Scholar]
- 182.Xu F, Straub WO, Pak W, et al. Antihypertensive effect of mechanism-based inhibition of renal arachidonic acid omega-hydroxylase activity. Am J Physiol Regul Integr Comp Physiol. 2002;283:R710–R720. doi: 10.1152/ajpregu.00522.2001. [DOI] [PubMed] [Google Scholar]
- 183.Chábová VC, Kramer HJ, Vanecková I, et al. Effects of chronic cytochrome P-450 inhibition on the course of hypertension and end-organ damage in Ren-2 transgenic rats. Vascul Pharmacol. 2007;47:145–159. doi: 10.1016/j.vph.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 184.Muthalif MM, Karzoun NA, Gaber L, et al. Angiotensin II-induced hypertension: contribution of Ras GTPase/Mitogen-activated protein kinase and cytochrome P450 metabolites. Hypertension. 2000;36:604–609. doi: 10.1161/01.hyp.36.4.604. [DOI] [PubMed] [Google Scholar]
- 185.Parmentier JH, Muthalif MM, Nishimoto AT, et al. 20-Hydroxyeicosatetraenoic acid mediates angiotensin ii-induced phospholipase d activation in vascular smooth muscle cells. Hypertension. 2001;37(2 pt 2):623–629. doi: 10.1161/01.hyp.37.2.623. [DOI] [PubMed] [Google Scholar]
- 186.Cheng J, Garcia V, Ding Y, et al. Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeico-satetraenoic acid-mediated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2012;32:1917–1924. doi: 10.1161/ATVBAHA.112.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Quigley R, Chakravarty S, Zhao X, et al. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiol. 2009;113:p23–p28. doi: 10.1159/000235774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fidelis P, Wilson L, Thomas K, et al. Renal function and vasomotor activity in mice lacking the Cyp4a14 gene. Exp Biol Med (Maywood) 2010;235:1365–1374. doi: 10.1258/ebm.2010.009233. [DOI] [PubMed] [Google Scholar]
- 189.Wu CC, Mei S, Cheng J, et al. Androgen-sensitive hypertension is associated with upregulated vascular CYP4A12-20-HETE synthase. J Am Soc Nephrol. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu X, Zhao Y, Wang L, et al. Overexpression of cytochrome P450 4F2 in mice increases 20-hydroxyeicosatetraenoic acid production and arterial blood pressure. Kidney Int. 2009;75:1288–1296. doi: 10.1038/ki.2009.67. [DOI] [PubMed] [Google Scholar]
- 191.Liu X, Wu J, Liu H, et al. Disturbed ratio of renal 20-HETE/EETs is involved in androgen-induced hypertension in cytochrome P450 4F2 transgenic mice. Gene. 2012;505:352–359. doi: 10.1016/j.gene.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 192.Zordoky BN, El-Kadi AO. Effect of cytochrome P450 polymorphism on arachidonic acid metabolism and their impact on cardiovascular diseases. Pharmacol Ther. 2010;125:446–463. doi: 10.1016/j.pharmthera.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 193.Gainer JV, Lipkowitz MS, Yu C, et al. AASK Study Group. Association of a CYP4A11 variant and blood pressure in black men. J Am Soc Nephrol. 2008;19:1606–1612. doi: 10.1681/ASN.2008010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fava C, Montagnana M, Almgren P, et al. The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension. 2008;52:373–380. doi: 10.1161/HYPERTENSIONAHA.108.114199. [DOI] [PubMed] [Google Scholar]
- 195.Mayer B, Lieb W, Götz A, et al. Association of the T8590C polymorphism of CYP4A11 with hypertension in the MONICA Augsburg echocardiographic substudy. Hypertension. 2005;46:766–771. doi: 10.1161/01.HYP.0000182658.04299.15. [DOI] [PubMed] [Google Scholar]
- 196.Williams JS, Hopkins PN, Jeunemaitre X, et al. CYP4A11 T8590C polymorphism, salt-sensitive hypertension, and renal blood flow. J Hypertens. 2011;29:1913–1918. doi: 10.1097/HJH.0b013e32834aa786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fu Z, Nakayama T, Sato N, et al. A haplotype of the CYP4A11 gene associated with essential hypertension in Japanese men. J Hypertens. 2008;26:453–461. doi: 10.1097/HJH.0b013e3282f2f10c. [DOI] [PubMed] [Google Scholar]
- 198.Lino Cardenas CL, Devos A, Toure A, et al. Arachidonic acid ω-hydroxylase CYP4A11: inter-ethnic variations in the 8590T>C loss-of-function variant. Mol Biol Rep. 2012;39:1503–1508. doi: 10.1007/s11033-011-0888-x. [DOI] [PubMed] [Google Scholar]
- 199.Zhang R, Lu J, Hu C, et al. A common polymorphism of CYP4A11 is associated with blood pressure in a Chinese population. Hypertens Res. 2011;34:645–648. doi: 10.1038/hr.2011.8. [DOI] [PubMed] [Google Scholar]
- 200.Sugimoto K, Akasaka H, Katsuya T, et al. A polymorphism regulates CYP4A11 transcriptional activity and is associated with hypertension in a Japanese population. Hypertension. 2008;52:1142–1148. doi: 10.1161/HYPERTENSIONAHA.108.114082. [DOI] [PubMed] [Google Scholar]
- 201.Liu H, Zhao YY, Gong W, et al. Correlation analysis and identification of G421C in regulatory region of CYP4F2 gene with essential hypertension. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:143–147. [PubMed] [Google Scholar]
- 202.Liu H, Zhao Y, Nie D, et al. Association of a functional cytochrome P450 4F2 haplotype with urinary 20-HETE and hypertension. J Am Soc Nephrol. 2008;19:714–721. doi: 10.1681/ASN.2007060713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stec DE, Roman RJ, Flasch A, et al. Functional polymorphism in human CYP4F2 decreases 20-HETE production. Physiol Genomics. 2007;30:74–81. doi: 10.1152/physiolgenomics.00003.2007. [DOI] [PubMed] [Google Scholar]
- 204.Munshi A, Sharma V, Kaul S, et al. Association of 1347 G/A cytochrome P450 4F2 (CYP4F2) gene variant with hypertension and stroke. Mol Biol Rep. 2012;39:1677–1682. doi: 10.1007/s11033-011-0907-y. [DOI] [PubMed] [Google Scholar]
- 205.Hu BC, Li Y, Li FH, et al. Peripheral and central augmentation indexes in relation to the CYP4F2 polymorphisms in Chinese. J Hypertens. 2011;29:501–508. doi: 10.1097/HJH.0b013e328342673c. [DOI] [PubMed] [Google Scholar]
- 206.Fava C, Ricci M, Melander O, et al. Hypertension, cardiovascular risk and polymorphisms in genes controlling the cytochrome P450 pathway of ara-chidonic acid: a sex-specific relation? Prostaglandins Other Lipid Mediat. 2012;98:75–85. doi: 10.1016/j.prostaglandins.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 207.Fu Z, Nakayama T, Sato N, et al. Haplotype-based case-control study of the human CYP4F2 gene and essential hypertension in Japanese subjects. Hypertens Res. 2008;31:1719–1726. doi: 10.1291/hypres.31.1719. [DOI] [PubMed] [Google Scholar]
- 208.Wiinberg N, Høegholm A, Christensen HR, et al. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8(10 pt 1):978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 209.Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 210.Martins D, Nelson K, Pan D, et al. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4:10–3. 20. [PubMed] [Google Scholar]
- 211.Ashton N, Balment RJ. Sexual dimorphism in renal function and hormonal status of New Zealand genetically hypertensive rats. Acta Endocrinol (Copenh) 1991;124:91–97. doi: 10.1530/acta.0.1240091. [DOI] [PubMed] [Google Scholar]
- 212.Chen YF, Meng QC. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci. 1991;48:85–96. doi: 10.1016/0024-3205(91)90428-e. [DOI] [PubMed] [Google Scholar]
- 213.Crofton JT, Share L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension. 1997;29(1 pt 2):494–499. doi: 10.1161/01.hyp.29.1.494. [DOI] [PubMed] [Google Scholar]
- 214.Ouchi Y, Share L, Crofton JT, et al. Sex difference in the development of deoxy-corticosterone-salt hypertension in the rat. Hypertension. 1987;9:172–177. doi: 10.1161/01.hyp.9.2.172. [DOI] [PubMed] [Google Scholar]
- 215.Reckelhoff JF, Zhang H, Srivastava K. Gender differences in development of hypertension in spontaneously hypertensive rats: role of the renin-angiotensin system. Hypertension. 2000;35(1 pt 2):480–483. doi: 10.1161/01.hyp.35.1.480. [DOI] [PubMed] [Google Scholar]
- 216.Reckelhoff JF, Zhang H, Srivastava K, et al. Gender differences in hypertension in spontaneously hypertensive rats: role of androgens and androgen receptor. Hypertension. 1999;34(4 pt 2):920–923. doi: 10.1161/01.hyp.34.4.920. [DOI] [PubMed] [Google Scholar]
- 217.Reckelhoff JF, Zhang H, Granger JP. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension. 1998;31(1 pt 2):435–439. doi: 10.1161/01.hyp.31.1.435. [DOI] [PubMed] [Google Scholar]