Abstract
Background
Differentiating heart failure (HF) induced renal dysfunction (RD) from intrinsic kidney disease is challenging. It has been demonstrated that biomarkers such as B-type natriuretic peptide (BNP) or the blood urea nitrogen to creatinine ratio (BUN/Creat) can identify high vs. low risk RD. Our objective was to determine if combination these biomarkers could further improve risk stratification and clinical phenotyping of patients with RD and HF.
Methods and Results
908 patients with a discharge diagnosis of HF were included. Median values were used to define elevated BNP (>1296 pg/ml) and BUN/Creat (>17). In the group without RD, survival was similar regardless of BNP and BUN/Creat (n=430, adjusted p=0.52). Similarly, in patients with both a low BNP and BUN/Creat, RD was not associated with mortality (n=250, adjusted HR=1.0, 95% CI 0.6-1.6, p=0.99). However, in patients with both an elevated BNP and BUN/Creat those with RD had a cardio-renal profile characterized by venous congestion, diuretic resistance, hypotension, hyponatremia, longer length of stay, greater inotrope use, and substantially worse survival compared to patients without RD (n=249, adjusted HR=1.8, 95% CI 1.2-2.7, p=0.008, p interaction=0.005).
Conclusions
In the setting of decompensated HF, the combined use of BNP and BUN/Creat stratifies patients with RD into groups with significantly different clinical phenotypes and prognosis.
Keywords: Cardio-renal syndrome, BNP, Blood urea nitrogen to creatinine ratio, decompensated heart failure
Introduction
In the setting of heart failure (HF), renal dysfunction has consistently been identified as one of the most powerful prognostic indicators available.[1, 2] However, as research in this area accumulates, it has become clear that not all forms of renal dysfunction are equivalent. Notably, worsening renal function that occurs as the result of initiation of renin angiotensin aldosterone system antagonism, titration of vasodilators, or successful decongestion appears to have limited prognostic importance.[3-6] Similarly, it has previously been described that the risk associated with a low estimated glomerular filtration rate (eGFR) is particularly pronounced in patients with high natriuretic peptide levels and we have found that an elevated blood urea nitrogen to creatinine ratio (BUN/Creat) can also identify higher risk forms of renal dysfunction.[7-9] The global interpretation of the above findings is that the mechanisms underlying renal dysfunction are critically important in determining the associated prognosis.
Although elevated natriuretic peptide levels (identifying patients with venous congestion and activation of compensatory cardio-renal pathways) and elevated BUN/Creat (signifying activation of sodium conserving pathways and renal neurohormonal activation) can each identify high risk renal dysfunction; factors such as diet, protein catabolism, age, and body habitus affect the levels of these markers independent of the cardio-renal axis. As a result, the specificity of each marker is somewhat limited. However, it is possible that a combination of these markers could more precisely identify patients with true HF-induced renal dysfunction by querying two relatively independent mechanisms for cardio-renal dysfunction. Therefore, we hypothesized that patients with acute decompensated HF and both an elevated B-type natriuretic peptide (BNP) and BUN/Creat should have a particularly pronounced risk for mortality attributable to renal dysfunction, in conjunction with a clinical phenotype typical of cardio-renal dysfunction. The primary objectives of this study were to: 1) validate the finding that natriuretic peptide levels can identify high and low risk renal dysfunction,[7] 2) evaluate if patients with renal dysfunction and elevated BNP and BUN/Creat will have a higher prevalence of findings thought typical of HF-induced renal dysfunction such as baseline venous congestion and diuretic non-responsiveness, and 3) determine if combination of BNP and BUN/Creat can identify high and low risk forms of renal dysfunction.
Methods
We reviewed the charts of all patients with a primary discharge diagnosis (determined using ICD codes) of congestive HF who had been admitted to non-interventional cardiology and internal medicine services at the Hospital of the University of Pennsylvania within the years of 2004 to 2009. Inclusion required a BNP level of > 100 pg/mL within 24 hours of admission and availability of admission blood urea nitrogen and creatinine levels. Patients with a length of stay ≤ 2 days (who likely underwent limited decongestion) and patients with length of stay > 14 days (who likely had either atypical degrees of congestion or non-HF problems driving the length of stay) were excluded from the cohort. Patients receiving renal replacement therapy were also excluded. In the event of multiple hospitalizations for a single patient, only the first admission meeting the above inclusion criteria was retained. Please see Supplementary Figure 1 for additional details on patient selection. The ultimate sample size of 908 represents a “convenience sample” as it was determined via the above patient availability and inclusion/exclusion criteria.
The four variable Modified Diet and Renal Disease equation was used to calculate eGFR.[10] Allcause mortality was determined via the Social Security Death Index.[11] Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide = 20 mg torsemide = 80 mg furosemide for oral diuretics, and 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide for intravenous diuretics.[12, 13] The relative diuretic efficiency in each patient was determined as the fluid output per mg of loop diuretic received (expressed as mL of net fluid output per 40 mg of furosemide equivalents). Forty milligrams of furosemide equivalents was chosen as a reference since this is a dose reported to produce near maximal rate of instantaneous natriuresis in a healthy volunteer naive to diuretics.[14] The initial assembly of the cohort was approved by the Institutional Review Board at the Hospital of the University of Pennsylvania and transfer of a version of this dataset stripped of patient identifiers was determined by the Yale University Institutional Review Board to not quality as human subject research.
Statistical Analysis
The primary goal of this analysis was to describe the clinical profile and prognosis of renal dysfunction in patients with low-low BNP-BUN/Creat or high-high BNP and BUN-Creat using patients without renal dysfunction as the reference. As such, the primary analysis focused on describing the clinical profile of these patients and determining the risk for all-cause mortality in the various groups. In order to minimize errors from multiple comparisons, the data is described in terms of 4 groups: 1) eGFR≥60 ml/min/1.73m2; 2) eGFR<60 ml/min/1.73m2 with a BNP and BUN/Creat below the median values; 3) eGFR<60 ml/min/1.73m2 with a BNP or BUN/Creat above the median values; and 4) eGFR<60 ml/min/1.73m2 with a BNP and BUN/Creat above the median values. A secondary objective was to validate the findings of van Kimmenade et al. regarding effect modification of BNP on the risk associated with renal dysfunction.[7] The primary outcome of this analysis was the interaction between BNP dichotomized about the median and an eGFR≥60 ml/min/1.73m2 with respect to all-cause mortality. Values reported are mean ± SD, median (quartile 1 - quartile 4) and percentile. The Kruskal-Wallis test was used to compare continuous variables across multiple groups. For comparison of continuous parameters between two groups the Mann-Whitney U test or t-test or was used. The Pearson chi-square was used to evaluate associations between categorical variables. The Jonckheere-Terpstra test for ordered alternatives was used as the test of trend. Correlations reported are Spearman’s r. Proportional hazards modeling was used to evaluate time-to-event associations with all-cause mortality. Candidate covariates entered in the model were baseline characteristics with univariate all-cause mortality associations p ≤ 0.2. Models were built using backward elimination (likelihood ratio) where all covariates with a p<0.2 were retained.[15] The proportional hazards assumption was examined using time dependent covariates. A post-hoc power calculation demonstrated that with an alpha of 0.05 and a power of 80% the subgroup analyzed with low BUN/Creat and low BNP (n= 250) an effect size of ≥1.43 would be detectable. Statistical analysis was performed with IBM SPSS Statistics version 19.0 (IBM Corp., Armonk, NY) and Stata 12.0 (Statacorp, College Station, Texas). A two sided p value of <0.05 was considered statistically significant aside from tests of interaction where a p<0.1 was considered significant.
Results
Overall, 908 patients were included in the analysis. Baseline and in-hospital characteristics of the overall cohort are presented in Supplementary Tables 1 and 2. The median admission serum BNP level was 1296 pg/mL (660-2387), the median eGFR was 57.9 ml/min/1.73m2 (39.5-75.9) and the median value of BUN/Creat was 17.0 (13.3-22.2). The strength of correlation between BUN/Creat and BNP was small (r=0.13, p<0.001) as was the correlation between eGFR and both BUN/Creat (r= −0.18, p<0.001) and BNP (r= −0.22, p<0.001). These modest correlations translated into 27.5% of the population having both a BUN/Creat and BNP below the median, 45.0% with one of the two parameters elevated, and 27.4% with both parameters elevated. Baseline and in-hospital parameters of patients with the various combinations of an eGFR<60, an elevated BNP, and/or an elevated BUN/Creat can be found in Tables 1 and 2 and Supplementary Tables 1 and 2. The change in BUN/Creat from admission to discharge was statistically significant but modest in magnitude (1.9 ± 6.4, p <0.001) and this change was not associated with mortality (HR=1.0 per 5 unit increase, 95% CI 0.95-1.1, p=0.50). There was a weak association between the increase in BUN/Creat from admission to discharge and a lower in-hospital cumulative diuretic efficiency (r=-0.08, p=0.039).
Table 1.
Baseline characteristics of patients grouped by renal dysfunction, B-type natriuretic peptide level, and the blood urea nitrogen to creatinine ratio
| Characteristics | eGFR ≥ 60 (n=430) |
eGFR < 60 ↓ BNP and ↓ BUN/Creat (n=105) |
p-value vs. eGFR ≥60 |
eGFR < 60 ↑ BNP and ↑ BUN/Creat (n=169) |
p-value vs. eGFR ≥60 |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y) | 56.9 ± 16.0 | 63.2 ± 14.3 | <0.001* | 71.3 ± 12.0 | <0.001* |
| White race | 25.3% | 31.4% | 0.206 | 53.8% | <0.001* |
| Male | 56.5% | 54.3% | 0.680 | 54.4% | 0.645 |
| Medical History | |||||
| Hypertension | 70.1% | 78.8% | 0.075 | 72.5% | 0.569 |
| Diabetes | 32.4% | 49.0% | 0.001* | 53.0% | <0.001* |
| Coronary artery disease | 31.1% | 48.1% | 0.001* | 63.5% | <0.001* |
| Ischemic etiology | 15.6% | 24.8% | 0.026* | 40.8% | <0.001* |
| Ejection fraction ≥ 40% | 33.1% | 49.5% | 0.002* | 24.8% | 0.052 |
| Admission Physical Exam | |||||
| Heart rate (beats/min) | 93.8 ± 20.2 | 90.2 ± 21.1 | 0.121 | 84.1 ± 17.9 | <0.001* |
| Systolic blood pressure (mm Hg) |
141.7 ± 32.7 | 151.9± 38.3 | 0.006* | 123.4 ± 30.9 | <0.001* |
| Jugular venous distention | 58.9% | 53.7% | 0.386 | 71.7% | 0.013* |
| Edema | 37.4% | 36.5% | 0.869 | 60.2% | <0.001* |
| Hepatojugular reflux | 15.3% | 19.8% | 0.335 | 25.6% | 0.013* |
| Echocardiographic parameters |
|||||
| Ejection fraction (%) | 25 (15, 45) | 38 (25, 60) | <0.001* | 23 (15,39) | 0.130 |
| Moderate to severe RV dysfunction |
40.8% | 20.2% | <0.001* | 50.9% | <0.027* |
| Moderate to severe RV dilation |
25.9% | 14.1% | 0.013* | 36.2% | 0.015* |
| Moderate to severe right atrial dilation |
6.9% | 9.3% | 0.424 | 17.0% | <0.001* |
| IVC inspiratory collapsibility < 50% |
44.7% | 33.8% | 0.076 | 65.5% | <0.001* |
| Absence of IVC inspiratory collapsibility |
13.9% | 7.5% | 0.122 | 21.1% | 0.049* |
| Right Heart Catheterization Parameters † |
|||||
| Right atrial pressure (mmHg) |
8.8± 5.7 | 8.2 ± 5.6 | 0.681 | 14.3 ± 7.7 | <0.001* |
| Pulmonary capillary wedge pressure (mmHg) |
20.8 ± 9.2 | 17.8 ± 9.5 | 0.165 | 25.4 ± 8.7 | 0.006* |
| Cardiac index (L/min/m2) | 2.1± 0.5 | 2.5 ± 0.8 | 0.062 | 2.0 ± 0.7 | 0.530 |
| Systemic vascular resistance (dyn·s/cm5) |
1663 ± 536 | 1482 ± 522 | 0.556 | 1546 ± 514 | 0.425 |
| Medications on Admission | |||||
| Beta-blocker | 59.8% | 61.9% | 0.688 | 82.8% | <0.001* |
| ACE inhibitor or ARB | 60.5% | 57.1% | 0.534 | 62.7% | 0.610 |
| Digoxin | 22.0% | 20.2% | 0.686 | 29.3% | 0.060 |
| Aldosterone antagonist | 13.8% | 10.6% | 0.350 | 19.8% | 0.085 |
| Thiazide | 8.2% | 6.7% | 0.619 | 21.6% | <0.001* |
| Loop diuretic dose (mg) | 40 (0, 80) | 40 (0, 80) | 0.338 | 80 (40, 160) | <0.001* |
| Laboratory Values on Admission |
|||||
| Serum sodium (mEq/L) | 138.7 ± 4.0 | 139.9 ± 3.3 | 0.003* | 137.1 ± 5.4 | <0.001* |
| Sodium ≤ 135 mEq/L | 15.9% | 10.6% | 0.174 | 30.8% | <0.001* |
| B-type natriuretic peptide (pg/mL) |
1068 (527, 1974) | 707 (361, 999) | <0.001* | 2670 (1832, 3891) | <0.001* |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.9 ± 0.9 | <0.001* | 2.0 ± 0.8 | <0.001* |
| eGFR (mL/min/1.73m2) | 83.7 ± 22.0 | 42.7 ± 13.7 | <0.001* | 37.5 ± 13.1 | <0.001* |
| BUN (mg/dL) | 17.0 ± 7.4 | 24.7 ± 12.7 | <0.001* | 50.8 ± 27.9 | <0.001* |
| BUN/Creat | 16.0(12.5,20.0) | 13.3(11.4, 15.4) | <0.001* | 22.7 (20.0, 30.0) | <0.001* |
| Hemoglobin (g/dL) | 12.7 ± 2.0 | 11.9± 2.0 | <0.001* | 11.6± 1.9 | <0.001* |
High vs. low BNP (>1296 pg/mL) and BUN/Creatinine (>17) defined as above or below the median value. ACE: Angiotensin converting enzyme inhibitor, ARB: Angiotensin receptor blocker, BNP: B-type natriuretic peptide, BUN: Blood urea nitrogen, Creat: Creatinine, eGFR: Estimated glomerular filtration rate, IVC: Inferior vena cava, RV: Right Ventricle.
Significant p value.
Table 2.
In-hospital characteristics of patients grouped by renal dysfunction, B-type natriuretic peptide level, and the blood urea nitrogen to creatinine ratio
| Characteristics | eGFR ≥ 60 (n=430) |
eGFR < 60 ↓ BNP and ↓ BUN/Creat (n=105) |
p-value vs. eGFR ≥60 |
eGFR < 60 ↑ BNP and ↑ BUN/Creat (n=169) |
p-value vs. eGFR ≥ 60 |
|---|---|---|---|---|---|
| In hospital diuresis related parameters | |||||
| Continuous diuretic infusion |
1.2% | 1.9% | 0.562 | 7.3% | <0.001* |
| Adjuvant thiazide diuretic | 4.9% | 10.6% | 0.029* | 24.4% | <0.001* |
| Net fluid loss (L) | 3.4 (1.3-6.8) | 3.0 (1.1 - 5.5) | 0.212 | 5.2 (1.6-9.0) | 0.017* |
| Average net daily fluid loss (L/day) |
0.68 (0.28 - 1.2) | 0.70 (0.24 - 1.2) | 0.540 | 0.64 (0.26- 1.1) | 0.286 |
| In-hospital inotropes | |||||
| Milrinone | 9.0% | 5.9% | 0.316 | 29.0% | <0.001* |
| Dobutamine | 1.2% | 0.0% | 0.217 | 2.5% | 0.257 |
| Admission to discharge change in laboratory parameters |
|||||
| eGFR (%) | −4.0 ± 20.4 | 2.3 ± 25.8 | 0.022* | 1I.9± 33.0 | <0.001* |
| BUN (%) | 28.9 ± 53.1 | 30.5 ± 56.1 | 0.792 | 2.5 ± 37.9 | <0.001* |
| Bicarbonate (%) | 10.2 ± 18.6 | 6.5 ± 16.9 | 0.061 | 12.4 ± 20.1 | 0.217 |
| Sodium (%) | −0.7 ± 2.7 | −0.9 ± 2.3 | 0.432 | −0.2 ± 3.6 | 0.121 |
| Hospital course | |||||
| Length of stay (days) | 5 (3, 7) | 5 (4, 7) | 0.693 | 8 (5, 11) | <0.001* |
High vs. low BNP (>1296 pg/ml) and BUN/Creatinine (>17) defined as above or below the median value. BNP: B-type natriuretic peptide, BUN: Blood urea nitrogen, eGFR: Estimated glomerular filtration rate.
Significant p value.
Combination of BNP and BUN/Creat identifies distinct phenotypes of renal dysfunction
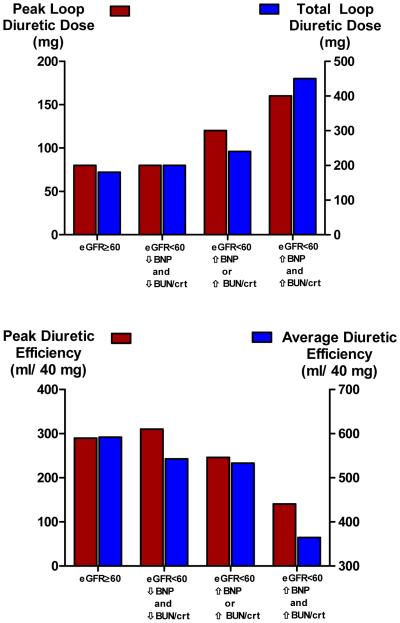
Compared to patients without renal dysfunction, patients with renal dysfunction and an elevated BUN/Creat and BNP consistently demonstrated findings commonly associated with HF induced renal dysfunction such as elevated right atrial pressure, right sided congestion/failure on echocardiography, physical examination findings consistent with venous congestion, a lower admission systolic blood pressure, and higher incidence of hyponatremia (Table 1). Furthermore these patients had evidence of reduced diuretic responsiveness with a higher peak and total loop diuretic doses, utilization of continuous diuretic infusions, utilization of adjuvant thiazide diuretics, and worsened diuretic efficiency (Table 2 and Figures 1A and 1B). Notably, these patients also had the greatest requirement for inotropes and longest hospital length of stay (Table 2). To the contrary, the majority of above parameters were not different between patients without renal dysfunction and those with renal dysfunction and low BNP and BUN/Creat (Tables 1 and 2, Figure 1). Interestingly, patients with renal dysfunction and low BNP and BUN/Creat had a higher ejection fraction, a higher prevalence of preserved ejection fraction, and higher admission blood pressure (Table 1).
Figure 1. Diuretic dose (Panel A) and diuretic efficiency (Panel B) across the cardio-renal spectrum of the cohort.
Peak loop diuretic refers to the maximum 24 hour amount intravenous of furosemide equivalents received. Total loop diuretic refers to the cumulative amount of intravenous loop diuretic received over the hospitalization. Diuretic efficiency is calculated as the net fluid output per 40 mg of furosemide equivalent received, peak and total corresponding to those doses of diuretics. High vs. low BNP and BUN/Creatinine defined as above or below the median value. BNP: B-type natriuretic peptide, BUN/Crt: Blood urea nitrogen to creatinine ratio, eGFR: Estimated glomerular filtration rate. P trend ≤ 0.001 for all comparisons
BNP identifies high and low risk forms of RD
Overall 51.6% of the population died over a median follow-up of 3.4 years (1.3-5.2). Similar to the findings of van Kimmenade et al., the risk associated with renal dysfunction was strongly dependent on the BNP level (Supplementary Table 3, p interaction = 0.006).[7] Notably, in patients with an admission BNP below the median, renal dysfunction was not associated with increased mortality (adjusted HR=1.1, 95% CI 0.78-1.4, p=0.72) whereas in patients with an elevated BNP, renal dysfunction was associated with significant risk (adjusted HR=1.5, 95% CI 1.1-2.0, p=0.012).
Combination of BUN/Creat and BNP identifies very low and very high risk renal dysfunction
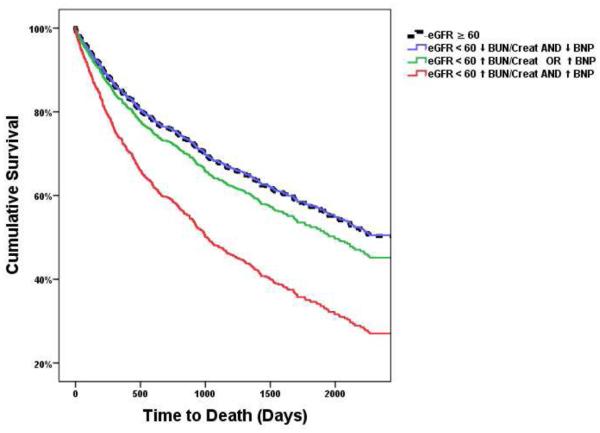
Patients with renal dysfunction but a normal BNP and BUN/Creat had the same risk for death as patients without renal dysfunction (Table 3 and Figure 2). However, patients with renal dysfunction and an elevated BNP and BUN/Creat had a substantially increased risk of death compared to patients without renal dysfunction (Table 3 and Figure 2). Patients with renal dysfunction but only one of the two parameters elevated had a trend toward increased mortality, but this did not reach significance (Table 3 and Figure 2). The three way interaction was highly significant between high vs. low BUN/Creat, BNP, and RD (Supplementary Table 4) Furthermore, analyzing the groups with an elevated BNP and RD or an elevated BUN/Creat and RD separately revealed no increased risk compared to patients without RD (Supplementary table 5). In a sensitivity analysis using the cut points of a BUN/Creat of 20 and a BNP of 1000 pg/ml, the results were essentially unchanged (Supplementary table 6 and Supplementary Figure 2). Furthermore, with eGFR, BNP, and BUN/Creat analyzed as continuous parameters a significant three way interaction remained (adjusted p interaction=0.037).
Table 3.
Covariates retained in the final model evaluating patients grouped by RD, BUN/Creatinine and BNP status as four groups
|
Characteristic |
HR |
95% confidence interval |
p value |
|
|---|---|---|---|---|
| eGFR ≥ 60 | reference | <0.001 | ||
| eGFR < 60 ↓ BNP and ↓ BUN/Creatinine | 0.99 | 0.71 | 1.39 | 0.973 |
| eGFR < 60 ↑ BNP or ↑ BUN/Creatinine | 1.16 | 0.89 | 1.51 | 0.278 |
| eGFR < 60 ↑ BNP and ↑ BUN/Creatinine | 1.91 | 1.46 | 2.49 | <0.001 |
| Age (per year) | 1.03 | 1.02 | 1.04 | <0.001 |
| Systolic blood pressure (per 10 mmHg) | 0.91 | 0.88 | 0.94 | <0.001 |
| Loop diuretic dose (per 100 mg) | 1.08 | 1.01 | 1.16 | 0.034 |
| Serum Sodium (per meq/L) | 0.98 | 0.96 | 1.00 | 0.114 |
| Black Race | 1.53 | 1.22 | 1.93 | <0.001 |
| Hypertension | 0.73 | 0.57 | 0.94 | 0.012 |
| Ischemic HF etiology | 1.21 | 0.97 | 1.51 | 0.085 |
| Edema | 1.27 | 1.04 | 1.55 | 0.017 |
| Digoxin use | 1.34 | 1.06 | 1.68 | 0.013 |
| Thiazide diuretic use | 1.23 | 0.93 | 1.61 | 0.140 |
| Ejection fraction (per %) | 1.89 | 1.15 | 3.12 | 0.012 |
High vs. low BNP and BUN/Creatinine defined as above or below the median value. BNP: B-type natriuretic peptide, BUN: Blood urea nitrogen, eGFR: Estimated glomerular filtration rate in ml/min/1.73m2.
Figure 2. Adjusted survival plots across the cardio-renal spectrum of the cohort.
Plots adjusted for: age, race, diabetes, hypertension, ischemic HF etiology, ejection fraction, heart rate, systolic blood pressure, edema, serum sodium, hemoglobin, and baseline medication use. High vs. low BNP and BUN/Creatinine defined as above or below the median value. BNP: B-type natriuretic peptide, BUN/Creat: Blood urea nitrogen to creatinine ratio, eGFR: Estimated glomerular filtration rate.
Subgroup analyses
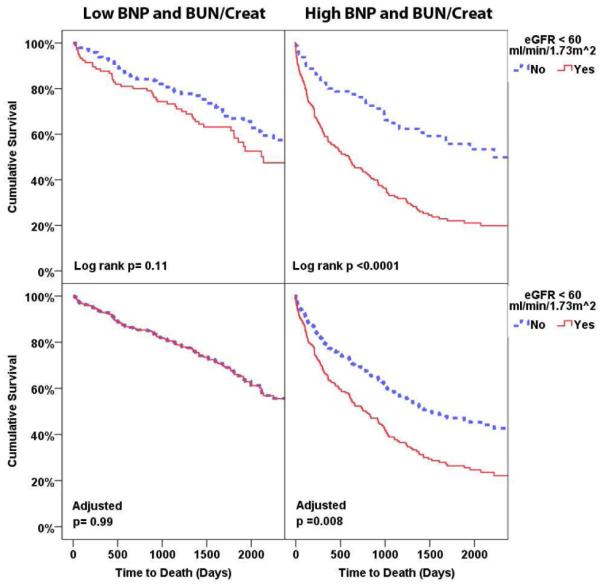
In patients with an eGFR ≥ 60 ml/min/1.73m2 (n=430, n events=177) there were no differences in survival amongst the different combinations of high vs. low BNP and BUN/Creat (adjusted p = 0.52) even when comparing patients with both markers high to both markers low (n events=88, adjusted HR=0.94, 95% CI 0.59-1.5, p=0.81). With respect to the risk specifically attributable to renal dysfunction across the groups of BNP and BUN/Creat: in the setting of a normal BNP and BUN/Creat, the presence or absence of renal dysfunction had no bearing on survival (subgroup n=250, n events=96, Figure 3A, adjusted HR=1.0, 95% CI 0.6-1.6, p=0.99). However, in patients with an elevated BUN/Creat and BNP level, there was a potent survival disadvantage associated with renal dysfunction (subgroup n=249, n events=168, Figure 3B, adjusted HR=1.8, 95% CI 1.2-2.7, p=0.008, p three way interaction=0.005). In patients with the intermediate phenotype of only one of the two parameters elevated, the survival disadvantage associated with renal dysfunction did not reach significance (subgroup n=409, n events=204, adjusted HR=1.2, 95% CI 0.9-1.6, p=0.34). The primary findings were similar between patients with or without an ejection fraction ≥ 40% (p interaction=0.741) or ≥ 50% (p interaction=0.950).
Figure 3. Unadjusted (top row) and adjusted (bottom row) survival plots grouped by presence of an eGFR above or below 60 ml/min/1.73m2 in patients with both BUN/Creat and BNP below (Panel A) or above (Panel B) the median.
Plots adjusted for: age, race, diabetes, hypertension, ischemic HF etiology, ejection fraction, heart rate, systolic blood pressure, edema, serum sodium, hemoglobin, and baseline medication use. High vs. low BNP and BUN/Creatinine defined as above or below the median value. BNP: B-type natriuretic peptide, BUN/Creat: Blood urea nitrogen to creatinine ratio, eGFR: Estimated glomerular filtration rate.
Discussion
The principal finding of this analysis is that in the setting of decompensated heart failure, the combined use of BNP and BUN/Creat identify what appear to be distinct phenotypes of renal dysfunction. Notably, patients with a low eGFR who had an elevated BNP and BUN/Creat on admission had multiple parameters consistent with HF-induced renal dysfunction including more frequent venous congestion, reduced diuretic responsiveness, hypotension, hyponatremia, longer length of stay, greater in-hospital requirement for inotropes, in addition to a substantial mortality disadvantage. To the contrary, patients with a low eGFR but normal BNP and BUN/Creat had a cardio-renal clinical profile and prognosis that was similar to patients without renal dysfunction. The striking differences in prognosis and clinical phenotype in patients with a similar degree of renal dysfunction but high vs. low BNP and BUN/Creat highlights the fact that renal dysfunction in heart failure is a heterogeneous phenomenon.
A classification scheme for different phenotypes of cardio-renal syndrome (CRS) has been put forth by Ronco et al., however, to date limited progress has been made in developing practical methods to differentiate these subtypes.[16] Simply dichotomizing CRS into cardio-renal (i.e., Type 1 and 2 CRS) vs. reno-cardiac (i.e, Type 3 and 4 CRS) could potentially be of great value for both research and clinical purposes, however no gold standard exists with which such tools can be evaluated. Although a low GFR in both cardio-renal and reno-cardiac syndrome theoretically could directly participate in the pathophysiology of heart failure progression, the relative prognosis of these conditions would be expected to differ. If heart failure is so severe as to cause peripheral organ dysfunction, this should have a worse prognosis than a similar degree of renal dysfunction caused by a comparatively more benign disease such as hypertensive nephropathy. Although both conditions would potentially have the negative direct effects from the low GFR, the patient with HF-induced renal dysfunction (i.e., Type 1 and 2 CRS) would not only have the low GFR but also the poor prognosis of very severe heart failure.
Unfortunately, since many of the same risk factors (i.e. hypertension and diabetes) are common to both chronic kidney disease and HF, any population of patients with a low GFR and HF will likely be comprised of a mixture of both cardio-renal and reno-cardiac syndrome. Not only does this inability to differentiate these conditions impede our ability to study this condition and develop therapeutic strategies, but clinical decisions such as which patients with severe renal dysfunction will reverse with advanced therapies remain challenging. The fact that approximately 40% of the patients with renal dysfunction fell into an intermediate category, with only one of two of BUN/Creat and BNP elevated, could be viewed as a limitation to the use of this approach. However, cardio-renal and reno-cardiac syndrome are not mutually exclusive disorders and it is highly probable, particularly in light of the shared risk factors, that a significant percentage of any HF population will actually have some degree of both HF-induced renal dysfunction and chronic kidney disease. In light of the lack of alternative tools or a gold standard with which to differentiate chronic kidney disease from HF-induced renal dysfunction, pending validation in additional cohorts, the combined use of BUN/Creat and BNP may be a potential method to approach this question from both a research and clinical standpoint.
An interesting observation from this and other studies is that, in the absence of renal dysfunction, both BNP and an elevated BUN/Creat appear to have limited to no prognostic importance.[7-9] Notably, in this cohort with acute decompensated heart failure, patients without renal dysfunction had similar survival even when comparing patients with both an elevated BNP and BUN/Creat to those with both parameters low. Unlike a low GFR, which could plausibly participate in the causal pathway for adverse outcomes, elevated BNP and BUN/Creat are most likely surrogates for disease severity. Both BNP and BUN/Creat are markers of activation of compensatory pathways; BUN/Creat sodium conserving pathways, and BNP natriuretic and renal preserving pathways.[17, 18] Given that the majority of compensatory pathways evolved to defend circulatory integrity, it does make sense that activation of such pathways at presentation for acute decompensated heart failure could, in the right circumstances, be appropriate and thus not always associated with a poor prognosis. However, when there is apparent failure of the cardio-renal axis to respond to these compensatory pathways it is intuitive that this would be a marker of poor prognosis. Notably, severe sodium avidity/natriuretic failure appeared to be prevalent in the patients with renal dysfunction and a high BUN/Creat and BNP evidenced by the substantial in-hospital diuretic resistance in these patients. Although this analysis cannot resolve the specific mechanisms, it is possible that the risk associated with activation of compensatory systems could be contingent on the primary target organ remaining responsive to the compensatory signals. Further research studying markers that evaluate different aspects of renal physiology which more directly query the target organ response, such as markers of sodium handling or urinary cyclic guanosine monophosphate, would likely better inform this question.
Limitations
There are several limitations that must be considered when interpreting these results. First, given the retrospective study design, causality is impossible to demonstrate and residual confounding cannot be excluded. Importantly, no gold standard exists with which to evaluate the sensitivity and specificity of BUN/Creat and BNP to differentiate HF-induced renal dysfunction from chronic kidney disease. Furthermore, due to this same lack of gold standard, the actual clinical profile of patients with HF-induced renal dysfunction and chronic kidney disease in an acute decompensated heart failure population is not well defined. As such, despite the fact that the prognosis was dramatically different and the clinical profile of the two extremes of BUN/Creat and BNP were consistent with what is thought typical of chronic kidney disease vs. HF-induced renal dysfunction, the possibility that the groups described in this manuscript represent distinct mechanisms cannot be proven or disproven. Although traditional wisdom holds that an elevated BUN/Creat is not a typical pattern observed in patients with chronic kidney disease, but rather is this pattern is restricted to patients with “pre-renal” physiology, this assumption has never been formally studied in patients with heart failure weakening the conclusions related to BUN/Creat. In some patients the profile of RD in the setting of a lower BNP may have been the result of pre-admission outpatient diuresis. Given that it has been reported that decongestion induced worsening renal function does not carry a similar poor prognosis, and we do not have information on pre-hospital diuresis, this represents a limitation of this analysis.[19] Although the purpose of the analysis was to describe cardio-renal profile and risk at admission, use of baseline information only while ignoring important changes in markers over the hospitalization limited inferences possible with respect to the outcomes. Receipt of an iodinated contrast agent was not an exclusion criterion. As a result, the reported changes in renal function during hospitalization could have been influenced by such procedures. Additionally, physicians were not blinded to measures of renal function and BNP and thus may have altered treatment decisions in response to these data. This analysis only incorporated BNP and BUN/Creat. It is unknown how the newer biomarkers such as galectin-3 and ST2 may have performed in these models. Although required to facilitate interpretability of the data, dichotomization of BUN/Creat and BNP makes the assumption that the hazard ratio does not change over the “high” or “low” categories, which may have influenced the results. Lack of information on repeat hospitalization and the lack of validation of vital status obtained using the social security death master file is a significant limitation. Lastly, non-neurohormonal factors such as diet and protein catabolism that influence urea reabsorption may have introduced potential uncontrolled confounding into BUN/Creat and parameters such as body mass, inflammation, and the renal dysfunction itself could have influenced BNP.
Conclusion
In the setting of acute decompensated HF, the combined use of BNP and BUN/Creat stratifies patients with renal dysfunction into groups with significantly different clinical phenotypes and prognosis. Additional research is necessary to understand potential mechanistic differences and therapeutic opportunities underlying these observations.
Supplementary Material
-Differentiating heart failure induced vs. intrinsic kidney disease is challenging
-BNP and BUN/creatinine may help distinguish these entities
-Renal dysfunction with low BNP and low BUN/creatinine had a good prognosis
-Renal dysfunction with high BNP and high BUN/creatinine had a poor prognosis
Acknowledgments
Funding sources: NIH Grants: K23HL114868 (JT), L30HL115790 (JT), and K24DK090203 (CRP): The funding source had no role in study design, data collection, analysis or interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors report no disclosures relevant to this work.
References
- [1].McAlister FA, Ezekowitz J, Tarantini L, Squire I, Komajda M, Bayes-Genis A, et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula. Circulation Heart failure. 2012;5:309–14. doi: 10.1161/CIRCHEARTFAILURE.111.966242. [DOI] [PubMed] [Google Scholar]
- [2].Bock JS, Gottlieb SS. Cardiorenal Syndrome: New Perspectives. Circulation. 2010;121:2592–600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- [3].Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of Angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circulation Heart failure. 2011;4:685–91. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–72. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. European journal of heart failure. 2011;13:877–84. doi: 10.1093/eurjhf/hfr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circulation Heart failure. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- [7].van Kimmenade RR, Januzzi JL, Jr., Baggish AL, Lainchbury JG, Bayes-Genis A, Richards AM, et al. Amino-terminal pro-brain natriuretic Peptide, renal function, and outcomes in acute heart failure: redefining the cardiorenal interaction? J Am Coll Cardiol. 2006;48:1621–7. doi: 10.1016/j.jacc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- [8].Brisco MA, Coca SG, Chen J, Owens AT, McCauley BD, Kimmel SE, et al. Blood urea nitrogen/creatinine ratio identifies a high-risk but potentially reversible form of renal dysfunction in patients with decompensated heart failure. Circulation Heart failure. 2013;6:233–9. doi: 10.1161/CIRCHEARTFAILURE.112.968230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Testani JM, Coca SG, Shannon RP, Kimmel SE, Cappola TP. Influence of renal dysfunction phenotype on mortality in the setting of cardiac dysfunction: analysis of three randomized controlled trials. European journal of heart failure. 2011;13:1224–30. doi: 10.1093/eurjhf/hfr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- [11].Quinn J, Kramer N, McDermott D. Validation of the Social Security Death Index (SSDI): An Important Readily-Available Outcomes Database for Researchers. West J Emerg Med. 2008;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- [12].Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–9. doi: 10.1038/ki.1984.153. [DOI] [PubMed] [Google Scholar]
- [13].Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–9. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- [14].Brenner BM, Rector FC. Brenner & Rector's the kidney. Saunders Elsevier; Philadelphia: 2008. [Google Scholar]
- [15].Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–37. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- [16].Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- [17].Lindenfeld J, Schrier RW. Blood urea nitrogen a marker for adverse effects of loop diuretics? J Am Coll Cardiol. 2011;58:383–5. doi: 10.1016/j.jacc.2011.01.054. [DOI] [PubMed] [Google Scholar]
- [18].Korinek J, Boerrigter G, Mohammed SF, Burnett JC., Jr Insights into natriuretic peptides in heart failure: an update. Current heart failure reports. 2008;5:97–104. doi: 10.1007/s11897-008-0016-y. [DOI] [PubMed] [Google Scholar]
- [19].Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.