Abstract
OBJECTIVE
To assess trends of tubal factor infertility and to evaluate risk of miscarriage and delivery of preterm or low birth weight (LBW) neonates among women with tubal factor infertility using assisted reproductive technology (ART).
METHODS
We assessed trends of tubal factor infertility among all fresh and frozen, donor, and nondonor ART cycles performed annually in the United States between 2000 and 2010 (N=1,418,774) using the National ART Surveillance System. The data set was then limited to fresh, nondonor in vitro fertilization cycles resulting in pregnancy to compare perinatal outcomes for cycles associated with tubal compared with male factor infertility. We performed bivariate and multivariable analyses controlling for maternal characteristics and calculated adjusted risk ratios (RRs) and 95% confidence intervals (CI).
RESULTS
The percentage of ART cycles associated with tubal factor infertility diagnoses decreased from 2000 to 2010 (26.02–14.81%). Compared with male factor infertility, tubal factor portended an increased risk of miscarriage (14.0% compared with 12.7%, adjusted RR 1.08, 95% CI 1.04–1.12); risk was increased for both early and late miscarriage. Singleton neonates born to women with tubal factor infertility had an increased risk of pre-term birth (15.8% compared with 11.6%, adjusted RR 1.27, 95% CI 1.20–1.34) and LBW (10.9% compared with 8.5%, adjusted RR 1.28, 95% CI 1.20–1.36). Significant increases in risk persisted for early and late preterm delivery and very low and moderately LBW delivery. A significantly elevated risk was also detected for twin, but not triplet, pregnancies.
CONCLUSION
Tubal factor infertility, which is decreasing in prevalence in the United States, is associated with an increased risk of miscarriage, preterm birth, and LBW delivery as compared with couples with male factor infertility using ART.
Tubal and peritoneal pathology, one of the most common causes of infertility, contributes to 30–35% of known infertility among women.1 Hydrosalpinx, accumulation of fluid within a diseased fallopian tube, present at the time of in vitro fertilization (IVF), decreases pregnancy rates significantly and has been linked with increased spontaneous miscarriage rates.2–6 The association persists with oocyte donation suggesting an etiology unrelated to oocyte quality.7 Infectious and inflammatory states, including prior pelvic infection, have been associated with preterm labor; however, studies investigating the effect of tubal disease on perinatal outcomes are limited.8–12 A secondary analysis of Centers for Disease Control and Prevention (CDC) surveillance data suggests an increased risk of preterm or low birth weight (LBW) delivery among singleton live births to women with tubal disease who underwent IVF in 2008.13
Preterm birth remains the largest contributor to perinatal morbidity and mortality worldwide.14 The elevated preterm delivery rate in developed countries may partially reflect increased numbers of multiple gestations secondary to heightened use of assisted reproductive technology (ART); however, evidence suggests that even singleton IVF pregnancies are at increased risk of adverse perinatal outcomes.15–20 Although the pathophysiology remains unclear, well-established risks exist between IVF and preterm labor and LBW among singleton pregnancies.15–20
Because infectious and inflammatory states have been associated with preterm labor,10–12 we hypothesize that among women who undergo IVF, those with tubal disease are at increased risk of miscarriage and of adverse perinatal outcomes, namely preterm and LBW delivery. The goal of this study is to use national data to examine trends of tubal factor infertility and to assess the risk of adverse perinatal outcomes associated with IVF cycles among women with tubal factor infertility as compared with IVF cycles conducted resulting from male factor infertility.
METHODS
Data used in this study were obtained from the National ART Surveillance System, a federally mandated reporting system that collects information about ART cycles performed in the United States (Fertility Clinic Success Rate and Certification Act of 1992, Public Law No. 102–493, October 24, 1992).21 Assisted reproductive technology procedures include those involving the laboratory handling of gametes, namely IVF–transcervical embryo transfer, gamete intrafallopian transfer, and zygote intrafallopian transfer. The National ART Surveillance System data on reported ART cycles include patient demographics, medical and obstetric history, infertility diagnoses, detailed parameters of each ART treatment cycle, and, if applicable, the resultant pregnancy outcome. Although 6–12% of ART clinics did not report data to the CDC in any given year between 2000 and 2010, we estimate that the National ART Surveillance System includes data from more than 95% of all ART cycles performed in the United States; most nonreporting clinics perform very few procedures annually.22 Additionally, for each of the study years, approximately 7–10% of reporting clinics were randomly selected for full validation. During validation, ART data reported by the clinics are compared with information recorded in medical records and discrepancy rates are calculated. Overall, discrepancy rates for the variables evaluated in the present study were less than 5%; however, diagnosis of infertility had higher discrepancy rates (up to 18%), mostly as a result of report of “other” or “unexplained” infertility instead of a specific cause. For these analyses, we used all available data meeting the criteria described subsequently.
To explore trends in tubal factor infertility, we included all fresh and frozen, donor, and nondonor ART cycles performed in the United States between 2000 and 2010 that did not use a gestational carrier (N=1,418,774). Clinicians may select one or multiple diagnoses to report tubal factor infertility. Options include “tubal factor,” “tubal ligation, not reversed,” “tubal disease, hydrosalpinx in place,” and “other tubal disease, not hydrosalpinx.” Although information on the methods of tubal factor infertility diagnosis was not collected, hydrosalpinx may be diagnosed by hysterosalpingogram, transvaginal ultrasonography, or at time of laparoscopy. In the trends analysis, we report the number and percent of cycles with any diagnosis of tubal factor, which includes cycles for which additional infertility diagnoses are reported.
To avoid misclassification and confounding, for all subsequent analyses, cycles were limited to those for which only one infertility diagnosis was reported (tubal factor only or male factor only). “Male factor infertility only” was chosen as the comparison group because it suggests a female without known infertility. Couples with only male factor infertility often require use of ART because of semen parameters that make intrauterine insemination with autologous semen unlikely to be successful. We only included fresh, nondonor ART cycles resulting in pregnancy to decrease potential confounding associated with frozen and donor cycles. Gestational carrier cycles were excluded because the intrauterine environment would not reflect the tubal factor diagnosis. Cycles were considered to result in pregnancy if they had an outcome of clinical intrauterine gestation or heterotopic pregnancy; cycles that had no indication of pregnancy from either β-human chorionic gonadotropin or ultrasonography or were biochemical or ectopic pregnancies were excluded. The National ART Surveillance System definition for a clinical intrauterine gestation is ultrasonographic confirmation of gestational sac(s) within the uterus, regardless of whether a heartbeat(s) is observed or fetal pole established. Without ultrasonographic data, confirmation is achieved through documented birth, spontaneous miscarriage, or induced abortion.
The three primary outcomes of interest were miscarriage, preterm delivery, and LBW delivery. A cycle was classified as a miscarriage if the patient was reported to have had a spontaneous miscarriage and the gestational age was less than 20 weeks. As a secondary analysis, we also explored early miscarriage (spontaneous miscarriages with a gestational age of less than 15 weeks) and late miscarriage (spontaneous miscarriages with a gestational age between 15 and 20 weeks). Birth outcomes (preterm delivery and LBW delivery) were analyzed at the neonate level; the outcome for each neonate of a multiple gestation pregnancy was thus counted as an individual unit. A neonate was defined as preterm if he had a gestational age of less than 37 weeks and LBW if he had a birth weight of less than 2,500 g at birth. As secondary analyses, we defined early pre-term birth to be less than 32 weeks of gestation and late preterm birth to be between 32 and 37 weeks of gestation. We defined very LBW as less than 1,500 g and moderately LBW between 1,500 and 2,500 g. To account for the contribution of plurality to gestational age and birth weight, the analyses of preterm birth and LBW were stratified by the number of neonates (singletons, twins, triplets, or more).
Bivariate analyses were conducted to explore the relationship between type of infertility (tubal factor only or male factor only) and other maternal characteristics. Bivariate analyses also were conducted to explore the relationship between each outcome of interest (miscarriage, preterm delivery, and LBW) and all maternal characteristics, including type of infertility. Secondary outcomes (early miscarriage, late miscarriage, early preterm birth, late preterm birth, very LBW, and moderate LBW) were included in the bivariate analyses for exploratory purposes. Risk ratios (RRs), 95% confidence intervals (CIs), and P values were generated using log-binomial modeling. In a few cases, the log-binomial model did not converge and a Poisson model with a robust error variance was used instead.23
Multivariable analyses were conducted to explore the relationship between each primary outcome of interest (miscarriage, preterm delivery, and LBW) and type of infertility, holding constant all other maternal characteristics included in the models. Secondary outcomes (early and late preterm birth, very and moderately LBW) were not included in the multivariable analysis. Maternal characteristics controlled for include maternal age, number of prior spontaneous miscarriages, number of prior preterm births, number of prior full term births, number of prior ART cycles, use of intracytoplasmic sperm injection, use of assisted hatching, the embryo stage at transfer, the number of embryos transferred, the number of supernumerary embryos cryopreserved, the number of oocytes retrieved, and year. Maximum number of fetal heartbeats was also controlled for in the preterm and LBW analyses but was excluded from the miscarriage analyses as a result of missing data among miscarriages that occurred at an early gestational age. Although the number of prior pregnancies was included in the bivariate tables, this variable was not controlled for in the multivariable models as a result of inclusion of the number of prior spontaneous miscarriages, preterm births, and full-term births. Race or ethnicity was also included in the bivariate tables but not in the multivariable analysis because over 40% of the observations were missing. However, because race or ethnicity is a known risk factor for adverse perinatal outcomes, we performed a secondary analysis including race. Risk ratios, 95% CI, and P values were generated using a Poisson model with a robust error variance. For all modeling, generalized estimating equations with an exchangeable correlation matrix were used to capture the clustering of twins and triplets for the neonatal outcomes.
Statistical significance was determined using an α of .05. All analyses were conducted using SAS 9.2. This study was approved by the institutional review board of the CDC.
RESULTS
In the United States between 2000 and 2010, physicians performed 274,525 fresh and frozen IVF cycles for which tubal disease was a contributing factor. Of these, 142,939 cycles were performed on patients with no additional infertility diagnoses. Over the 11-year period, the percentage of total ART cycles performed in which tubal factor was included as a diagnosis but may not have been the primary indication for therapy decreased from 26.0% to 14.8% (Fig. 1). The absolute number decreased slightly from 25,615 to 22,433 cycles per year. A test for trend in the proportion of cycles with primary or secondary diagnosis of tubal factor infertility indicated a significant decline (P<.001).
Fig. 1.
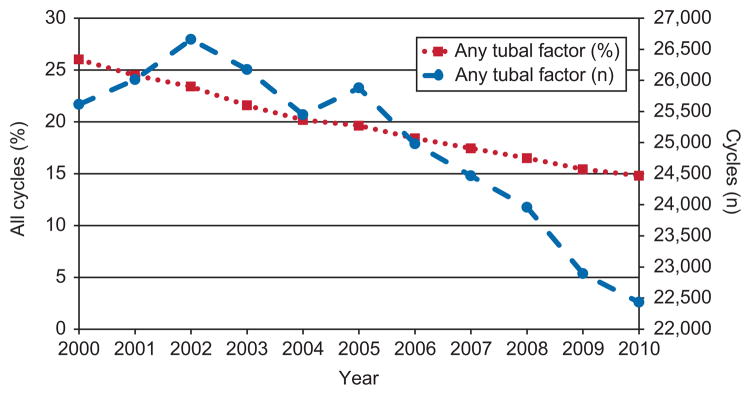
Trends over time in tubal factor infertility among all women undergoing assisted reproductive technology, including fresh and frozen, donor, and nondonor cycles, 2000–2010. Gestational carriers excluded.
Kawwass. Perinatal Risk After ART in Tubal Factor Infertility. Obstet Gynecol 2013.
In the United States between 2000 and 2010, physicians performed 40,046 fresh nondonor IVF cycles resulting in pregnancy for which the only infertility diagnosis was tubal factor and 78,308 cycles for which the sole diagnosis was male factor infertility (Table 1). The amount of missing data was less than 0.5% for all variables except race (41.3%) and number of fetal heartbeats (5.7%). All maternal characteristics reported for these pregnancies, except embryo stage at the time of transfer, were significantly different between those for which tubal factor was the reported cause of infertility as compared with male factor infertility. Notably, those with tubal factor infertility were more likely to be older and to have had prior pregnancies (miscarriages, preterm births, and full-term births). Those with tubal factor infertility were also more likely to have fewer oocytes retrieved, to have a larger number of embryos cryopreserved, to have a larger maximum number of fetal heartbeats, and to have a cycle resulting in no live births. Those with tubal factor infertility were less likely to be non-Hispanic white, to have a history of ART use, and to have used intracytoplasmic sperm injection or assisted hatching.
Table 1.
Maternal Characteristics of Pregnancies Resulting From Fresh, Nondonor In Vitro Fertilization Cycles by Type of Infertility, 2000–2010
| Characteristic | Tubal Factor Only | Male Factor Only | P |
|---|---|---|---|
| Total | 40,046 | 78,308 | |
| Maternal age (y)* | <.001 | ||
| Younger than 30 | 5,678 (14.18) | 15,944 (20.36) | |
| 30–34 | 15,716 (39.24) | 32,959 (42.09) | |
| 35–39 | 15,630 (39.03) | 25,253 (32.25) | |
| 40 or older | 3,022 (7.55) | 4,152 (5.30) | |
| Race or ethnicity† | <.001 | ||
| Non-Hispanic white | 16,497 (69.58) | 36,882 (80.55) | |
| Non-Hispanic black | 2,968 (12.52) | 1,832 (4.00) | |
| Asian or Pacific Islander | 1,428 (6.02) | 3,611 (7.89) | |
| Hispanic | 2,756 (11.62) | 3,391 (7.41) | |
| Other | 62 (0.26) | 69 (0.15) | |
| No. of prior pregnancies* | <.001 | ||
| 0 | 11,616 (29.03) | 44,425 (56.83) | |
| 1 | 8,715 (21.78) | 21,005 (26.87) | |
| 2 or more | 19,680 (49.19) | 12,736 (16.29) | |
| No. of prior miscarriages* | <.001 | ||
| 0 | 25,101 (62.90) | 61,574 (79.01) | |
| 1 | 8,611 (21.58) | 12,449 (15.97) | |
| 2 or more | 6,196 (15.53) | 3,910 (5.02) | |
| No. of prior preterm births* | <.001 | ||
| 0 | 38,601 (96.76) | 76,517 (98.33) | |
| 1 or more | 1,291 (3.24) | 1,303 (1.67) | |
| No. of prior full-term births* | <.001 | ||
| 0 | 23,162 (57.97) | 59,871 (76.71) | |
| 1 | 8,011 (20.05) | 15,412 (19.75) | |
| 2 or more | 8,779 (21.97) | 2,763 (3.54) | |
| No. of prior ART cycles* | <.001 | ||
| 0 | 25,525 (63.76) | 48,298 (61.71) | |
| 1 | 6,897 (17.23) | 14,400 (18.40) | |
| 2 or more | 7,610 (19.01) | 15,572 (19.90) | |
| Use of intracytoplasmic sperm injection* | <.001 | ||
| Used | 15,963 (39.90) | 73,471 (93.84) | |
| Did not use | 24,048 (60.10) | 4,822 (6.16) | |
| Use of assisted hatching* | <.001 | ||
| Used | 13,899 (34.71) | 28,476 (36.36) | |
| Did not use | 26,147 (65.29) | 49,832 (63.64) | |
| Embryo stage at transfer* | 0.869 | ||
| Day 3 | 24,187 (65.91) | 47,463 (66.06) | |
| Day 5 | 12,511 (34.09) | 24,390 (33.94) | |
| Other | 3,179 (7.97) | 6,194 (7.94) | |
| No. of embryos transferred* | <.001 | ||
| 1 | 1,738 (4.34) | 4,147 (5.30) | |
| 2 | 19,423 (48.50) | 40,883 (52.21) | |
| 3 | 12,122 (30.27) | 22,068 (28.18) | |
| 4 or more | 6,763 (16.89) | 11,210 (14.32) | |
| No. of supernumerary embryos cryopreserved* | <.001 | ||
| 0 | 20,915 (52.45) | 41,908 (53.72) | |
| 1–2 | 5,578 (13.99) | 12,061 (15.46) | |
| 3–4 | 5,424 (13.60) | 10,668 (13.67) | |
| 5 or more | 7,960 (19.96) | 13,374 (17.14) | |
| No. of oocytes retrieved* | <.001 | ||
| 0–10 | 15,029 (37.53) | 25,476 (32.53) | |
| 11–20 | 18,531 (46.27) | 38,646 (49.35) | |
| 21 or more | 6,486 (16.20) | 14,186 (18.12) | |
| No. of fetal heartbeats at first ultrasonography‡ | <.001 | ||
| 1 | 23,527 (62.51) | 47,033 (63.58) | |
| 2 | 12,066 (32.06) | 23,461 (31.71) | |
| 3 or more | 2,044 (5.43) | 3,485 (4.71) | |
| No. of births (live birth and stillbirth)* | <.001 | ||
| 0 | 6,758 (16.88) | 11,501 (14.69) | |
| 1 | 21,858 (54.58) | 44,664 (57.04) | |
| 2 | 10,430 (26.05) | 20,539 (26.23) | |
| 3 or more | 1,000 (2.50) | 1,604 (2.05) |
ART, assisted reproductive technology.
Data are n (%) unless otherwise specified.
Less than 0.54% missing data.
41.28% missing data.
5.69% missing data.
The bivariate analysis indicated a slight increase in the risk of miscarriage for pregnancies resulting from ART cycles among women with tubal factor infertility (14.0%) as compared with cycles among women whose partners had male factor infertility (12.7%) (RR 1.11, 95% CI 1.07–1.14, P<.001) (Table 2). The risk of early and, to a greater extent, late miscarriage was also higher in the tubal factor group, 12.9% compared with 12.0% (RR 1.08, 95% CI 1.04–1.11, P<.001) in the early group and 1.5% compared with 0.9% (RR 1.61, 95% CI 1.43–1.81, P<.001) in the late group. The adjusted relative risk estimate for miscarriage from multivariable analysis for all cycles was similar to the unadjusted estimate (adjusted RR 1.08, 95% CI 1.04–1.12, P=.001). Of note, a significant (P=.003) interaction was identified between miscarriage and history of spontaneous miscarriage. Among women with no previous miscarriage, tubal factor portends a significantly increased risk of miscarriage (adjusted RR 1.12, 95% CI 1.07–1.17, P<.001); however, for women with a history of miscarriage, tubal factor does not appear to further increase the risk of subsequent miscarriage.
Table 2.
Association Between Infertility and Miscarriage for Fresh, Nondonor InVitro Fertilization Cycles Resulting in Pregnancy, 2000–2010
| Outcome | Tubal Factor†‡ | Male Factor†‡ | Bivariate Analysis
|
Multivariable Analysis*
|
||
|---|---|---|---|---|---|---|
| RR (95% CI) | P | Adjusted RR (95% CI) | P | |||
| Any miscarriage (less than 20 wk of gestation) | 5,491 (13.99) | 9,785 (12.65) | 1.11 (1.07–1.14) | <.001 | 1.08 (1.04–1.12) | .001 |
| Early miscarriage (less than 15 wk of gestation) | 4,989 (12.87) | 9,163 (11.94) | 1.08 (1.04–1.11) | <.001 | ||
| Late miscarriage (15–20 wk of gestation) | 502 (1.47) | 622 (0.91) | 1.61 (1.43–1.81) | <.001 | ||
RR, relative risk; CI, confidence interval. Data are n (%) unless otherwise specified.
Controlled for maternal age, number of prior spontaneous miscarriages, number of prior preterm births, number of prior full-term births, number of prior assisted reproductive technology cycles, use of intracytoplasmic sperm injection, use of assisted hatching, the embryo stage at transfer, the number of embryos transferred, the number of supernumerary embryos cryopreserved, the number of oocytes retrieved, and year.
The number of cycles with no miscarriage was 33,762 among the tubal factor group and 67,558 among the male factor group.
Percent any miscarriage=100×number any miscarriage/(number any miscarriage+number no miscarriage); percent early miscarriage=100×number early miscarriage/(number early miscarriage+number no miscarriage); percent late miscarriage=100×number late miscarriage/(number late miscarriage+number no miscarriage).
Singletons and twins born to women with tubal factor infertility were found to have an increased risk of preterm delivery (Table 3). Among singletons, the bivariate results indicated that the preterm birth rate for neonates born to women with tubal disease was 15.8% compared with 11.6% in the male factor group, (RR 1.37, 95% CI 1.32–1.43, P<.001). An increased risk was observed for early (RR 1.67, 95% CI 1.52–1.84, P<.001) and late (RR 1.34, 95% CI 1.28–1.40, P<.001) preterm birth. The increased risk for preterm delivery did not substantially change after controlling for maternal characteristics (adjusted RR 1.27, 95% CI 1.20–1.34, P<.001). Similarly, among twin births, an increased risk of preterm delivery was detected for neonates born to women with tubal factor, although the magnitude of the increased risk was smaller. For the bivariate analysis, again, the most notable increase in risk was for early preterm birth (RR 1.32, 95% CI 1.24–1.40, P<.001). The adjusted relative risk estimate from multivariable analysis of the twin data did not differ from the unadjusted estimate (adjusted RR 1.06, 95% CI 1.03–1.08, P<.001). For triplets, there was no evidence of a significant increase in risk of preterm birth among neonates born to women with tubal factor as compared with male factor infertility. The relative risk approached one for all bivariate and multivariable results for triplet pregnancies.
Table 3.
Association Between Infertility and Preterm Birth for Fresh, Nondonor In Vitro Fertilization Cycles Resulting in Pregnancy, Stratified by Number of Fetuses, 2000–2010
| No. of Births | Outcome | Tubal Factor†‡ | Male Factor†‡ | Bivariate Analysis
|
Multivariable Analysis*
|
||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P | Adjusted RR (95% CI) | P | ||||
| Singleton | Any preterm birth | 3,432 (15.83) | 5,131 (11.55) | 1.37 (1.32–1.43) | <.001 | 1.27 (1.20–1.34) | <.001 |
| Early preterm birth | 715 (3.77) | 907 (2.26) | 1.67 (1.52–1.84) | <.001 | |||
| Late preterm birth | 2,717 (12.96) | 4,224 (9.71) | 1.34 (1.28–1.40) | <.001 | |||
| Twins | Any preterm birth | 13,214 (64.03) | 24,550 (60.23) | 1.06 (1.04–1.08) | <.001 | 1.06 (1.03–1.08) | <.001 |
| Early preterm birth | 2,552 (25.58) | 3,908 (19.42) | 1.32 (1.24–1.40) | <.001 | |||
| Late preterm birth | 10.662 (58.95) | 20,642 (56.01) | 1.05 (1.03–1.08) | <.001 | |||
| Triplets or more | Any preterm birth | 2,903 (96.7) | 4,671 (97.17) | 0.99 (0.98–1.01) | .48 | 0.99 (0.98–1.01) | .428 |
| Early preterm birth | 1,110 (91.8) | 1,735 (92.73) | 0.99 (0.90–1.03) | .56 | |||
| Late preterm birth | 1,793 (94.77) | 2,936 (95.57) | 0.99 (0.97–1.01) | .44 | |||
RR, relative risk; CI, confidence interval.
Any preterm birth, 20 to less than 37 wk of gestation; early PTB, 20 to less than 32 wk of gestation; late preterm birth, 32 to less than 37 wk of gestation.
Controlled for maternal age, number of prior spontaneous miscarriages, number of prior preterm births, number of prior full-term births, number of prior assisted reproductive technology cycles, use of intracytoplasmic sperm injection, use of assisted hatching, the embryo stage at transfer, the number of embryos transferred, the number of supernumerary embryos cryopreserved, the number of oocytes retrieved, and year.
For singletons, the number of full-term fetuses was 18,245 among the tubal factor group and 39,299 among the male factor group; for twins, 7,424 among the tubal factor group and 16,212 among the male factor group; for triplets, 99 among the tubal factor group and 136 among the male factor group.
Percent any preterm birth=100×number any preterm birth/(number any preterm birth+number full term); percent early preterm birth-=100×number early preterm birth/(number early preterm birth+number full term); percent late preterm birth=100×number late pre-term birth/(number late preterm birth+number full term).
Similarly, singletons and twins born to women with tubal factor infertility were found to have an increased risk of delivering a LBW neonate (Table 4). Among singleton pregnancies, LBW delivery was more likely for neonates born to women with tubal factor compared with male factor infertility (RR 1.28, 95% CI 1.22–1.34, P<.001). An increased risk was also observed for very low (RR 1.70, 95% CI 1.52–1.90, P<.001) and moderately low (RR 1.21, 95% CI 1.14–1.28, P<.001) birth weight. The relative risk estimate for LBW delivery from the multivariable model did not differ from the unadjusted estimate (adjusted RR 1.28, 95% CI 1.20–1.36, P<.001). Among twin births, again, neonates born to women with tubal factor had an increased risk of LBW delivery, although the magnitude of the risk estimate was smaller. For the bivariate analysis, the most notable increase in risk was for very LBW (RR 1.22, 95% CI 1.15–1.30, P<.001). The multivariable analysis also indicated a significantly increased risk in LBW delivery (adjusted RR 1.08, 95% CI 1.05–1.10, P<.001) for twins after adjusting for maternal characteristics. For triplets, there was no evidence of a significant increase in risk of LBW delivery among those neonates born to mothers with tubal factor as compared with male factor infertility. The relative risk approached one for all bivariate and multivariable results for triplet pregnancies.
Table 4.
Association Between Infertility and Low Birth Weight for Fresh, Nondonor In Vitro Fertilization Cycles Resulting in Pregnancy, Stratified by Number of Fetuses, 2000–2010
| No. of Births | Outcome | Tubal Factor†‡ | Male Factor†‡ | Bivariate Analysis
|
Multivariable Analysis*
|
||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P | Adjusted RR (95% CI) | P | ||||
| Singleton | Any LBW | 2,310 (10.86) | 3,708 (8.49) | 1.28 (1.22–1.34) | <.001 | 1.28 (1.20–1.36) | <.001 |
| Very LBW | 538 (2.76) | 661 (1.63) | 1.70 (1.52–1.90) | <.001 | |||
| Moderate LBW | 1,772 (8.55) | 3,047 (7.08) | 1.21 (1.14–1.28) | <.001 | |||
| Twins | Any LBW | 11,692 (58.13) | 22,238 (55.83) | 1.04 (1.01–1.06) | <.001 | 1.08 (1.05–1.10) | <.001 |
| Very LBW | 2,042 (19.52) | 3,238 (15.55) | 1.22 (1.15–1.30) | <.001 | |||
| Moderate LBW | 9,650 (53.40) | 19,000 (51.93) | 1.03 (1.01–1.05) | .007 | |||
| Triplets or more | Any LBW | 2,747 (94.82) | 4,377 (94.8) | 1.00 (0.99–1.01) | .99 | 1.01 (0.99–1.02) | .574 |
| Very LBW | 964 (86.54) | 1,551 (86.6) | 1.00 (0.95–1.05) | .995 | |||
| Moderate LBW | 1,783 (92.24) | 2,826 (92.17) | 1.00 (0.98–1.02) | .98 | |||
RR, relative risk; CI, confidence interval, LBW, low birth weight.
Any LBW, less than 2,500 g; very LBW, less than 1,500 g; moderate LBW (1,500–2,500 g).
Controlled for maternal age, number of prior spontaneous miscarriages, number of prior preterm births, number of prior full-term births, number of prior assisted reproductive technology cycles, use of intracytoplasmic sperm injection, use of assisted hatching, the embryo stage at transfer, the number of embryos transferred, the number of supernumerary embryos cryopreserved, the number of oocytes retrieved, and year.
For singletons, the number of full-term fetuses was 18,958 among the tubal factor group and 39,985 among the male factor group; for twins, 8,421 among the tubal factor group and 17,591 among the male factor group; for triplets, 150 among the tubal factor group and 240 among the male factor group.
Percent any LBW=100×number any LBW/(number any LBW+number full term); percent very LBW=100×number very LBW/(number very LBW+number full term); percent moderate LBW=100×number moderate LBW/(number moderate LBW+number full term).
The relationships between tubal factor and miscarriage, preterm delivery, and LBW persisted when race was incorporated into the multivariable analysis. More specifically, the adjusted RR for miscarriage changed minimally from 1.08 to 1.06 (95% CI 1.01–1.12, P=.031) after also controlling for race or ethnicity in the multivariate model. The adjusted RR for pre-term delivery changed only slightly for singleton, twin, and triplet neonates when controlling for race or ethnicity. Among singletons, the adjusted RR decreased slightly from 1.27 to 1.20 (95% CI 1.12–1.28, P<.001); among twins, the adjusted RR remained the same at 1.06 (95% CI 1.02–1.09, P=.001); among triplets, the adjusted RR also remained the same at 0.99 (95% CI 0.97–1.01, P=0.22). Similarly, the heightened risk of LBW delivery among singleton neonates born to women with tubal factor remained significant but of a slightly smaller magnitude with the adjusted RR decreasing from 1.28 to 1.15 (95% CI 1.06–1.25, P=.001) after including race or ethnicity. For twins, the adjusted RR also decreased slightly from 1.08 to 1.04 (95% CI 1.01–1.07, P=.011), whereas for triplets, the adjusted RR was not significant.
DISCUSSION
The annual percentage of ART cycles associated with a primary or secondary diagnosis of tubal factor infertility decreased from 2000 to 2010. Our study suggests that tubal factor portended a small but significant increased risk of any, early, and late miscarriage. Our results also suggest that singleton neonates born to women with tubal factor infertility had an increased risk of preterm and LBW delivery. These findings persisted for early and late preterm delivery and for very low and moderately LBW delivery. Notably, the greatest magnitude of increased risk was observed for the most severe categories of the outcomes, namely late miscarriage, early preterm birth, and very LBW. The associations remained significant for twin but not triplet pregnancies.
The temporal decrease in the annual percentage of ART cycles performed secondary to tubal factor is likely the result of an increasing number of alternative indications for use of ART making tubal factor a smaller portion of total cycles performed. The absolute number of tubal factor patients has also decreased slightly over the 11-year period but to a lesser degree than the percentage. This decline may result from improved treatment of pelvic infection because the rates of pelvic inflammatory disease in the United States have been trending downward.24
Our results are consistent with the previously described relationship between hydrosalpinx present at the time of IVF and increased risk of spontaneous miscarriage.2,3,5–7 Our observed risk is likely underestimated. As a result of limitations in categorizing detailed tubal status, our study population included not only patients with untreated hydrosalpinx, but also those with a history of hydrosalpinx who have undergone a salpingectomy, tubal ligation, or hysteroscopic tubal occlusion. When clinicians submit data to the CDC, it is feasible that they could have placed a patient with a history of excised or occluded hydrosalpinx in any of these tubal categories: “tubal factor,” “tubal ligation, not reversed,” or “other tubal disease, not hydrosalpinx.” Those with treated hydrosalpinx may have a lower risk of miscarriage and poor perinatal outcomes compared with their untreated counterparts. Moreover, patients with untreated hydrosalpinx may be at an increased risk, potentially greater than that calculated in this study, of not only miscarriage, but also of delivering a preterm or LBW neonate.
Increased risk of delivering a preterm or LBW neonate was most notable in singleton pregnancies. This difference likely reflects the innate risk of preterm and LBW delivery associated with multiple gestations, making the contribution of tubal factor to poor outcomes less apparent in twin and, more so, in triplet pregnancies. Additionally, because our control group was also infertile and used ART, the observed association between tubal factor and perinatal outcomes would likely be even greater if it were compared with spontaneously conceived neonates who, as a group, do not possess the elevated risk of preterm and LBW delivery detected for ART-conceived neonates.15–20
As with any study using a national database, we are limited by the accuracy of data entered by individual clinics. Additionally, because data collection is cycle-based and is not linked over time, women who underwent more than one fresh IVF cycle that resulted in a pregnancy in the 11-year period would likely have been included more than once in these data. Ideally, we would also have controlled for additional medical and social history characteristics such as presence or absence of hypertensive disorder or diabetes, patient body mass index, or tobacco use status. The CDC recently has begun to collect some of this additional data, but it was unavailable for all study years of interest. Future studies incorporating more detailed patient information may help to affirm our current findings.
The study is strengthened by the large sample size and by the high compliance of clinics with nationally mandated fertility clinic reporting. We were able to control for maternal age, obstetric history, number of prior ART cycles, number of oocytes retrieved, use of intracytoplasmic sperm injection or assisted hatching, embryo stage and number at transfer, number of supernumerary embryos cryopreserved, number of fetal heartbeats, and year of treatment in our multivariable analysis. Additionally, despite the large proportion of missing data for patient race and ethnicity, we were able to perform a secondary analysis incorporating race. This affirmed our initial findings and suggests that race is not a confounding factor in the elevated risk associated with tubal disease.
Women with tubal disease are at known risk of pregnancy failure and miscarriage15–20; our findings suggest they may also be at increased risk of delivering preterm or LBW neonates. The apparent further heightened risk of the more severe of these perinatal outcomes, namely, late miscarriage and early pre-term and very LBW delivery, warrants further study because additional monitoring or follow-up with a maternal fetal medicine specialist may be indicated. Prior pelvic infection with resultant tubal disease has been shown to be associated with persistently elevated chlamydial IgG antibody titers and with heat shock proteins that act as proinflammatory mediators suggesting a long-lasting inflammatory state among women with history of pelvic infection.8,9 An infectious or inflammatory state may contribute to pre-term delivery; our findings lend support to the need for basic science research exploring the etiology and pathophysiology of these poor obstetric outcomes, which could potentially help address calls from the World Health Organization and the March of Dimes to decrease preterm delivery.25 Finally, our findings provide evidence regarding potential sequelae of untreated cervicitis. Ascending pelvic infection and resultant tubal disease contribute not only to infertility, but may also contribute to poor obstetric outcomes, both of which may be prevented by physician compliance with sexually transmitted infection screening and prompt treatment of pelvic infection.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Miller JH, Weinberg RK, Canino NL, Klein NA, Soules MR. The pattern of infertility diagnoses in women of advanced reproductive age. Am J Obstet Gynecol. 1999;181:952–7. doi: 10.1016/s0002-9378(99)70331-5. [DOI] [PubMed] [Google Scholar]
- 2.Andersen AN, Yue Z, Meng FJ, Petersen K. Low implantation rate after in-vitro fertilization in patients with hydrosalpinges diagnosed by ultrasonography. Hum Reprod. 1994;9:1935–8. doi: 10.1093/oxfordjournals.humrep.a138362. [DOI] [PubMed] [Google Scholar]
- 3.Camus E, Poncelet C, Goffinet F, Wainer B, Merlet F, Nisand I, et al. Pregnancy rates after in-vitro fertilization in cases of tubal infertility with and without hydrosalpinx: a meta-analysis of published comparative studies. Hum Reprod. 1999;14:1243–9. doi: 10.1093/humrep/14.5.1243. [DOI] [PubMed] [Google Scholar]
- 4.Strandell A, Waldenstrom U, Nilsson L, Hamberger L. Hydrosalpinx reduces in-vitro fertilization/embryo transfer pregnancy rates. Hum Reprod. 1994;9:861–3. doi: 10.1093/oxfordjournals.humrep.a138606. [DOI] [PubMed] [Google Scholar]
- 5.Vandromme J, Chasse E, Lejeune B, Van Rysselberge M, Delvigne A, Leroy F. Hydrosalpinges in in-vitro fertilization: an unfavourable prognostic feature. Hum Reprod. 1995;10:576–9. doi: 10.1093/oxfordjournals.humrep.a135992. [DOI] [PubMed] [Google Scholar]
- 6.Zeyneloglu HB, Arici A, Olive DL. Adverse effects of hydrosalpinx on pregnancy rates after in vitro fertilization-embryo transfer. Fertil Steril. 1998;70:492–9. doi: 10.1016/s0015-0282(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MA, Lindheim SR, Sauer MV. Hydrosalpinges adversely affect implantation in donor oocyte cycles. Hum Reprod. 1999;14:1087–9. doi: 10.1093/humrep/14.4.1087. [DOI] [PubMed] [Google Scholar]
- 8.Stephens AJ, Aubuchon M, Schust DJ. Antichlamydial antibodies, human fertility, and pregnancy wastage. Infect Dis Obstet Gynecol. 2011;2011:525182. doi: 10.1155/2011/525182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linhares IM, Witkin SS. Immunopathogenic consequences of Chlamydia trachomatis 60 kDa heat shock protein expression in the female reproductive tract. Cell Stress Chaperones. 2010;15:467–73. doi: 10.1007/s12192-010-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40:1837–45. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 11.Klein LL, Gibbs RS. Infection and preterm birth. Obstet Gynecol Clin North Am. 2005;32:397–410. doi: 10.1016/j.ogc.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–78. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Joshi N, Kissin D, Anderson JE, Session D, Macaluso M, Jamieson DJ. Trends and correlates of good perinatal outcomes in assisted reproductive technology. Obstet Gynecol. 2012;120:843–51. doi: 10.1097/AOG.0b013e318269c0e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 15.Dhont M, De Sutter P, Ruyssinck G, Martens G, Bekaert A. Perinatal outcome of pregnancies after assisted reproduction: a case-control study. Am J Obstet Gynecol. 1999;181:688–95. doi: 10.1016/s0002-9378(99)70514-4. [DOI] [PubMed] [Google Scholar]
- 16.Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–7. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- 17.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A, et al. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–48. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 18.Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328:261. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–63. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 20.Halliday J. Outcomes of IVF conceptions: are they different? Best Pract Res Clin Obstet Gynaecol. 2007;21:67–81. doi: 10.1016/j.bpobgyn.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Adashi EY, Wyden R. Public reporting of clinical outcomes of assisted reproductive technology programs: implications for other medical and surgical procedures. JAMA. 2011;306:1135–6. doi: 10.1001/jama.2011.1249. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention ASfRM, Society for Assisted Reproductive Technology. 2010 assisted reproductive technology fertility clinic success rates report. Atlanta (GA): U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 23.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 24.Sutton MY, Sternberg M, Zaidi A, St Louis ME, Markowitz LE. Trends in pelvic inflammatory disease hospital discharges and ambulatory visits, United States, 1985–2001. Sex Transm Dis. 2005;32:778–84. doi: 10.1097/01.olq.0000175375.60973.cb. [DOI] [PubMed] [Google Scholar]
- 25.Born too soon: the global action report on preterm birth. Geneva (Switzerland): March of Dimes, PMNCH, Save the Children, WHO; 2012. [Google Scholar]


