Abstract
We utilized a transgenic mouse model where nuclear factor kappa B (NF-κB) is selectively inhibited in glial fibrillary acidic protein (GFAP) expressing cells. The transgene, GFAP-IκBα-dn, overexpresses a dominant negative form of the inhibitor of NF-κB (IκBα) under the control of the GFAP promoter. In the present work, we sought to understand the impact of glial NF-κB inhibition on the expression of pain mediating sensory neuropeptides galanin and calcitonin gene related peptide (CGRP) in a model of neuropathic pain in mice. Chronic constriction injury (CCI) of the left sciatic nerve was performed on wild type (WT) and GFAP-IκBα-dn transgenic mice. RT-PCR and immunohistological staining were performed in sciatic nerve and/or L4-L5 DRG tissue for galanin, CGRP and macrophage marker CD11b. GFAP-IκBα-dn mice had less mechanical and thermal hyperalgesia compared to WT mice post-CCI. After CCI, we observed galanin upregulation in DRG and sciatic nerve, which was less in GFAP-IκBα-dn mice. CGRP gene expression in the DRG increased transiently on day 1 post-CCI in WT but not in GFAP-IκBα-dn mice, and no evidence of CGRP upregulation in sciatic nerve post-CCI was found. After CCI, upregulation of CD11b in sciatic nerve was less in GFAP-IκBα-dn mice compared to WT mice, indicative of less macrophage infiltration. Our results showed that glial NF-κB inhibition reduces galanin and CGRP expression, which are neuropeptides that correlate with pain behavior and inflammation after peripheral nerve injury.
Keywords: Inflammation, Astrocytes, Neuropathic pain, Chronic constriction injury
1. Introduction
Behavioral and electrophysiological studies show that galanin inhibits the transmission of nociceptive information in the spinal cord (Wiesenfeld-Hallin and Xu, 2001; Zhang et al., 2000a; Zhang et al., 2000b). Upregulation of galanin expression in dorsal root ganglion (DRG) neurons occurs in various peripheral nerve lesion models including chronic constriction injury (CCI) of the sciatic nerve, axotomy, and partial sciatic nerve transection (Ma and Bisby, 1997; Villar et al., 1989; Hokfelt et al., 1987). Holmberg et al. (2005) showed that galanin over-expressing mice had a reduction in the total number of DRG neurons lost following axotomy compared to WT mice, indicating a rescue effect from the high levels of galanin after peripheral nerve injury. The effect of inflammatory factors on galanin expression has not been thoroughly investigated, but several reports have shown that less upregulation of galanin expression in the DRG or dorsal horn occurs after peripheral nerve injury in interleukin-6 (IL-6) knockout and leukemia inhibitory factor (LIF) knockout mice (Ramer et al., 1998; Corness et al., 1996).
Calcitonin gene related peptide (CGRP) is another neuropeptide which is produced in small diameter neurons in the dorsal root ganglion (DRG) giving rise to unmyelinated (C) and thinly myelinated (Aδ) fibers (Zhang et al., 1997). In fact, primary afferent fibers have been implicated in the transmission of pain, temperature sensation, and other modalities including noxious and non-noxious mechanical stimuli (Klein et al., 1990). Spinal application of CGRP facilitates nociceptive behavior (Bird et al., 2006; Oku et al., 1987; Sun et al., 2004) and sensitizes the responses of dorsal horn neurons to innocuous and noxious peripheral stimulation (Sun et al., 2004; Biella et al., 1991). Furthermore, Lee and Kim (2007) showed that CGRP from central terminals of intact sensory neurons after peripheral nerve injury contributes to the development and maintenance of neuropathic pain. However, Na et al. (2001) provided data indicating that spinal CGRP levels will decrease after peripheral nerve injury and their data showed that the decrease in spinal CGRP levels after peripheral nerve injury are not related to neuropathic pain (Na et al., 2001). While Schafers et al. (2002) reports that tumor necrosis factor (TNF) and CGRP are transported to the injured sciatic nerve in an anterograde fashion, the relationship between CGRP expression and the inflammatory response is unclear.
The transcriptional regulator nuclear factor kappa B (NF-κB) plays a major role in regulating inflammatory mediators and nociceptive sensory responses after peripheral nerve injury (Niederberger and Geisslinger, 2008). Previously, we showed that transgenic glial NF-κB inhibition decreased formalin pain (Fu et al., 2007) and mechanical/thermal hyperalgesia after chronic constriction injury (CCI) of the sciatic nerve (Fu et al., 2010). In those studies, the transgene GFAP-IκBα-dn overexpresses the dominant negative (dn) form of the inhibitor of kappa B alpha (IκBα) in glial cells, under the control of the glial fibrillary acidic protein (GFAP) promoter (Brambilla et al., 2005). The GFAP-IκBα-dn transgene is present not only in astrocytic glial cells in the CNS but also in peripheral glial cells such as non-myelinating Schwann cells (Jessen et al., 1990; Triolo et al., 2006) and DRG satellite cells (Miyagi et al., 2006; Ohtori et al., 2004; Woodham et al., 1989). We also showed that glial NF-κB inhibition decreased cytokine and chemokine inflammation in DRG and sciatic nerve following CCI of the sciatic nerve (Fu et al., 2010). While inflammation of the peripheral nervous system appears to trigger the changes of sensory neuropeptide release, it remains unclear what happens to galanin and CGRP expression in specific situations in which NF-κB is downregulated. Therefore, we sought to determine the effects of selective inactivation of NF-κB in glial cells on galanin and CGRP expression after peripheral nerve injury.
2. Results
2.1. Mechanical and thermal hyperalgesia
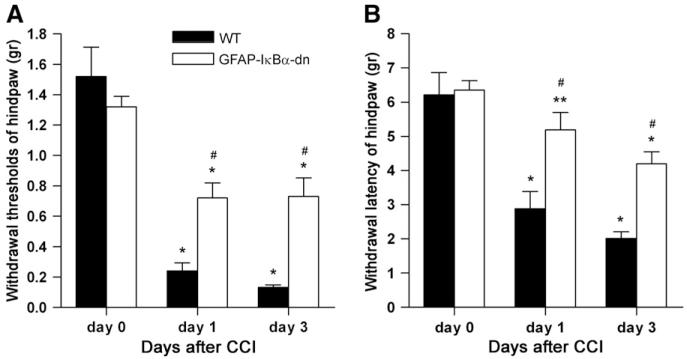
Pre-surgical mechanical thresholds and thermal latencies were similar in both WT and GFAP-IκBα-dn animals (Fig. 1). In WT mice, chronic constriction of the sciatic nerve led to a neuropathic pain syndrome characterized by a marked reduction of the paw withdrawal thresholds to mechanical and thermal stimuli on days 1 and 3 post-CCI. GFAP-IκBα-dn mice also showed a reduction in withdrawal thresholds to mechanical and thermal stimuli, but mechanical and thermal hyperalgesia were significantly less compared to WT mice. A longer time course describing the mechanical and thermal behavior in WT and GFAP-IκBα-dn mice after CCI is described in our previous work (Fu et al., 2010).
Fig. 1.
(A) Withdrawal thresholds for the affected paw (hindpaw) at baseline (day 0), day 1 and day 3 post-CCI in WT and GFAP-IκBα-dn mice. (B) Withdrawal latencies to radiant heat of the affected paw at baseline (day 0), day 1 and day 3 post-CCI in WT and GFAP-IκBα-dn mice. Values are expressed as mean±SEM of 6–10 animals per group. *p<0.001, **p<0.05, vs. corresponding day 0; #p<0.001 vs. corresponding WT, one-way ANOVA Tukey test.
2.2. Galanin and CGRP gene expression in the DRG following CCI
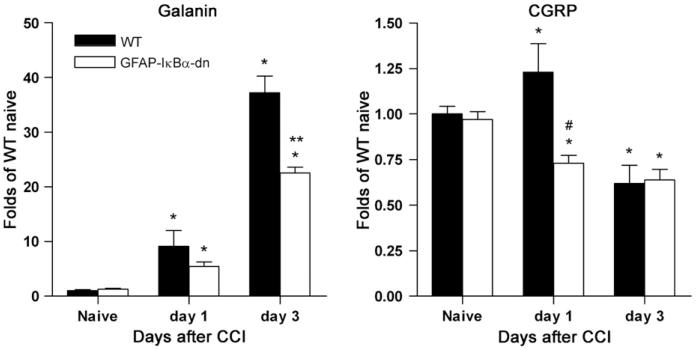
The expression of galanin and CGRP was evaluated by real-time RT-PCR at 1 and 3 days following nerve injury in the DRGs of WT and GFAP-IκBα-dn mice. In both WT and transgenic mice a significant time-dependent upregulation of galanin was observed as a consequence of nerve injury (Fig. 2). However, such increase was significantly reduced in GFAP-IκBα-dn mice compared to WT at 3 days post-CCI, when the peak of gene expression occurred (37.21±2.88 vs. 22.57±0.79; p<0.001, one-way ANOVA, Tukey test). As for CGRP, a rather small but significant difference in gene expression was detected at 1 day post-injury between WT and transgenic mice, with a transient increase in WT mice which was completely prevented in GFAP-IκBα-dn mice (1.23±0.16 vs. 0.73±0.04; p<0.05, one-way ANOVA, Tukey test). On day 3 after CCI, gene expression was downregulated in both WT and GFAP-IκBα-dn mice compared to naïve mice and no intergroup difference was observed between the genotypes.
Fig. 2.
Galanin and CGRP gene expression in DRGs from WT and GFAP-IκBα-dn mice following CCI. *p<0.05 vs. corresponding naïve; **p<0.05 vs. WT day 3; #p<0.05 vs. WT day 1, one-way ANOVA Tukey test.
2.3. Immunohistochemical detection of galanin, CGRP and CD11b following CCI
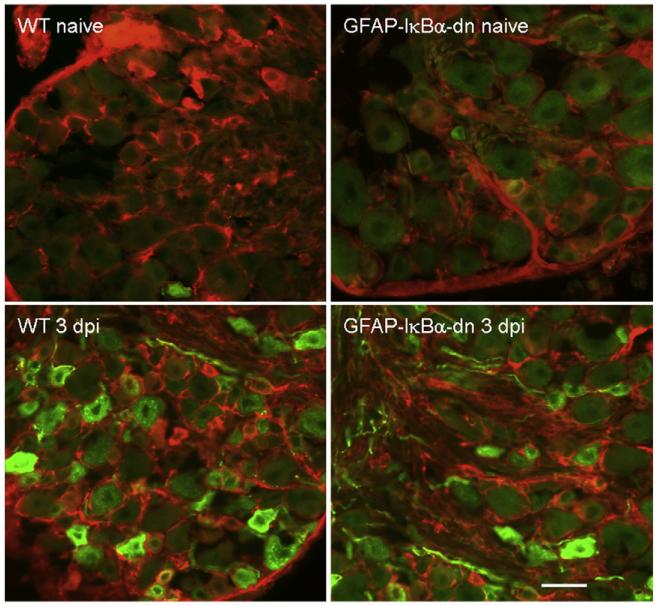
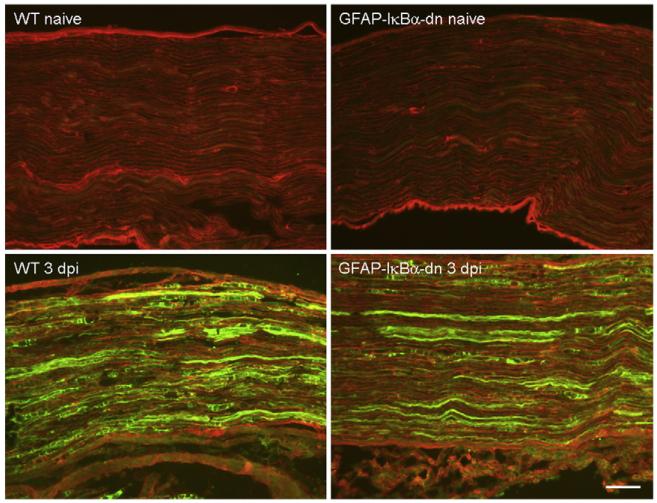
We examined galanin expression by immunohistochemistry in DRG and sciatic nerve sections of naïve and injured mice (day 3 post-CCI) double-labeled with an antibody against GFAP. In naïve animals, no differences were found in galanin immunostaining between WT and GFAP-IκBα-dn mice. Following CCI, galanin immunostaining increased in DRG (Fig. 3) and sciatic nerve (Fig. 4) of both WT and GFAP-IκBα-dn mice. However, expression was significantly reduced in GFAP-IκBα-dn mice compared to WT, as determined by quantification of the galanin immunoreactivity (Table 1).These results are consistent with the gene expression data obtained by RT-PCR (Fig. 2). We also evaluated by immunohistochemistry the expression of CGRP and, although we found expression both in sciatic nerve and DRG, we did not detect changes as a result of CCI in either genotype (Table 1). Furthermore, no differences were found between WT and transgenic mice in both naïve and injured conditions (Table 1).
Fig. 3.
Immunofluorescent staining of galanin (green) in combination with GFAP (red) in the DRGs of WT and GFAP-IκBα-dn mice 3 days after CCI; dpi=days post-injury. Scale bar=50 μm.
Fig. 4.
Immunofluorescent staining of galanin (green) in combination with GFAP (red) in the sciatic nerve of WT and GFAP-IκBα-dn mice 3 days after CCI; dpi=days post-injury. Scale bar=100 μm.
Table 1.
Quantitative analysis of galanin and CGRP immunostaining in DRGs and sciatic nerves of WT and GFAP-IκBα-dn mice at day 0 (Naive) and day 3 following CCI (CCI 3 dpi).
| DRG (% of total DRG neurons) |
Sciatic nerve (signal intensity) |
||||
|---|---|---|---|---|---|
| WT | GFAP-IκBα-dn | WT | GFAP-IκBα-dn | ||
| Galanin | Naive | 7.80±1.63 | 7.61±1.10 | 10.97±0.26 | 10.57±1.14 |
| CCI 3 dpi | 24.71±2.19* | 18.30±2.02*,* | 27.27±1.89* | 19.21±0.67*,* | |
| CGRP | Naive | 19.40±2.04 | 20.10±2.25 | 12.27±2.29 | 10.95±2.67 |
| CCI 3 dpi | 18.71±1.69 | 19.80±1.86 | 8.78±0.41 | 9.28±0.55 | |
In the DRG, analysis was conducted by stereological quantification of the number of positive neurons and results are expressed as percent of total number of DRG neurons. In the sciatic nerve, analysis was conducted by measuring the intensity of the immunofluorescence signal with the software NIH Image J. Four animals per group were analyzed; dpi: days post-injury.
p <0.05 vs. corresponding naïve
p<0.05 vs. WT at CCI 3 dpi, one-way ANOVA, Tukey test.
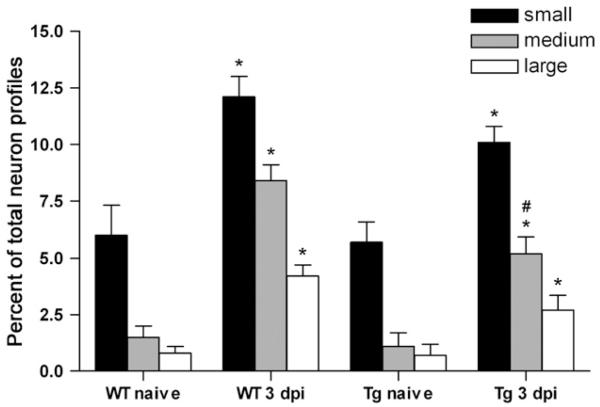
In order to determine whether the observed changes in the DRG were specific to a subpopulation of neurons, the distribution of galanin-positive neuron profiles was determined in naïve and injured mice. On day 3 after CCI, an increase in the number of galanin expressing neuron profiles was detected in all cell sizes in the DRGs of WT and GFAP-IκBα-dn mice (Fig. 5). However, such increases were significantly lower in GFAP-IκBα-dn mice compared to WT. Particularly, the percentage of medium size galanin-positive neuron profiles was significantly greater in WT mice than in GFAP-IκBα-dn mice (8.40±0.71 vs. 5.20±0.73; p<<0.05, one-way ANOVA, Tukey test).
Fig. 5.
Quantification of the numbers of galanin-positive neuron profiles in the DRG of WT and GFAP-IκBα-dn mice following CCI. DRG neuron profiles are defined as small (<600 μm2), medium (between 600 and 1200 μm2), and large (>1200 μm2) according to their cross-sectional area. Results are expressed as percent±SEM of total neuron profiles. *p<0.05, compared to corresponding naïve; #p<0.05, compared to WT same size (medium) neuron profiles.
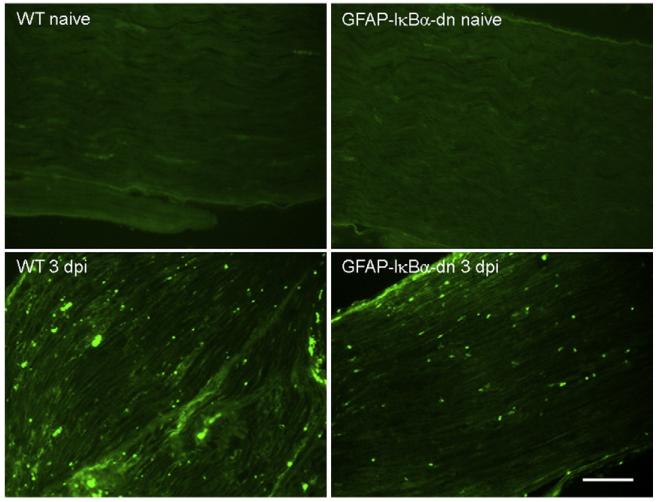
Since macrophage infiltration of the sciatic nerve following CCI could be associated with nerve degeneration and development of neuropathic pain, we determined the presence of invading macrophages by CD11b immunostaining. CD11b positive cells were detected in both WT and GFAP-IκBα-dn mice on day 3 post-CCI (Fig. 6). However, quantification of the numbers of CD11b positive cells showed that the extent of infiltration was significantly reduced in GFAP-IκBα-dn mice compared to WT (WT=48.88±4.68 vs. GFAP-IκBα-dn=33.75± 3.13, p<0.01, one-way ANOVA). Furthermore, minimal presence of CD11b positive cells was found in naïve mice and no difference between the two genotypes was detected (WT=5.50±0.42 vs. GFAP-IκBα-dn=4.65±0.60).
Fig. 6.
Immunofluorescent staining of CD11b positive cells in the sciatic nerve of WT and GFAP-IκBα-dn mice 3 days after CCI; dpi=days post-injury. Scale bar=100 μm.
3. Discussion
Immune and inflammatory responses occurring in an injured nerve have been generally believed to contribute to the generation and maintenance of neuropathic pain. In this study, glial NF-κB inhibition reduced neuropathic pain at 1 and 3 days after CCI with reduced galanin expression in the DRG and sciatic nerve on day 3. On day 1 after CCI, upregulation of CGRP mRNA was not present in GFAP-IκBα-dn mice in the DRG. Therefore, glial NF-κB inhibition appears to decrease galanin and CGRP expression in DRG, implicating inflammatory mediators downstream of NF-κB activation as important regulators of not only neurobehavioral responses but also neuropeptide expression following CCI. We chose to measure expression of these neuropeptides in DRG and sciatic nerve since our previous study showed that glial NF-κB inhibition altered cytokine and chemokine expression in these tissues (Fu et al., 2010).
In the adult rat, galanin is expressed in a population of small neurons in DRG under normal circumstances (Skofitsch and Jacobowitz, 1985). In WT mice, we found substantial upregulation of galanin mRNA in DRG on day 3 after CCI. Our immunohistochemistry findings showed that at this time point, sciatic nerve injury induced galanin upregulation in small, medium and large size dorsal root ganglion neurons in WT mice. This is in agreement with reports from other investigators, who found that, following peripheral nerve injury, increases in galanin immunoreactivity occur in large and intermediate DRG neurons (Corness et al., 1996; Ma and Bisby, 1997). Galanin was found to be upregulated also in GFAP-IκBα-dn mice, although to a lesser degree, specifically in small and medium (not in large) DRG neurons after CCI. Galanin immunoreactivity in the sciatic nerve also increased following CCI in both WT and GFAP-IκBα-dn mice, but again the increase was significantly lower in GFAP-IκBα-dn mice compared to WT. Based on these findings, it appears that selective suppression of NF-κB in glial cells attenuates galanin upregulation in the peripheral nervous system in response to injury. This, particularly in the medium-sized DRG population, may be explained by the localization of the injury on the peripheral nerve, and nerve fibers projecting from medium-sized DRG neurons (likely Aδ fibers) are severely damaged.
Our previous work (Fu et al., 2010) showed that upregulation of NF-κB-dependent cytokines and chemokines in the sciatic nerve was significantly reduced in GFAP-IκBα-dn mice compared to their WT counterpart. This could potentially explain the parallel reduction in the expression of galanin which, as several authors have previously reported, is regulated by proinflammatory cytokines following nerve injury. Indeed, Shadiack et al. (1998) found that galanin expression in sympathetic and sensory neurons is dependent on the induction of the cytokine LIF. Injection of LIF and IL6 into the intact sciatic nerve of adult rats significantly increases the percentage of immunoreactive galanin neurons after sciatic nerve axotomy in the L4 and L5 DRGs (Thompson et al., 1998). Furthermore, IL6 knockout mice show decreased galanin expression in the spinal cord (Ramer et al., 1998). These data suggest that NF-κB-dependent proinflammatory cytokines can modulate the expression of galanin and support the hypothesis that in GFAP-IκBα-dn mice the reduced expression of galanin could result from the suppression of NF-κB-dependent cytokines (Fu et al., 2010).
Although the precise role played by galanin in the peripheral nervous system remains unclear, galanin has inhibitory effects on nociceptive transmission, and high levels of galanin have a rescue effect on neurons after peripheral nerve injury (Holmberg et al., 2005; Holmes et al., 2003). Based on this evidence, it would be reasonable to expect an upregulation of galanin in association with reduced neuropathic pain behavior. In our GFAP-IκBα-dn mice, however, despite the reduced pain (Fu et al., 2010), we found a significantly reduced galanin expression compared to WT mice. Similar effects were reported by Ramer et al. (1998), who found that in IL6 knockout mice displaying reduced neuropathic pain behavior, galanin immunoreactivity was also reduced as compared to WT mice. It appears that galanin upregulation occurs as a natural protection of nerve cells against degeneration after injury. However, since NF-κB inhibition limits such upregulation while still significantly reducing neuropathic pain, we can conclude that the protective effect of directly limiting the inflammatory response by blocking NF-κB in GFAP expressing cells is sufficient to attenuate the pain response, overwriting the potentially neuroprotective effect of galanin.
Our findings also revealed a mild but significant increase in CGRP gene expression in the DRGs of WT mice on day 1 after CCI, which did not occur in GFAP-IκBα-dn mice. On day 3, down-regulation of CGRP was present in both genotypes. Nahin et al. (1994) observed in rats that CGRP gene expression was initially upregulated after CCI followed by downregulation of CGRP gene expression 7 days after CCI. These changes in CGRP gene expression, however, did not translate into increased protein expression levels. Previous reports by Schafers et al. (2003) and Ma and Bisby (1998) also showed that CGRP protein immunoreactivity was not increased following CCI even though CGRP immunostaining was present in the DRG of naïve mice.
The inflammatory response following CCI also involves the recruitment of leukocytes into the sciatic nerve (Gomez-Nicola et al., 2008). We found that following CCI the number of CD11b positive cells in the sciatic nerve was lower in GFAP-IκBα-dn mice compared to WT, suggesting a reduced macrophage infiltration as a consequence of NF-κB inactivation. Even though the relationship between leukocyte recruitment and sensory neuropeptide regulation remains unclear, several studies have shown that upregulation of galanin accompanies macrophage infiltration following nerve injury (Wallace et al., 2007; Peters et al., 2005). Our data are in agreement with these reports and, when galanin upregulation is reduced as in our GFAP-IκBα-dn mice, so is the presence of CD11b positive macrophages.
In conclusion, our findings show that selective NF-κB inhibition in GFAP expressing cells reduces galanin and CGRP expression along with attenuation of neuropathic pain and macrophage infiltration following peripheral nerve injury. We conclude that glial NF-κB is an important regulator of sensory neuropeptides in the development of neuropathic pain after peripheral nerve injury.
4. Experimental procedures
4.1. Animal preparation
The studies were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Miami. Wild type (WT) and transgenic GFAP-IκBα-dn mice, where NF-κB was selectively inactivated in GFAP expressing cells by overexpression of a truncated form the inhibitory protein IκBα, were developed and bred at the University of Miami Transgenic Core Facility. Details pertaining to the generation of GFAP-IκBα-dn mice on a C57BL/6 background were previously described (Brambilla et al., 2005). All mice used in our experiments were 2–4 months old males, weighing 25–35 g, and were obtained by breeding heterozygous male GFAP-IκBα-dn males with WT C57BL/6 females. Male WT littermates were used as controls in all experiments. All animals were housed in a 12 h light/dark cycle in a virus/antigen-free facility with controlled temperature.
4.2. Chronic constriction injury of the sciatic nerve
Neuropathic pain was induced in mice after peripheral nerve injury according to the procedure of sciatic nerve chronic construction injury (CCI) as originally described in rats by Bennett and Xie (1988). Various investigators have made modifications of the CCI model in mice by using silk or prolene sutures with suture sizes ranging from 6.0 to 10.0 ISP (Sommer and Schafers, 1998; Fu et al., 2010; Uceyler et al., 2010; Caspani et al., 2009). In this study, the left sciatic nerve was exposed at the level of the mid thigh. Three loose 6.0 silk ligatures were placed around the dissected nerve with a 1.0-1.5 mm interval between each ligature. The ligation was carefully manipulated so that the ligature was loosely tied around the nerve. The wound was closed with 6-0 Ethilon monofilament nylon in layers. The same procedure was used for sham control surgeries.
4.3. Assessment of neuropathic pain behavior
4.3.1. Mechanical hyperalgesia
Mice were habituated to the behavioral testing paradigms for 3–5 days before initiating data collection. Each mouse was placed individually beneath an inverted transparent plastic cage with a wire mesh bottom. Von Frey filaments (Touch-Test Sensory Evaluator, North Coast Medical, Morgan Hill, CA) with incremental stiffness (0.1–1.5 g) were applied serially to the paw in ascending or descending order of stiffness depending on the foot withdrawal response of the mouse. The maximum and minimum cut-offs were at 1.5 and 0.1 g, respectively. A single trial of stimuli consists of five applications of von Frey filaments to the plantar surface of the paw perpendicularly for about 4–5 s. Foot withdrawals in response to the stimuli were considered positive. Depending on the positive or negative response, subsequent filaments were applied in order of descending and ascending intensity, respectively. The withdrawal threshold was expressed as the tolerance level in grams.
4.3.2. Thermal hyperalgesia
Mice were placed in a transparent Perspex box on a thin glass platform. The hindpaws were in contact with a 1/4 in. thick glass plate that is maintained at room temperature. A mobile infrared heat lamp device or plantar apparatus (UGO BASILE 7371 Plantar Test Apparatus, Italy) was then positioned underneath the targeted hind paw. The plantar apparatus directs a focused radiant light source from below the glass onto the plantar surface of one hindpaw. The paw withdrawal latency (PWL) was determined for the left and right hindpaw, with a 5-min inter-trial interval. The infrared intensity was set at 50 (corresponding to 196 mW/cm2), which produced baseline paw withdrawal latencies of 5–10 s. The mean latency of withdrawal response of each hind paw was determined by 6 tests. A cut off of 20 s was used.
4.3.3. Behavioral assessment
A blinded observer measured withdrawal threshold to mechanical stimuli and latency withdrawal to thermal stimuli. Baseline measurements were obtained 2 days prior to CCI surgery. Behavioral tests were performed at baseline, day 1 and day 3 post-CCI to evaluate the effects of CCI on mechanical and thermal hyperalgesia.
4.4. Total RNA isolation and RT-PCR
For real-time RT-PCR analyses, L4-L5 DRGs were removed at day 0 (naïve), day 1, and day 3 post-CCI, freshly frozen in dry ice and maintained at −80 °C until use for RNA isolation. Total RNA from DRGs was extracted with the RNeasy Micro Kit from Qiagen according to the manufacturer’s instructions. RNA (1 μg) was reverse transcribed with SuperScript II (Invitrogen) to obtain cDNA. Real-time PCR was performed with the Rotor-Gene 3000 (Corbett Research, Mortlake, Australia) on cDNA samples using the TAQurate green real-time PCR master mix (Epicentre Biotechnologies, Madison, WI, USA). For amplification the following primers were used: galanin, 5′-TGC AGT AAG CGA CCA TCC AG-3′ (forward) and 5′-AGC ACA GGA CAC ACG TGC AC-3′ (reverse); CGRP, 5′-CAC CAA TGT GGG CTC TGA AG-3′ (forward) and 5′-CCG CTT GAG GTT TAG CAG AG-3′ (reverse); β-actin, 5′-CTA GAC TTC GAG CAG GAG ATG-3′ (forward) and 5′-CAA GAA GGA AGG CTG GAA AAG AG-3′ (housekeeping gene for normalization).
4.5. Immunohistochemistry and immunofluorescence quantification
The animals were sacrificed at day 0 (Naive) and day 3 post-CCI. For immunohistochemistry, L4-L5 DRGs and sciatic nerves were removed, fixed in 4% paraformaldehyde in PBS (pH 7.4) overnight, cryoprotected in 0.1 M PBS containing 20% sucrose and cryostat cut into 10 μm thick sections. Sections were incubated overnight at 4 °C with a primary antibody-anti-GFAP (Dako, USA; 1:2000), anti-galanin (Peninsula laboratories, Inc.; 1:200), anti-CGRP (Sigma; 1:3000) or anti-CD11b (Serotec; 1:100) followed by secondary species-specific fluorescent antibodies (FITC or Rhodamine, Jackson ImmunoResearch Lab, Inc. USA) for 1 h at 22 °C.
For quantification of the immunofluorescence signal, two sections of the sciatic nerve or DRG were randomly selected for each mouse and analyzed in a blinded fashion with the software NIH Image J (developed by the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/ij). Four animals per experimental condition were analyzed. To distinguish cell size-specific changes, we divided the DRG neurons into small (area<600 μm2), medium (area between 600 and 1200 μm2), and large (area>1200 μm2) neurons according to their cross-sectional area.
4.6. Statistical analysis
Data are presented as mean±SEM. Behavior, real-time PCR assessment, and fluorescence quantification were analyzed with one-way ANOVA with Tukey’s multiple comparison test. p values of less than <0.05 were designated as statistically significant.
Acknowledgments
This work was supported by NIH grants NS051709 and NS065479 to J.R.B. and by the Miami Project To Cure Paralysis.
REFERENCES
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Biella G, Panara C, Pecile A, Sotgiu ML. Facilitatory role of calcitonin gene-related peptide (CGRP) on excitation induced by substance P (SP) and noxious stimuli in rat spinal dorsal horn neurons. An iontophoretic study in vivo. Brain Res. 1991;559:352–356. doi: 10.1016/0006-8993(91)90024-p. [DOI] [PubMed] [Google Scholar]
- Bird GC, Han JS, Fu Y, Adwanikar H, Willis WD, Neugebauer V. Pain-related synaptic plasticity in spinal dorsal horn neurons: role of CGRP. Mol. Pain. 2006;2:31. doi: 10.1186/1744-8069-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS ONE. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corness J, Shi TJ, Xu ZQ, Brulet P, Hokfelt T. Influence of leukemia inhibitory factor on galanin/GMAP and neuropeptide Y expression in mouse primary sensory neurons after axotomy. Exp. Brain Res. 1996;112:79–88. doi: 10.1007/BF00227180. [DOI] [PubMed] [Google Scholar]
- Fu ES, Zhang YP, Sagen J, Yang ZQ, Bethea JR. Transgenic glial nuclear factor-kappa B inhibition decreases formalin pain in mice. NeuroReport. 2007;18:713–717. doi: 10.1097/WNR.0b013e3280d9e869. [DOI] [PubMed] [Google Scholar]
- Fu ES, Zhang YP, Sagen J, Candiotti KA, Morton PD, Liebl DJ, Bethea JR, Brambilla R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain. 2010;148:509–518. doi: 10.1016/j.pain.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Nicola D, Valle-Argos B, Suardiaz M, Taylor JS, Nieto-Sampedro M. Role of IL-15 in spinal cord and sciatic nerve after chronic constriction injury: regulation of macrophage and T-cell infiltration. J. Neurochem. 2008;107:1741–1752. doi: 10.1111/j.1471-4159.2008.05746.x. [DOI] [PubMed] [Google Scholar]
- Holmberg K, Kuteeva E, Brumovsky P, Kahl U, Karlstrom H, Lucas GA, Rodriguez J, Westerblad H, Hilke S, Theodorsson E, Berge OG, Lendahl U, Bartfai T, Hokfelt T. Generation and phenotypic characterization of a galanin overexpressing mouse. Neuroscience. 2005;133:59–77. doi: 10.1016/j.neuroscience.2005.01.062. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Morgan L, Stewart HJS, Mirsky R. Three markers of adult non-myelin-forming Schwann cells, 217c (Ran-1), A5E3 and GFAP: development and regulation by neuron-Schwann cell interactions. Development. 1990;109:91–103. doi: 10.1242/dev.109.1.91. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Wiesenfeld-Hallin Z, Villar M, Melander T. Increase in galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci. Lett. 1987;83:217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol. Sci. 2003;24:580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Klein CM, Coggeshall RE, Carlton SM, Westlund KN, Sorkin LS. Changes in calcitonin gene-related peptide immunoreactivity in the rat dorsal horn following electrical stimulation of the sciatic nerve. Neurosci. Lett. 1990;115:149–154. doi: 10.1016/0304-3940(90)90446-g. [DOI] [PubMed] [Google Scholar]
- Lee SE, Kim JH. Involvement of substance P and calcitonin gene-related peptide in development and maintenance of neuropathic pain from spinal nerve injury model of rat. Neurosci. Res. 2007;58:245–249. doi: 10.1016/j.neures.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Differential expression of galanin immunoreactivities in the primary sensory neurons following partial and complete sciatic nerve injuries. Neuroscience. 1997;79:1183–1195. doi: 10.1016/s0306-4522(97)00088-2. [DOI] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive intestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience. 1998;86:1217–1234. doi: 10.1016/s0306-4522(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Ohtori S, Ishikawa T, Aoki Y, Ozawa T, Doya H, Saito T, Moriya H, Takahashi K. Up-regulation of TNFα in DRG satellite cells following lumbar facet injury in rats. Eur. Spine J. 2006;15:953–958. doi: 10.1007/s00586-005-1031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na HS, Kim HJ, Sung B, Back SK, Kim DY, Kim JS, Hong SK. Decrease in spinal CGRP and substance P is not related to neuropathic pain in a rat model. NeuroReport. 2001;12:175–178. doi: 10.1097/00001756-200101220-00042. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Ren K, De Leon M, Ruda M. Primary sensory neurons exhibit altered gene expression in a rat model of neuropathic pain. Pain. 1994;58:95–108. doi: 10.1016/0304-3959(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Niederberger E, Geisslinger G. The IKK-NF-kappaB pathway: a source for novel molecular drug targets in pain therapy? FASEB J. 2008;22:3432–3442. doi: 10.1096/fj.08-109355. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-α and TNF-α receptor type I upregulation in glia and neurons after peripheral nerve injury. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res. 1987;403:350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- Peters CM, Ghilardi JR, Keyser CP, Kubota K, Lindsay TH, Luger NM, Mach DB, Schwei MJ, Sevcik MA, Mantyh PW. Tumor-induced injury of primary afferent sensory nerve fibers in bone cancer pain. Exp. Neurol. 2005;193:85–100. doi: 10.1016/j.expneurol.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Ma W, Murphy PG, Richardson PM, Bisby MA. Galanin expression in neuropathic pain: friend or foe? Ann. NY Acad. Sci. 1998;863:390–401. doi: 10.1111/j.1749-6632.1998.tb10709.x. [DOI] [PubMed] [Google Scholar]
- Schafers M, Geis C, Brors D, Yaksh TL, Sommer C. Anterograde transport of tumor necrosis factor-alpha in the intact and injured rat sciatic nerve. J. Neurosci. 2002;22:536–545. doi: 10.1523/JNEUROSCI.22-02-00536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur. J. Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- Shadiack AM, Vaccariello SA, Sun Y, Zigmond RE. Nerve growth factor inhibits sympathetic neurons’ response to an injury cytokine. Proc. Natl Acad. Sci. USA. 1998;95:7727–7730. doi: 10.1073/pnas.95.13.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Galanin-like immunoreactivity in capsaicin sensitive sensory neurons and ganglia. Brain Res. Bull. 1985;15:191–195. doi: 10.1016/0361-9230(85)90135-2. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J. Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- Thompson SW, Priestley JV, Southall A. gp130 cytokines, leukemia inhibitory factor and interleukin-6, induce neuropeptide expression in intact adult rat sensory neurons in vivo: time-course, specificity and comparison with sciatic nerve axotomy. Neuroscience. 1998;84:1247–1255. doi: 10.1016/s0306-4522(97)00553-8. [DOI] [PubMed] [Google Scholar]
- Triolo D, Dina G, Lorenzetti I, Malaguti M, Del Carro U, Comi G, Messing A, Quattrini A, Previtali SC. Loss of glial fibrillary acidic protein (GFAP) impairs Schwann cell proliferation and delays nerve regeneration. J. Cell Sci. 2006;119:3981–3993. doi: 10.1242/jcs.03168. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Gobel K, Meuth SG, Ortler S, Stoll G, Sommer C, Wiendl H, Keinschnitz C. Exp. Neurol. 2010;222:153–160. doi: 10.1016/j.expneurol.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Villar MJ, Cortes R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Hokfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neursocience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Pheby T, Segerdahl AR, Davies M, Hasnie F, Hall S, McMahon SB, Rice AS. Pharmacological, behavioural and mechanistic analysis of HIV-1 gp120 induced painful neuropathy. Pain. 2007;133:47–63. doi: 10.1016/j.pain.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ. Neuropeptides in neuropathic and inflammatory pain with special emphasis on cholecystokinin and galanin. Eur. J. Pharmacol. 2001;429:49–59. doi: 10.1016/s0014-2999(01)01305-x. [DOI] [PubMed] [Google Scholar]
- Woodham P, Anderson PN, Nadim W, Turmaine M. Satellite cells surrounding axotomized rat dorsal horn ganglion cells increase expression of a GFAP-like protein. Neurosci. Lett. 1989;98:8–12. doi: 10.1016/0304-3940(89)90364-9. [DOI] [PubMed] [Google Scholar]
- Zhang X, Shi T, Holmberg K, Landry M, Huang W, Xiao H, Ju G, Hokfelt T. Expression and regulation of the neuropeptide Y Y2 receptor in sensory and autonomic ganglia. Proc. Natl Acad. Sci. USA. 1997;94:729–734. doi: 10.1073/pnas.94.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YP, Lundeberg T, Yu LC. Interactions of galanin and morphine in the spinal antinociception in rats with mononeuropathy. Brain Res. 2000a;852:485–487. doi: 10.1016/s0006-8993(99)02236-2. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Yu LC, Lundeberg T. An interaction of opioids and galanin in dorsal horn of the spinal cord in mononeuropathic rats. Regul. Pept. 2000b;86:89–94. doi: 10.1016/s0167-0115(99)00091-9. [DOI] [PubMed] [Google Scholar]