Abstract
Objective
To describe the prevalence of obesity-related noncommunicable diseases (NCDs) and associated risk factors in a sample of Samoan adults studied in 2010 as part of a genome-wide assocation study (GWAS) for obesity related traits.
Methods
Anthropometric and biochemical data collected from n = 3,475 participants (n = 1,437 male; n = 2,038 female) aged 24.5 to <65 years were used to describe the prevalence of obesity, diabetes, hypertension, and dyslipidemia within the study sample. One way analysis of variance, χ2 tests, and binary logistic regression were used to identify differences in disease and risk factor prevalence by 10-year age group, gender, or by census region of residence.
Results
Obesity was highly prevalent among the study sample; 64.6% of females and 41.2% of males were obese according to Polynesian cutoffs (BMI ≥ 32 kg/m2). Females were less likely than males to have hypertension (31.7% vs. 36.7%) but equally likely to have diabetes (17.8% vs. 16.4%). With the exception of obesity and low HDL-cholesterol in females only, there were significant differences in the prevalence of all NCDs and associated risk factors by age group, with the oldest age group (55 to <65 years) most affected. In both sexes, residents of the Apia Urban Area were at significantly greater risk of obesity, diabetes, low HDL-cholesterol, and high triglycerides than residents of the more rural Savaii region.
Conclusions
The phenotypic characteristics of this sample provide evidence of a continuation of previously reported temporal trends toward obesity and its associated disorders. Attention must be paid to the critical NCD situation in Samoa.
Samoans are characterized by high levels of adiposity and associated noncommunicable diseases (NCDs) such as Type 2 diabetes mellitus and cardiovascular disease (McGarvey, 2001, 2005). There has been a documented rise in the prevalence of these conditions over the last 30 years related to economic modernization, rapid urbanization, and shifts in lifestyle toward increased caloric intake and more sedentary behavior (Keighley et al., 2007). For example, in our prior studies, we found that body mass index (BMI) increased sharply from 1979 to 2003 in adults above the age of 35 years, especially among those aged 45–54 years. Between 1979 and 2003, the mean BMI of this age group changed from 27.4 to 32.9 kg/m2 in men and from 30.8 to 36.5 kg/m2 in women (Keighley et al., 2007). Addressing the high and rising chronic NCD burden is a top national health priority in Samoa (World Health Organization, 2011). It is important to continue to report on the basic descriptive patterns of NCDs, as time and modernization forces proceed to exert their effects on the Samoan population and report on their association with fundamental sociodemographic factors such as age, sex, and rural–urban factors. These patterns provide the crucial context for etiologic and individual level investigations for the high and still rising level of cardiometabolic conditions among Samoans.
While modernization and consequent changes in lifestyle patterns are clearly associated with the increase in adiposity and abnormalities in cardiometabolic traits, there is strong evidence from numerous studies that genetic factors influence our response to environmental exposures such as changing lifestyle and diet (Bouchard, 2008; Qi and Cho, 2008). Our prior research in Samoans showed that genetic variation is associated with adiposity and cardiometabolic risk factors (Åberg et al., 2008, 2009a, 2009b) as well as modulation of those risks by gene–environment interaction (Baylin et al., 2012). Precise identification of loci involved in these complex traits has, however, been difficult, with candidate gene and family-based linkage studies having limited success. With the identification of millions of genetic variants (single nucleotide polymorphisms) human genetics moved into the era of genome-wide association studies (GWAS). Although GWAS have identified hundreds of sequence variants associated with risks of complex traits, they have only explained a small fraction of genetic variance and, in particular, associations across populations of varied ancestries have been equivocal. For example, the most strongly obesity associated gene, FTO, identified by GWAS (Dina et al., 2007; Frayling et al., 2007) with replication across populations of European, African, and Hispanic ancestries, showed inconclusive association in populations of Asian descent (Li et al., 2008; Ohashi et al., 2007; Yajnik et al., 2009). Our earlier study among the Samoans found no significant evidence of association of FTO variants with obesity-related phenotypes (Karns et al., 2012). These different findings may have resulted from differences in allele frequencies as well as in mechanisms underlying energy balance across populations. Rigorous studies in well-defined populations with a high risk of adiposity are required to elucidate the genetic architecture of obesity and other complex NCD traits. Isolated populations of recent evolutionary history and reduced genetic diversity provide advantages in understanding the genetic basis of complex diseases (Peltonen et al., 2000). These populations may likely provide novel insight into the genetics of adiposity and cardiometabolic phenotypes that are present in smaller strata of larger cosmopolitan populations but difficult to detect (Kristiansson et al., 2008; Rosenberg et al., 2010). Understanding the inherited basis of adiposity and cardiometabolic traits is essential in elucidating the biological pathways associated with obesity as well as designing effective interventions to prevent or limit the morbidity and mortality resulting from obesity (McCarthy et al., 2008).
The overall objective of this research progam is to use GWAS methods to define the detailed genetic variation that underlies adiposity and related phenotypes among adult Samoans and understand how this variation interacts with environmental exposures. The specific purpose of this report is to describe in detail the GWAS study sample, describe the methodologic approach, and provide an initial description of several NCDs and associated risk factors from the population sample studied as part of the GWAS for obesity related conditions in Samoa in 2010. Thus this report serves both as a description of the levels of NCDs and their basic sociodemographic covariates in a representative study sample of Samoa and as a reference for our later reports on the genetic findings from the ongoing GWAS investigations.
METHODS
Study population, design, and recruitment
Our study population is derived from the independent nation of Samoa. In 2010, Samoa’s population was estimated to be 186,405 with 38% <15 years of age, 5% aged 65 years and above, and life expectancy at birth was 74.2 years (Samoa Bureau of Statistics, 2012). Currently classified as a lower-middle income economy (World Bank, 2013), Samoa was ranked 94th of 182 countries according to the Human Development Index (UNDP, 2009). Approximately, 58% of men, age 15 years and above, are economically active, and 46% of this group works in subsistence related farming and fishing. For women, 23% are economically active, and 86% of them work in clerical, service, industrial and professional positions according to the 2011 census (Samoa Bureau of Statistics, 2012).
The study had a population-based design targeting approximately 3,500 adults aged 24.5 to <65 years with target participation numbers for each Samoan census region based upon the national population distribution described in the 2006 census (Samoa Bureau of Statistics, 2008). The aim was to sample adults from villages within all four census regions of Samoa: Apia Urban Area (AUA), Northwest Upolu (NWU), Rest of Upolu (ROU), and Savaii (SAV).
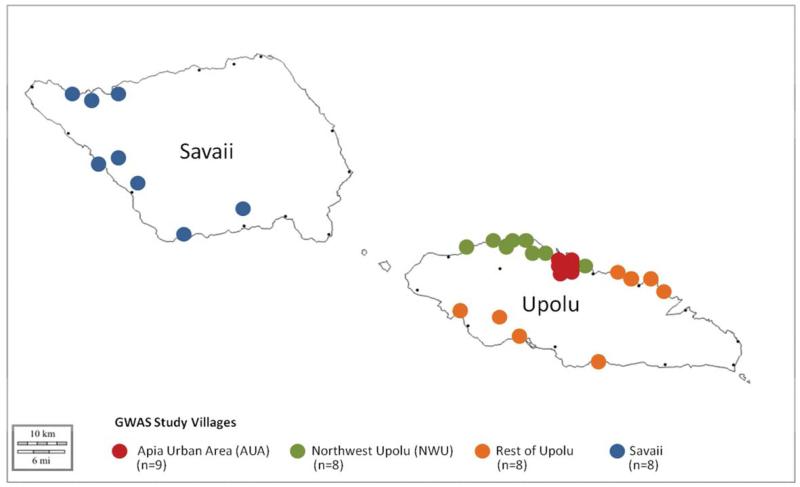
The recruitment and measurement of the study participants took place from February to July 2010. Thirty-three villages were included in the study: nine from the AUA, eight from NWU, eight from ROU, and eight from SAV. For ease of data collection, large villages (with a population ≥500) were targeted, paying attention to geographic distribution. The geographic distribution of villages can be seen in Figure 1. Following consultation with local experts, the decision was made not to recruit from the Southeastern region of Upolu which was affected by a September 2009 tsunami. The burden of accommodating a research project of this scale was considered detrimental to the rebuilding process in these villages.
Fig. 1.
Study village locations.
The Samoan Bureau of Statistics arranged for village level permission to spend 2-3 days in each village completing study activities, including early morning fasting blood collections, anthropometry, blood pressure and body composition examinations, and several interview modules. Recruitment within villages was undertaken with the co-operation of village leaders and completed using a study orator, whose role was to explain the purpose and procedures of the study in order to promote interest in participation. Participation in the study was voluntary. The study site was usually a centrally located building, provided by village leaders, women’s health committees and other civic organizations. A field team of six investigators from Brown University and six locally trained field assistants conducted the survey.
Informed consent
Participants were given detailed information about the study, the data collection protocols, and their rights as participants. The study was first explained verbally, in Samoan, by trained fieldworkers, and consenting participants signed a paper consent form (also presented in the Samoan language). Because the core objective of the study was an investigation of genetic factors that may increase susceptibility to cardiometabolic phenotypes, and the US National Institutes of Health (NIH), National Heart, Lung & Blood Institute (NHLBI) funding agency required uploading of de-identified genetic and phenotypic data to the dbGaP website (http://www.ncbi.nlm.nih.gov/gap), informed consent documents focused on participant understanding of this, with a specific check box certifying their agreement. Research protocols and the informed consent were approved both by the Brown University Institutional Review Board and the Health Research Committee of the Samoan Ministry of Health.
Sample and inclusion criteria
A total of 3,504 participants were recruited into the study. To be included in the study, participants were of Samoan origin, which was determined by having four Samoan grandparents (based on self-report), being 24.5 to <65years old, non-pregnant, with no severe physical or cognitive impairment (which would prohibit completion of either the anthropometric or questionnaire measures), and willing and able to complete the interview portion of the study in Samoan. A total of 29 enrolled participants were later excluded from study analyses: 27 based on the inclusion criteria (15 were or were possibly pregnant, 7 were under 24.5 years of age, 5 were over 64.9 years of age) and 2 based on a lack of complete data.
Of the 3,475 eligible participants, 99.4% completed the questionnaire and anthropometry portion, 91.1% gave a blood sample for DNA processing, and a fasting serum sample was analyzed for 84.6% of participants. There were no significant demographic differences between those who gave DNA samples and those who did not. The level of refusal did not differ between census regions. The proportion of participants for whom a serum sample was analyzed was lowered firstly because a small amount of participants did not adhere to fasting protocols, and secondly due to a blood specimen handling error in the first surveyed village which may have affected the accuracy of those results.
Sample representativeness
While effort was made to recruit a nationally representative sample, the final distribution of males and females in the GWAS sample did not reflect the population as represented by the 2011 census (Samoa Bureau of Statistics, 2012). There were a greater proportion of women included in the GWAS sample (58.6%) than in the population as a whole (48.0%). The more rural ROU and SAV regions were over-represented in the GWAS sample. In ROU, 32.4% of the eligible population was surveyed and 33.1% in SAV versus 15.9% and 19.6% in the urban and semiurban areas, respectively. Older participants were also over-represented in the GWAS sample with the proportion of participants known to be eligible actually volunteering for inclusion in the study rising with each five year age group. Of the 24.5–29 year olds identified by the census as being eligible for inclusion, 16.1% volunteered to participate compared to 42.2% of 60–64 year olds. Willingness and availability to participate in the study likely reflected the age and gender associations with full-time employment. Study villages were surveyed Monday-Friday, meaning that older and female residents tended to be at home in the village, and agreed to participate.
The study sample was representative of the wider Samoan population in terms of marital status, educational attainment, and access to basic household amenities (as compared to the 2006 census since the publication format of the 2011 census data did not allow us to perform these more stratified comparisons) (Samoa Bureau of Statistics, 2008). The disease/risk factor prevalence results presented here in the tables are unadjusted and reflect the age and census region trends within our sample. In an attempt to account for the over-representation of women and older participants in our sample, and to allow comparison with other populations, sex-specific, age-adjusted prevalence estimates are given in the text for each disease outcome and NCD risk factor. Since the age-distribution of the sample differed across census regions, we also present age-adjusted risk of disease based upon residence in each of the census regions in Table 2. These prevalence estimates were calculated based on the 2011 census (Samoa Bureau of Statistics, 2012).
TABLE 2. ORs for NCDs & Risk Biomarkers by Census Region (adjusted for age).
| Obesity | Hypertension | Diabetes | High total cholesterol |
High LDL-cholesterol |
Low HDL-cholesterol |
Triglycerides | |
|---|---|---|---|---|---|---|---|
| Males | |||||||
| AUA | - | - | - | - | - | - | - |
| NWU | 0.56 (0.41, 0.76) | 1.07 (0.78, 1.46) | 0.87 (0.57, 1.34) | 0.85 (0.61, 1.18) | 0.80 (0.47, 1.37) | 0.72 (0.50, 1.05) | 0.71 (0.50, 1.01) |
| ROU | 0.60 (0.44, 0.82) | 1.03 (0.74, 1.43) | 0.63 (0.40, 0.99) | 0.98 (0.71, 1.37) | 0.70 (0.41, 1.18) | 0.50 (0.34, 0.74) | 0.69 (0.49, 0.98) |
| SAV | 0.44 (0.32, 0.62) | 0.67 (0.47, 0.95) | 0.41 (0.25, 0.68) | 1.29 (0.91, 1.84) | 1.58 (0.82, 3.06) | 0.26 (0.16, 0.41) | 0.46 (0.32, 0.68) |
| Females | |||||||
| AUA | - | - | - | - | - | - | - |
| NWU | 0.85 (0.65, 1.12) | 1.01 (0.76, 1.34) | 0.96 (0.66, 1.39) | 0.88 (0.65, 1.18) | 1.10 (0.67, 1.79) | 0.79 (0.60, 1.04) | 0.78 (0.55, 1.10) |
| ROU | 0.62 (0.47, 0.82) | 0.94 (0.71, 1.26) | 1.05 (0.73, 1.50) | 1.12 (0.84, 1.50) | 0.92 (0.57, 1.46) | 0.81 (0.62, 1.06) | 0.84 (0.60, 1.17) |
| SAV | 0.51 (0.39, 0.68) | 0.91 (0.68, 1.23) | 0.63 (0.42, 0.93) | 1.42 (1.05, 1.91) | 1.32 (0.78, 2.22) | 0.32 (0.24, 0.43) | 0.63 (0.44, 0.90) |
AUA = Apia Urban Area; NWU =Northwest Upolu; ROU =Rest of Upolu; SAV =Savaii. Significance is denoted in bold text. Polynesian cutoffs: Obesity = BMI >32 kg/m2, hypertension = systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking medication for hypertension, diabetes = fasting blood glucose ≥126 mg/dl or currently taking medication for diabetes, high total cholesterol = ≥ 5.2 mmol/l, high LDL-cholesterol = ≥ 2.59 mmol/l, low HDL-cholesterol = HDL-cholesterol ≤ 1.29 mmol/l, high triglycerides = >1.69 mmol/l.
Measures
Questionnaire
A questionnaire was administered, in Samoan, to all participants. The questionnaire collected socio-demographic information, a detailed health history, including exogenous hormone use in females, alcohol and tobacco consumption, physical activity estimations, a household assets inventory, and a brief acculturation assessment. A 104 item food frequency questionnaire (FFQ) was administered to assess dietary intake, cooking oil and fat use, and fruit and vegetable consumption. This was an expanded version of a shorter Samoan FFQ whose reliability and validity were established using the method of triads with 24-hour dietary recalls, FFQs, and urinary potassium measurements (DiBello et al., 2009).
Anthropometric measures
All anthropometric measures were taken with participants in light island clothing. Height was measured to the nearest 0.1 mm using a portable GPM anthropometer (Pfister Imports, New York, NY) and weight to the nearest 0.1 kg using a Tanita HD 351 digital weight scale (Tanita Corporation of America, IL). Categorical definitions of BMI based upon Polynesian cutoffs were used to define overweight (BMI 26–32 kg/m2) and obesity (BMI >32 kg/m2) (Swinburn et al., 1999). Skinfold thicknesses were taken with a Lange skinfold caliper (Cambridge Scientific Industries, Cambridge, MD) to the nearest 0.1 mm. Duplicate measurements were taken at the tricep, forearm, subscapular, abdominal, and suprailiac sites. Measurements exceeding the maximum capacity of the skinfold calipers (>65 mm) were recorded as doing so in participant records. Mid-upper arm, abdomen, hip, and calf circumferences were also measured in duplicate. Duplicate measures were averaged for use in analyses. Bioelectrical impedance (BIA) measures of resistance and reactance were obtained with an RJL BIA-101Q device (RJL Systems, MI) using standard procedures. Fat mass and body fat percentage were calculated using the equations established from direct body composition studies using the duel-energy X-ray absorptiometry method in Polynesians residing in New Zealand (Keighley et al., 2006; Swinburn et al., 1999). After a 10-minute seated rest period blood pressure (BP) was measured three times, with 3-minute rest periods between measurements, using an Omron HEM907 XL digital blood pressure monitor (Omron Healthcare, IL). Results of the second and third blood pressure measurements were averaged for use in analyses. A random finger-prick blood glucose was also taken using a Bayer Contour glucometer (Bayer Healthcare, IN).
Serum sampling and analysis
Fasting whole blood specimens were collected by local phlebotomists after a 10-hour overnight fast. Samples for serum analysis were collected in 10 ml vacutainers spray coated with silica and containing polymer gel for serum separation. Serum was separated by centrifugation in the field and then stored at −40°C before transport on dry ice to Northwest Lipid Labs, Seattle, WA for analysis. Quantitative determination of total cholesterol, LDL- and HDL-cholesterol and triglycerides were performed by enzymatic in vitro tests using Roche reagents on a Roche Double Modular P Analytics automated analyzer. The Roche methods are standardized to the Centers for Disease Control and Prevention reference methods. Analyses of the fasting blood glucose samples were performed enzymatically on a Hitachi 917 Clinical Chemistry auto-analyzer. This instrument executes the glucose hexokinase method described by Bergmeyer et al. (1974) and Peterson and Young (1968).
Risk factor definitions
Polynesian cutoffs were used to classify participants as normal weight, overweight, and obese based on BMI ranges of <26 kg/m2, 26–32 kg/m2, and >32 kg/m2, respectively (Keighley et al., 2006, 2007; Swinburn et al., 1999). For comparison to other populations, we also report the standard World Health Organization (WHO) criteria of overweight as 25 to <30 kg/m2 and obesity as ≥30 kg/m2 (WHO, 2000). Hypertension was defined as having either a systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg, or currently taking medication for hypertension (Chobanian et al., 2003). Type 2 diabetes was defined as fasting serum glucose ≥126 mg/dl or currently taking medication for diabetes (American Diabetes Association, 2012). American Heart Association (AHA) criteria for high total cholesterol (≥5.2 mmol/l), high LDL cholesterol (≥2.59 mmol/l), low HDL cholesterol (≤1.03 mmol/l (men); ≤1.29 mmol/l (women), and high triglycerides (>1.69 mmol/l) were applied (National Cholesterol Education Program, 2001).
DNA sampling and analysis
Whole blood samples were collected in a 7-ml ethylenediaminetetraacetic acid (EDTA) treated vacutainer. The samples were processed in the field to the cell lysate stage of DNA extraction using Red Blood Cell Lysis, Protein Precipitation and Cell Lysis solutions following manufacturer’s protocols (5PrimeT-MArchivePure™ obtained from Fischer Scientific). Cell lysates were then transported at room temperature, according to standard operating procedures to the University of Cincinnati, where further extraction steps were completed. The first stage of the genotyping was completed in November 2011. Initial genome-wide association analyses with 13 adiposity and other NCD phenotypic traits have been conducted, and we are currently performing replication genotyping and analysis of regions in which we observed strong signals. Additional follow-up genotyping will continue over the 5-year grant award period. Analysis of the interaction of genetic variants with environmental exposures, such as diet or cigarette smoking, and NCDs will be ongoing throughout the 5-year project.
Participant feedback
Participant feedback occurred in two stages. At the time of anthropometric assessment, feedback was given to participants about their weight status, their BP, and their random blood glucose. Following laboratory analysis of the serum samples, participants then received further feedback on their total cholesterol and fasting blood glucose. At both stages of feedback, if a participant was found to meet local clinical criteria for hypertension, diabetes or high cholesterol, they were referred to local healthcare providers for further investigation.
Statistical analysis
NCD characteristics and the prevalence of NCD risk factors were described by sex and 10-year age groups. Age group differences in NCD characteristics (i.e., BMI, fasting blood glucose, etc.) were evaluated using ANOVA. For the purpose of prevalence analyses, all NCDs and lipid and lipoprotein biomarkers were dichotomized based upon the risk factor definitions described above. Gender-specific age group and regional differences in NCD prevalence were evaluated using χ2 statistics. Risk of having NCDs or their risk factors based on residing in each census region (adjusted for age distribution within each census region) were calculated with binary logistic regression. All statistical analyses were performed using SPSS version 18.0 (SPSS, IL). P-values less than 0.05 were considered statistically significant.
To ensure that relatedness among participants in the study sample did not inflate the significance of our findings, sensitivity analyses were conducted using a subsample of “unrelated” participants (n = 1,829) with a maximum kinship estimate of 6.01% (less than first cousins). Kinship coefficients were calculated from the GWAS markers based on the degree of allele sharing between each pair of individuals in the study, such that the greater the allele sharing the closer the relationship of the pair. The coefficients were estimated using GenABEL (Aulchenko et al., 2007). These analyses are presented in Supplementary material (Tables 1–3).
RESULTS
Risk factors for NCDs
Table 1 shows the NCD characteristics of the study sample by age and sex. With the exception of HDL-cholesterol levels in males, there were significant differences by age group among all NCD risk factors. BMI, BP, and fasting blood glucose as well as fasting levels of cholesterol and triglycerides increased with age in both sexes.
TABLE 1. NCD characteristics by sex and age.
| 24.5 to <35 years |
35 to <45 years |
45 to <55 years |
55 to <65 years |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | |
| Males | ||||||||||
| BMI (kg/m2)** | 357 | 29.4 (5.4) | 329 | 31.1 (5.4) | 390 | 32.1 (5.9) | 345 | 32.3 (6.3) | 1,421 | 31.3 (5.9) |
| Systolic BP (mm Hg) ** | 350 | 127.4 (11.7) | 331 | 127.8 (12.4) | 390 | 133.2 (16.2) | 343 | 138.5 (21.3) | 1,413 | 131.8 (16.5) |
| Diastolic BP (mm Hg)** | 350 | 76.1 (10.4) | 331 | 80.7 (11.6) | 390 | 84.3 (12.5) | 343 | 84.9 (13.9) | 1,413 | 81.5 (12.7) |
| Fasting blood glucose (mg/dl)** | 278 | 86.1 (21.5) | 274 | 96.6 (40.8) | 328 | 116.6 (57.4) | 307 | 124.7 (71.0) | 1,187 | 106.9 (54.2) |
| Total cholesterol (mmol/l)** | 278 | 4.7 (0.9) | 274 | 5.2 (0.8) | 328 | 5.4 (1.0) | 307 | 5.4 (1.1) | 1,187 | 5.2 (1.0) |
| LDL-cholesterol(mmol/l)** | 276 | 3.4 (0.9) | 274 | 3.9 (0.9) | 327 | 4.0 (1.0) | 304 | 4.1 (1.2) | 1,181 | 3.8 (1.1) |
| HDL-cholesterol (mmol/l) | 278 | 1.3 (0.3) | 274 | 1.3 (0.3) | 328 | 1.3 (0.4) | 307 | 1.3 (0.3) | 1,187 | 1.3 (0.3) |
| Triglycerides (mmol/l)* | 278 | 1.4 (1.3) | 274 | 1.5 (0.9) | 328 | 1.7 (1.2) | 307 | 1.7 (1.5) | 1,187 | 1.6 (1.3) |
| Females | ||||||||||
| BMI (kg/m2)* | 483 | 34.0 (6.6) | 560 | 35.0 (6.5) | 559 | 35.1 (6.8) | 428 | 35.1 (7.2) | 2,030 | 34.8 (6.8) |
| Systolic BP (mm Hg)** | 481 | 115.1 (12.1) | 555 | 120.3 (16.2) | 551 | 130.2 (20.4) | 421 | 136.4 (22.0) | 2,008 | 125.2 (19.7) |
| Diastolic BP (mm Hg)** | 481 | 77.0 (10.3) | 555 | 79.6 (12.4) | 551 | 84.4 (13.4) | 421 | 85.5 (13.5) | 2,008 | 81.5 (12.9) |
| Fasting blood glucose (mg/dl)** | 405 | 86.0 (22.6) | 478 | 98.6 (48.0) | 493 | 121.6 (66.7) | 376 | 125.5 (66.3) | 1,752 | 107.9 (56.5) |
| Total cholesterol (mmol/l)** | 405 | 4.7 (0.8) | 478 | 4.9 (0.9) | 493 | 5.5 (0.9) | 376 | 5.6 (1.0) | 1,752 | 5.2 (0.9) |
| LDL-cholesterol(mmol/l)** | 405 | 3.4 (0.8) | 477 | 3.7 (0.9) | 493 | 4.2 (1.0) | 376 | 4.3 (1.1) | 1,751 | 3.9 (1.0) |
| HDL-cholesterol (mmol/l)* | 405 | 1.4 (0.4) | 478 | 1.4 (0.3) | 493 | 1.4 (0.3) | 376 | 1.4 (0.3) | 1,752 | 1.4 (0.3) |
| Triglycerides (mmol/l)** | 405 | 1.1 (0.5) | 478 | 1.2 (1.3) | 493 | 1.5 (0.8) | 376 | 1.5 (0.7) | 1,752 | 1.3 (0.9) |
Polynesian cutoffs: overweight = BMI 26–32 kg/m2, obesity = BMI >32 kg/m2; hypertension = systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg; diabetes = fasting blood glucose ≥126 mg/dl; high total cholesterol = total cholesterol ≥ 5.2 mmol/l; high LDL-cholesterol = LDL-cholesterol ≥ 2.59 mmol/l; low HDL-cholesterol = HDL-cholesterol ≤ 1.03 mmol/l (males) or ≤ 1.29 mmol/l (females); high triglycerides = triglycerides > 1.69 mmol/l. Comparison between age groups: ANOVA: main effects significant:
P < 0.05.
P < 0.01.
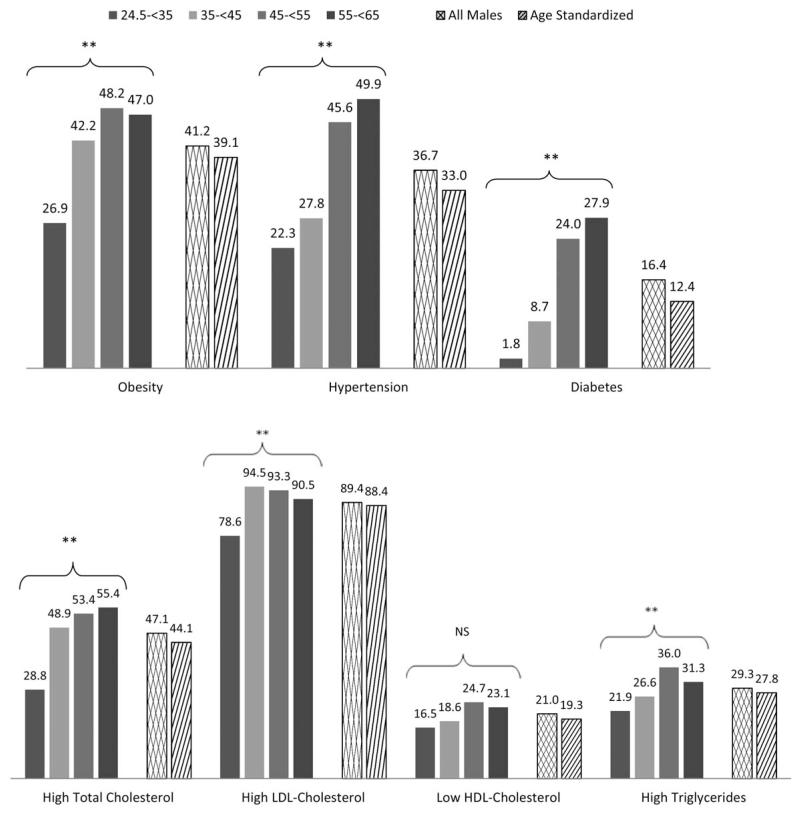
Figure 2 shows the prevalence of NCD risk factors in male and female Samoans. In all males, the total prevalence of overweight and obesity (combined), according to Polynesian cutoffs, was 80.4%. Using the alternative WHO criteria, the male prevalence of overweight and obesity was 86.5%. The prevalence of obesity was significantly different between age groups (P < 0.001). A substantially lower proportion of 24.5 to <35 year-old males had a BMI greater than 32 kg/m2 compared to other age groups. Hypertension and diabetes prevalence also significantly differed by age group (P < 0.001 for both) with the oldest age group (55 to <65 years) being most affected.
Fig. 2.
Prevalence of NCD risk factors in males by age group (years). Main effects significant: *, P < 0.05; **, P < 0.01; NS, nonsignificant. Polynesian cutoffs: Obesity = BMI >32 kg/m2, hypertension = systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking medication for hypertension, diabetes = fasting blood glucose ≥126 mg/dl or currently taking medication for diabetes, high total cholesterol = ≥5.2 mmol/l, high LDL-cholesterol = ≥2.59 mmol/l, low HDL-cholesterol = HDL-cholesterol ≤ 1.03 mmol/l, high triglycerides = > 1.69 mmol/l.
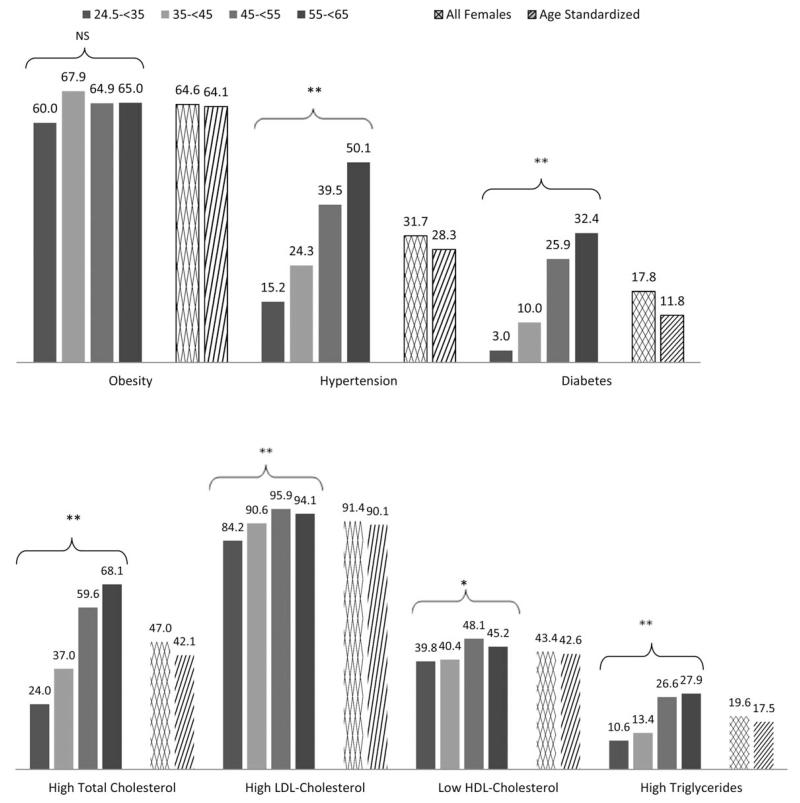
Using Polynesian cutoffs, 91.3% of all females had a BMI which was above the normal range (Fig. 3). According to the WHO criteria, the total prevalence of over-weight and obesity among females was 93.5%. Unlike the striking age trend in males, the prevalence of obesity did not differ significantly between female age groups (P = 0.23). Hypertension and diabetes prevalence in females did significantly differ by age group (P < 0.001 for both), with the oldest age group most affected.
Fig. 3.
Prevalence of NCD risk factors in females by age group (years). Main effects significant: * P < 0.05; ** P < 0.01; NS, non-significant. Polynesian cutoffs: Obesity = BMI >32 kg/m2, hypertension = systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking medication for hypertension, diabetes = fasting blood glucose ≥126 mg/dl or currently taking medication for diabetes, high total cholesterol = ≥5.2 mmol/l, high LDL-cholesterol = ≥2.59 mmol/l, low HDL-cholesterol = HDL-Cholesterol ≤ 1.29 mmol/l, high triglycerides = > 1.69 mmol/l.
The prevalence of overweight and obesity (combined) differed significantly by sex (χ2 [1, n = 3,451] = 86.83, P < 0.001) and was higher in females than in males (91.3% versus 80.4%). Hypertension prevalence also differed significantly by sex (χ2 [1, n = 3,425] = 9.20, P = 0.002). Females were less likely than males to be affected by hypertension (31.7% vs. 36.7%). There was no significant difference in the prevalence of diabetes by sex (χ2 [1, n = 2,975] = 1.11, P = 0.29; 17.8% [females] vs. 16.4% [males]).
High total serum cholesterol was found in 47.1% of the total sample. In both males and females, the prevalence of high total cholesterol differed significantly by age group (P < 0.001 for both), with the prevalence increasing with age. There were comparable rates of high LDL-cholesterol among males and females (χ2 [1, n = 2,932] = 3.18, P = 0.075). In both sexes, the prevalence of high LDL-cholesterol significantly differed between age groups (P < 0.001 for both). Prevalence of low HDL-cholesterol, however, did significantly differ by sex (χ2 [1, n = 2,939] = 158.2, P < 0.001). Females were more likely to exhibit low HDL-cholesterol; 43.4% versus 21.0% of males. The prevalence of low-HDL cholesterol only differed significantly between age groups in females (P = 0.031). High triglyceride levels were significantly more prevalent in males, 29.3%, than females, 19.6% (χ2 [1, n = 2,939] = 37.3, P < 0.001). In both males and females, the prevalence of high triglycerides differed significantly by age group (P < 0.001 in both). The prevalence of high triglycerides increased with age.
Regional variation in NCD risk
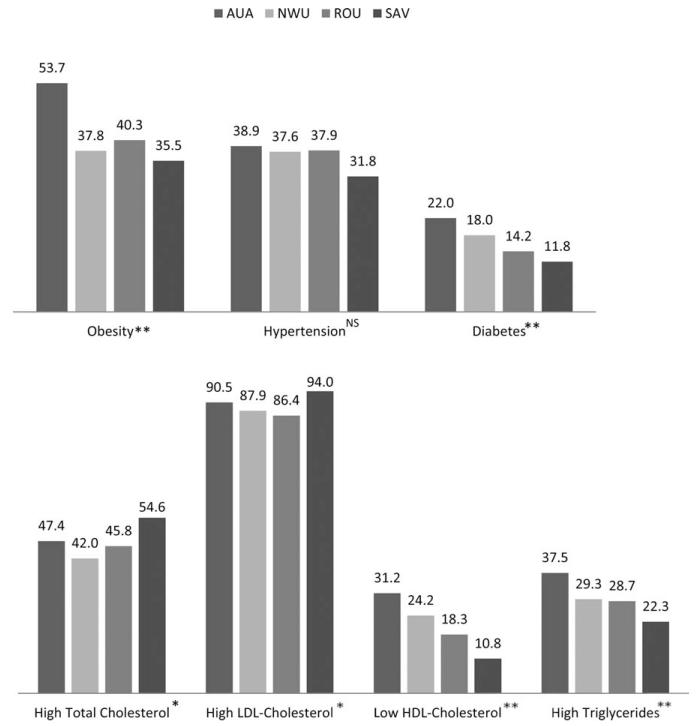
Among males, the prevalence of obesity differed significantly between census regions (P < 0.001) (Fig. 4). Prevalence was highest in the AUA (53.7%) and lowest in the more rural SAV region (35.5%). There were similar differences in prevalence by census region for diabetes (P = 0.008); the prevalence of diabetes in males from the AUA (21.0%) was close to double the prevalence in males from the SAV region (11.8%). Hypertension prevalence was not significantly different among census regions although prevalence was lowest in the SAV region. The relative risks of having each of these NCD, based on residence in each of the census regions after adjusting for the differing age composition of each census region, are presented in Table 2. Compared to those resident in the AUA, male participants residing in the three other census regions had a significantly lower risk of obesity. In the SAV region male participants also had a lower risk of hypertension and diabetes. Male ROU residents also had a lower risk of having diabetes compared to the AUA.
Fig. 4.
Prevalence of NCD risk factors in all males by census region. AUA = Apia Urban Area; NWU = Northwest Upolu; ROU = Rest of Upolu; SAV = Savaii. Main effects significant: * P < 0.05; ** P < 0.01; NS, nonsignificant. Polynesian cutoffs: Obesity = BMI >32 kg/m2, hypertension = systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking medication for hypertension, diabetes = fasting blood glucose ≥126 mg/dl or currently taking medication for diabetes, high total cholesterol = ≥5.2 mmol/l, high LDL-cholesterol = ≥2.59 mmol/l, low HDL-cholesterol = HDL-cholesterol ≤ 1.03 mmol/l, high triglycerides = > 1.69 mmol/l.
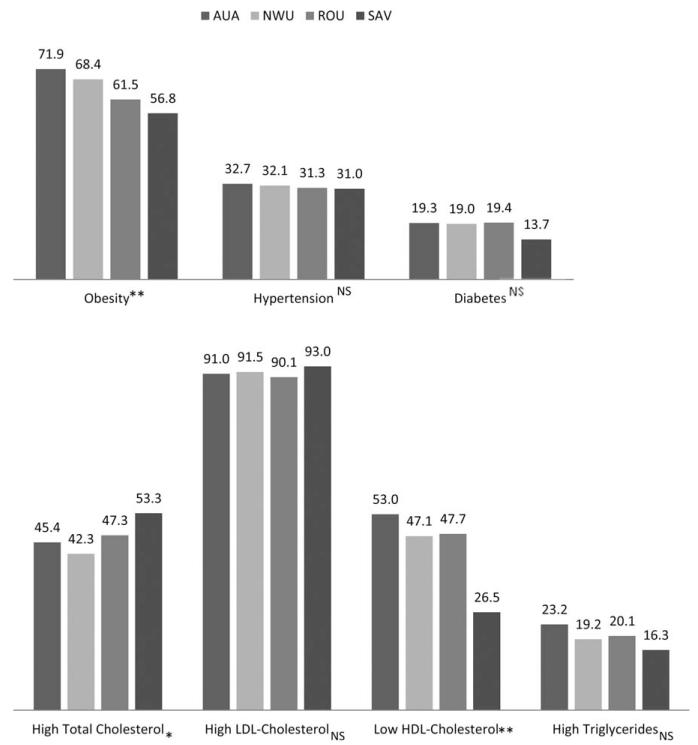
In females, there was similar regional variation in obesity prevalence (Fig. 5). Prevalence differed significantly by census region (P < 0.001) with the greatest prevalence in the AUA (71.9%) and the lowest in SAV (56.8%). Hypertension and diabetes prevalence did not differ among census regions. After adjusting for age within each of the census regions (Table 2), female residents of ROU and SAV had significantly less risk of obesity than residents of the AUA. Female SAV residents were also less likely to have diabetes. There were no significant differences in the risk for hypertension according to census region.
Fig. 5.
Prevalence of NCD risk factors in all females by census region. AUA = Apia Urban Area; NWU = Northwest Upolu; ROU = Rest of Upolu; SAV: Savaii. Main effects significant: * P < 0.05; ** P < 0.01; NS, non-significant. Polynesian cutoffs: Obesity = BMI >32 kg/m2, hypertension = systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking medication for hypertension, diabetes = fasting blood glucose ≥126 mg/dl or currently taking medication for diabetes, high total cholesterol = ≥5.2 mmol/l, high LDL-cholesterol = ≥2.59 mmol/l, low HDL-cholesterol = HDL-cholesterol ≤ 1.29 mmol/l, high triglycerides = > 1.69 mmol/l.
In both sexes, the prevalence of high total cholesterol differed significantly by census region (P = 0.020 and P = 0.010 for males and females, respectively) and was greater in the SAV region than in any of the Upolu regions (Figs. 4 and 5). The same was true of high LDL-cholesterol, although the regional differences only reached significance in males (P = 0.017). Low HDL-cholesterol prevalence did, however, differ between census regions in both males and females (P < 0.001 for both) with the SAV region having the lowest prevalence of low HDL-cholesterol. Prevalence of high triglycerides was highest in the AUA and lowest in SAV in both males and females, although again, differences between the regions were only significant among males (P = 0.002). After adjusting for the age composition of the census regions (Table 2), the risk of having high total cholesterol or high LDL-cholesterol was similar among males in all census regions. In the ROU and SAV regions, males were less likely to have low-HDL cholesterol or high triglycerides compared to males in the referent AUA. Women in SAV were significantly more likely to have high total cholesterol but less likely to have low HDL-cholesterol and high triglycerides than females in the AUA.
Sensitivity analyses including only unrelated participants (n = 1,829) demonstrated very similar findings for gender-specific age group and regional differences in NCD characteristics and prevalence, although several significant associations were attenuated by the reduced sample size (Supplementary Tables 1–3). Similarly, when logistic regression analyses were repeated with the “unrelated” sample (data not shown), the ORs for 5 of the 16 significant associations in the whole sample became nonsignificant due to reduced sample size but retained their direction and estimate of the OR itself: (1) hypertension in men from SAV; (2) diabetes and high triglycerides in men from ROU; (3) high total cholesterol and high triglycerides in women from SAV.
DISCUSSION
The phenotypic characteristics reported here provide evidence of a continuation of previously reported temporal trends toward obesity and its associated disorders (Keighley et al., 2007). In 2011, a global review of trends in BMI reported that countries in Oceania had demonstrated the greatest increase in mean BMI between 1980 and 2010 of all regions in the world (Finucane et al., 2011). The mean increase in BMI in the region as a whole was reported to be 1.3 kg/m2 per decade for males and 1.8 kg/m2 per decade for females which is more than three times the rate of the global mean (0.4 kg/m2 per decade) (Finucane et al., 2011). The data presented here suggest that Samoans are contributing to this trend. While obesity levels in Samoa (66.4% of the total sample were obese according to the WHO criteria; BMI ≥30 kg/m2) have not risen to those reported in Nauru, where 78.5% of the adult population are obese according to the same cutoffs, they are higher than in Tonga (56%), Kiribati (50.6%), and substantially greater than in Fiji (23.9%) and Vanuatu (15.9%) (World Health Organization, 2013).
The last study of this scale in Samoa was the nationally representative WHO STEPS survey, which was undertaken in 2002 and surveyed 2,817 adults of the same age range. The STEPS survey used WHO criteria for obesity and reported that 44.8% of males aged 25–64 years were obese, as were 66.3% of females (World Health Organization, 2009). Our study showed that, in 2010, the prevalence of obesity was 53.4% in males, an increase of 8.6% in the 8 years between surveys, and 75.5% in females, an increase of 9.2%. This study replicated the STEPS study finding that obesity was greatest in the urban areas and lowest in the rural Savaii region. The STEPS survey reported that obesity prevalence increased with age, and while that is true in the males surveyed here, obesity was prevalent in similar proportions across all age groups in the females we surveyed, suggesting younger females to be at greater risk than their male counterparts.
Rates of hypertension have also risen in the Samoan population since the STEPS survey. In 2002, the STEPS survey reported the prevalence of hypertension in males aged 25–64 years to be 23.1% using the same criteria as were used here (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking medication for hypertension) (World Health Organization, 2009). Our finding that the prevalence of hypertension in 2010 was 36.7% in males of the same age group represents a striking and significant increase. The increase in hypertension in females was greater, rising from 18.8% in 2002 to 31.7% in 2010. These increases in hypertension are likely a reflection of the temporal increases in obesity and reflect the continual rise in hypertension in Samoa and American Samoa from the 1970s to the present (Keighley et al., 2006, 2007; see especially Tables 8.10 and 8.11 in Keighley et al., 2007). Considering the rapid changes in imported prepared foods in Samoa, more work is needed to determine how dietary patterns and specific nutrients such as sodium are related to these blood pressure and hypertension patterns (Seiden et al., 2012). Comparability with the STEPS survey of diabetes prevalence is problematic due to a difference in the testing methodology and the diagnostic criteria used. Using a fasting cutoff of >100 mg/dl (capillary blood) or ≥126 mg/dl (venous blood) or a current use of diabetes medication, the STEPS survey indicated that in 2002, 20.7% of males and 22.4% of females had diabetes (World Health Organization, 2009). We included only those with a fasting blood glucose measurement ≥126 mg/dl or a current use of diabetes medication and report a lower prevalence of diabetes (16.4% in males; 17.8% in females), which we suggest is likely as a result of the differing survey methodologies and diagnostic criteria rather than an actual decrease in diabetes prevalence since 2002.
As was the case for diabetes and hypertension, older participants in this study were more likely to be affected by dyslipidemia. Females were significantly more likely than males to have HDL-cholesterol levels which were too low (≥1.03 mmol/l in males or ≥1.29 mmol/l in females), which is concerning considering the role of HDL-cholesterol in protecting against coronary artery disease and its thrombotic complications (Di Angelantonio et al., 2009).
The internal patterns of disease prevalence shown here reflect social and behavioral patterns widely reported in both this and other populations (Ezeamama et al., 2006; Kannel and McGee, 1979; McGarvey, 2001). In many developing nations, the body composition of women is affected by changes in lifestyle years before that of men, for reasons including a more rapid shift toward sedentary employment activities, multiple pregnancies, and the cultural significance attached to body image, such as the preference for larger female body size. The regional variation in obesity and disease prevalence reported here likely reflect the maintenance of a more traditional lifestyle in the rural regions where residents are more likely to be involved in high energy farming activities and somewhat less exposed to the more calorie dense, fast foods widely available in the AUA. The finding that the prevalence of high cholesterol was greatest in the most rural region is likely a reflection of decreased access to medical care and less rigorous screening than is available in the urban areas. We are also investigating the effect of dietary intake on lipid and lipoprotein levels, especially the putative role of dietary coconut products.
In conclusion, this initial report of the national survey data of NCDs from the 2010 GWAS research shows that we were able to provide representative estimates of the levels and frequencies of key risk factors and NCDs themselves. Further research will focus on, and report, more detailed associations with social, demographic, economic, and behavioral factors. The addition of comprehensive genotyping will allow investigators to explore the genetic basis of NCD in this apparently susceptible population and to begin to elucidate the interaction of genes and environment in determining disease status.
Perhaps most importantly, this survey from 2010 indicates that serious attention must be paid to the critical NCD situation in Samoa. There are alarming levels among adults in obesity, type 2 diabetes, hypertension, and lipidemia, which reflect an unfortunate temporal trend (Keighley et al., 2007). National and local resources must be mobilized to help individuals, communities and organizations address the high NCD levels with priorities to: (1) identify and treat high risk individuals; (2) promote healthful ways of life in diet and exercise and other known risk factors such as cigarette smoking; and (3) plan health interventions in adults and youth to reduce NCD risk levels. Our ongoing translational intervention research on type 2 diabetes in neighboring American Samoa may be a guide for such interventions and the necessary evaluation research (DePue et al., 2010, 2013a, 2013b; Hamid et al., 2013).
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge the Samoan Ministry of Health and the Samoan Bureau of Statistics for their guidance and support in the conduct of this study. Author Contributions: S.T.M., D.E.W., and R.D. designed the GWAS study; N.L.H. led the data collection with the local support of S.V. and M.S.R; N.L.H. and R.L.M. collated data and, with advice from DEW, conducted the statistical analyses. All authors contributed to the interpretation of data and writing and editing of the manuscript.
Contract grant sponsor: U.S. National Institutes of Health; Contract grant number: R01-HL093093.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Åberg K, Dai F, Keighley ED, Sun G, Indugula SR, Roberts ST, Smelser D, Viali S, Tuitele J, Jin L, Deka R, Weeks DE, McGarvey ST. Susceptibility loci for adiposity phenotypes on 8p, 9p, and 16q in American Samoa and Samoa. Obesity. 2009a;17:518–524. doi: 10.1038/oby.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åberg K, Dai F, Sun G, Keighley ED, Indugula SR, Bausserman L, Viali S, Tuitle J, Deka R, Weeks DE, McGarvey ST. A genome-wide linkage scan identifies multiple chromosomal regions influencing serum lipid levels in the population on the Samoan islands. J Lipid Res. 2008;49:2169–2178. doi: 10.1194/jlr.M800194-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åberg K, Dai F, Viali S, Tuitele J, Sun G, Indugula SR, Deka E, Weeks DE, McGarvey ST. Suggestive linkage detected for blood pressure related traits on 2q and 22q in the population on the Samoan Islands. BMC Med Genet. 2009b;10:107–116. doi: 10.1186/1471-2350-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23:1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- Baylin A, Deka R, Tuitele J, Viali S, Weeks DE, McGarvey ST. INSIG2 variants, dietary patterns and metabolic risk in Samoa. Eur J Clin Nutr. 2012;67:101–107. doi: 10.1038/ejcn.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU, Bernt E, Schmidt F, Stork H. d-Glucose determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Academic Press; New York: 1974. pp. 1196–1201. [Google Scholar]
- Bouchard C. Gene–environment interactions in the etiology of obesity: defining the fundamentals. Obesity. 2008;16:S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Rocella EJ, The National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- DePue JD, Dunsiger S, Seiden AD, Blume J, Rosen RK, Goldstein MG, Nu’usolia O, Tuitele J, McGarvey ST. Nurse-community health worker team improves diabetes care in American Samoa: results of a randomized controlled trial. Diabetes Care. 2013b;36:1947–1953. doi: 10.2337/dc12-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePue JD, Rosen RK, Batts-Turner M, Bereolos N, House M, Held RF, Nu’usolia O, Tuitele J, Goldstein MG, McGarvey ST. Cultural translation of interventions: diabetes care in American Samoa. Am J Pub Health. 2010;100:2085–2093. doi: 10.2105/AJPH.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePue JD, Rosen RK, Seiden A, Bereolos N, Chima ML, Goldstein MG, Nu’usolia O, Tuitele J, McGarvey ST. Implementation of a culturally tailored diabetes intervention with community health workers in American Samoa. Diabetes Educ. 2013a;39:761–771. doi: 10.1177/0145721713504630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J, Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBello JR, McGarvey ST, Kraft P, Goldberg R, Campos H, Quested C, Laumoli TS, Baylin A. Dietary patterns are associated with metabolic syndrome in Adult Samoans. J Nutr. 2009;139:1933–1943. doi: 10.3945/jn.109.107888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina C, Meyre D, Gallina S, Durand E, Körner A, Jacobson P, Carlsson LMS, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougnères P, Kovacs P, Marre M, Balkau B, Cauchi S, Chèvre J, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Ezeamama A, Viali S, Tuitele J, McGarvey ST. The influence of socioeconomic factors on cardiovascular disease risk factors in the context of economic development in the Samoan archipelago. Soc Sci Med. 2006;63:2533–2545. doi: 10.1016/j.socscimed.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associates with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S, Dunsiger S, Seiden AD, Nu’usolia O, Tuitele J, DePue JD, McGarvey ST. Impact of a diabetes control and management intervention on healthcare utilization in American Samoa. Chronic Illn. 2013 doi: 10.1177/1742395313502367. [Epub ahead of print] doi: 10.1177/1742395313502367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, McGee DL. Diabetes and cardiovascular risk factors in the Framingham study. Circulation. 1979;59:8–13. doi: 10.1161/01.cir.59.1.8. [DOI] [PubMed] [Google Scholar]
- Karns R, Viali S, Tuitele J, Sun G, Cheng H, Weeks DE, McGarvey ST, Deka R. Common variants in FTO are not significantly associated with obesity-related phenotypes among Samoans of Polynesia. Ann Hum Genet. 2012;76:17–24. doi: 10.1111/j.1469-1809.2011.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keighley ED, McGarvey ST, Quested C, McCuddin C, Viali S, Maga UA. Nutrition and health in modernizing Samoans: temporal trends and adaptive perspectives. In: Ohtsuka R, Ulijaszek SJ, editors. Health change in the Asia-Pacific region: biocultural and epidemiological approaches. Cambridge University Press; Cambridge, NY: 2007. pp. 147–191. [Google Scholar]
- Keighley ED, McGarvey ST, Turituri P, Viali S. Farming and adiposity in Samoan adults. Am J Hum Biol. 2006;18:112–121. doi: 10.1002/ajhb.20469. [DOI] [PubMed] [Google Scholar]
- Kristiansson K, Naukkarinen J, Peltonen L. Isolated populations and complex disease gene identification. Genome Biol. 2008;9:109–117. doi: 10.1186/gb-2008-9-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, Hirshhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- McGarvey ST. Cardiovascular disease (CVD) risk factors in Samoa and American Samoa, 1990–1995. Pac Health Dialog. 2001;8:157–162. [PubMed] [Google Scholar]
- McGarvey ST, Bausserman L, Viali S, Tufa J. Prevalence of the metabolic syndrome in Samoans. Am J Phys Anthropol. 2005;40:S14–S15. [Google Scholar]
- National Cholesterol Education Program . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) National Institutes of Health; Bethesda: 2001. NIH Publication No. 01-3670. [PubMed] [Google Scholar]
- Ohashi J, Naka I, Kimura R, Natsuhara K, Yamauchi T, Furusawa T, Nakazawa M, Ataka Y, Patarapotikul J, Nuchnoi P, Tokunaga K, Ishida T, Inaoka T, Matsumura Y, Ohtsuka R. FTO polymorphisms in oceanic populations. J Hum Genet. 2007;52:1031–1035. doi: 10.1007/s10038-007-0198-2. [DOI] [PubMed] [Google Scholar]
- Peltonen L, Palotie A, Lange K. Use of population isolates for mapping complex traits. Nat Rev Genet. 2000;1:182–190. doi: 10.1038/35042049. [DOI] [PubMed] [Google Scholar]
- Peterson JL, Young DS. Evaluation of the hexokinase-glucose-6-phosphate dehydrogenase method of determination of glucose in urine. Anal Biochem. 1968;23:301–316. doi: 10.1016/0003-2697(68)90361-8. [DOI] [PubMed] [Google Scholar]
- Qi L, Cho YA. Gene-environment interaction and obesity. Nutr Rev. 2008;66:684–694. doi: 10.1111/j.1753-4887.2008.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg NA, Huang L, Jewett EM, Szpiech ZA, Jankovic I, Boehnke M. Genome-wide association studies in diverse populations. Nat Rev Genet. 2010;11:356–366. doi: 10.1038/nrg2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoa Bureau of Statistics [Accessed December 2013];Population and Housing Census 2006. 2008 Available at http://www.sbs.gov.ws/index.php?option=com_advlisting&view=download&fileId=5956&Itemid=164.
- Samoa Bureau of Statistics [Accessed December 2013];Population and Housing Census 2011 Analytical Report. 2012 Available at http://www.sbs.gov.ws/index.php?option=com_advlisting&view=download&fileId=6004&Itemid=164.
- Seiden A, Hawley N, Schulz D, Raifman S, McGarvey ST. Long-term trends in food availability, food prices, and obesity in Samoa. Am J Hum Biol. 2012;24:286–295. doi: 10.1002/ajhb.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Ley SJ, Carmichael HE, Plank LD. Body size and composition in Polynesians. Int J Obes. 1999;23:1178–1183. doi: 10.1038/sj.ijo.0801053. [DOI] [PubMed] [Google Scholar]
- United Nations Development Program (UNDP) Human Development Report 2009. Palgrave Macmillan; New York: 2009. [Google Scholar]
- World Bank [Accessed Dec 2013];2013 Available at http://data.worldbank.org/about/country-classifications/country-and-lending-groups.
- World Health Organization . Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organization; Geneva: 2000. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- World Health Organization [Accessed December 2013];Samoa STEPS Survey Factsheet. 2009 Available at http://www.who.int/chp/steps/2002_Samoa_FactSheet.pdf.
- World Health Organization [Accessed December 2013];Country Health Information Profiles: Samoa. 2011 Available at http://www.wpro.who.int/countries/wsm/29SMApro2011_finaldraft.pdf.
- World Health Organization [Accessed December 2013];Global Database on BMI. 2013 Available at http://apps.who.int/bmi/index.jsp.
- Yajnik CS, Janipalli CS, Bhaskar S, Kulkarni SR, Freathy RM, Prakash S, Mani KR, Weedon MN, Kale SD, Deshpande J, Krishnaveni GV, Veena SR, Fall CHD, McCarthy MI, Frayling TM, Hattersley AT, Chandak GR. FTO gene variants are strongly associated with Type 2 Diabetes but only weakly with obesity in South Asian Indians. Diabetologia. 2009;52:247–252. doi: 10.1007/s00125-008-1186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.