Abstract
Background and Aim
A major cause of cancer-related deaths is the development of liver metastasis. To better understand the metastatic process, we studied the cotton top tamarin as an animal model, which spontaneously develops colorectal cancer but rarely liver metastasis.
Method
DNA was extracted from primates and Hot-Start PCR was performed. Sequencing was achieved with Big-Dye Terminator™ Sequencing Kit. Tissue expression and glycosylation studies were also performed for carcinoembryonic antigen family proteins.
Results
Sixty-three percent of tamarin carcinoembryonic antigen had PELPK changes essential for carcinoembryonic antigen hepatic uptake. Tamarin carcinoembryonic antigen showed minimal glycosylation. Cotton top tamarin livers showed reduced carcinoembryonic antigen-receptor expression and were devoid of CEACAM1 (BGP) as compared to human liver despite positive expression in cotton top tamarin gallbladder mucosa. Peritumoral regions showed more CEACAM1 in human hepatocyte cytoplasm than in biliary canaliculi (P < 0.05). Therefore, tamarins may evade liver metastasis through mechanisms of decreased hepatic uptake by altered PELPK sequences, reduced glycosylation and reduced carcinoembryonic antigen-receptor expression. Furthermore, the absence of cotton top tamarin hepatocyte CEACAM1 may lead to alteration of the liver milieu creating an inhospitable “infertile-field” for metastases.
Conclusions
Four hypotheses explain a complex mechanism for the lack of liver metastasis: (1) carcinoembryonic antigen PELPK-encoding nucleotide sequence changes, (2) minimal carcinoembryonic antigen glycosylation, (3) reduced carcinoembryonic antigen-receptor expression, and (4) reduced CEACAM1 distribution, a putative vascular endothelial growth factor. While these hypotheses are not necessarily causal they are testable and therefore are feasible targets for prevention of hepatic metastasis in man.
Keywords: Cotton top tamarin, Carcinoembryonic antigen, CEACAM1, Sequencing, Metastasis
Introduction
The treatment of cancer involves ablating the primary tumor and treatment of distant metastases. Unfortunately, hepatic metastases often herald a fatal outcome [1] despite considerable effort in clinical research with modest gains [2]. Currently, most in vivo models of colorectal liver metastasis development have used rodents [3]. However, models in higher primates are likely to provide more representation of the human metastatic process. Saguinus oedipus, the cotton top tamarin (CTT), is one such primate known to contract colitis in the feral state and in captivity. Colorectal cancer (CRC) develops spontaneously after the emergence of chronic inflammatory bowel disease as in man [4]. While the primary disease entities and tumor antigenic profiles are very similar in man and the CTT [5], a striking difference between the CTT and humans is the paucity of liver metastasis in the CTT [6]. The CTT develops CRC complicating colitis with one-third dying of CRC, while another member of the Callitrichidae family, Callithrix jacchus, the common marmoset (CM), develops colitis alone and may be considered as a natural control in terms of development of malignancy.
The metastatic process requires multiple interactions between selected cancer cells (seed) and specific organ microenvironments (soil) as originally suggested by Paget [7]. The interaction of cells with their environment is mediated by proteins collectively known as intracellular adhesion molecules (ICAMs). While ICAMs are found in several different molecular families, the majority identified to date belong to the immunoglobulin (Ig) supergene family [8]. Among the Ig superfamily, carcinoembryonic antigen (CEA) is a well-known tumor marker as important in hepatic implantation of circulating CRC cells and correlates with the metastatic potential in an animal model [9]. CEA uptake is medicated by a specific receptor (CEAR) on the liver macrophage (Kupffer cell) via the peptide sequence, Pro-Glu-Leu-Pro-Lys (PELPK). CEA uptake activates Kupffer cells which then produces inflammatory cytokines that influence tumor cell retention in the hepatic sinusoids [10]. Changes in the sequence of the PELPK region can lead to loss of CEA binding capacity [11]. We hypothesize that changes from the human sequence in this region may be the mechanism at least in part for the paucity of liver metastasis in the CTT. We previously characterized CEA family expression in the CTT and CM [12]. We therefore undertook to determine the DNA sequences flanking the PELPK region in the CTT for changes that would affect Kupffer cell binding and activation.
Among the other Ig superfamily moieties shared by Callitrichidae and humans is CEACAM1, a member of the CEA subfamily, also known variously as biliary glycoprotein (BGP) and CD66a [13]. It has been demonstrated that CEACAM1 may function as a down stream effector of vascular endothelial growth factor (VEGF) in the early stages of angiogenesis [14, 15]. Because such a function may be important in metastasis, we also analyzed CEACAM1 expression in CTT liver and compared it with human liver samples.
Multiple factors may be involved in metastasis, one of which may be mediated through the binding of extracellular matrix components to cell surface receptors [16]. Alternatively, species differences may confound a cause and effect and wherever possible should be controlled for.
In this study, we describe potential pathways for the avoidance of hepatic metastasis by the CTT. To our knowledge, this is the first systematic study of such factors in a primate model.
Materials and Methods
Sequencing of Primate Genomic DNA
Archival extracts from post mortem specimens of Callitrichidae representative of normal and tumor tissues were obtained from the University of Tennessee Marmoset Research Colony (MARCOR, Oak Ridge, TN) as described previously [5, 12, 17]. Tissues were stored at −70°C with reproducibility studies performed before and after prolonged storage to document stability.
Blood samples from Old- and New-World primates and normal-appearing CTT and CM colonic tissues were collected. Two hundred microliters of sample were centrifuged at 14,000 × g and genomic DNA was extracted from pellets using the DNeasy Tissue Kit™ (QIAGEN, Valencia, CA). We performed Hot-Start PCR using 200 ng of genomic DNA with AmpliTaq Gold DNA Polymerase (Applied Biosystems, Foster City, CA) in a final volume of 50 µl as previously described [18]. PCR was performed under initial denaturing cycle at 95°C for 5 min, then 30 cycles of 94, 60 and 72°C for 30 s each, and a final extension cycle was at 72°C for 5 min. The primers were based on the DNA sequences coding for the PELPK region of CEA. The first set was designed for the region straddling intron 2 and the A1-domain hinge (5′-TGGAAGGTGC CAACTCTGAT-3′ and 5′-CTGGGTTCTGGGTTTCACA T-3′). The second set was based on the region straddling most of the N-domain and the upstream region of intron 2 (5′-GTGCTGGTTGTTGTGCTGTC-3′ and 5′-TTGGGTC GACACTAGTACGC-3′). We performed sequencing with Big-Dye Terminator™ Cycle Sequencing Ready Reaction Kit and the ABIPRISM 377® system (Applied Biosystems, Foster City, CA). Only changes resulting in deduced amino acid substitutions (nonsynonymous) were considered.
CEA Determination and Western Blotting
We used the AIA-PACK CEA Kit (Tosoh Medics Inc., San Francisco, CA) for CEA quantification (threshold 0.05 ng/ml). This kit was used because other available commercial kits do not react with the CTT CEA epitope recognized by this kit [17]. It incorporates a solid phase anti-CEA antibody of Gold group IV and a more specific tracer, anti-CEA Gold group I antibody [19]. Serum CEA was determined in 10 Callitrichidae and 25 research study patients from Kaiser Permanente Medical Center (Sacramento, CA) as previously described [12, 17]. Reproducibility studies were performed on samples after prolonged storage.
CEA deglycosylation experiments were conducted as described previously [20]. Briefly, tissue extracts from CTT were run on 12% SDS–PAGE before and after treatment with N-glycosidase (Roche, Indianapolis, IN) as per manufacturer’s instructions and similarly treated human CEA was used as positive controls. After transfer to PVDF membranes (Schleicher and Schuell Bioscience, Keene, NH), Western blotting with 1:500 highly specific anti-CEA monoclonal antibody, T84.66, was performed [12]. The human CEA and anti-CEA antibody were kind gifts of Dr. J. Shively (Beckman Research Institute, City of Hope, Duarte, CA). For CEAR expression study, we used anti-heterogeneous nuclear ribonucleoproteins M4 (hnRNP M4; Upstate, Lake Placid, NY) monoclonal antibody [21, 22].
Immunohistochemistry
Immunostaining was achieved as described in Hsu et al. [23], and graded as previously described [24] for each component of the hepatic parenchyma. The anti-CEACAM1 antibody 4D1/C2 was graciously provided by Dr. C. Wagener (University of Freiburg, Germany). We labeled 4-µ tissue sections from archival liver biopsies from patients with cirrhosis (n = 16), chronic active hepatitis without cirrhosis (n = 18), and liver metastasis from diverse primary tumors (n = 6). Thirteen sections from Callitrichidae livers, with gallbladder mucosa included in some, serving as an internal positive control, were similarly stained. These tissues originated from the New England Regional Primate Center (Boston, MA). Human livers were also stained with T84.66 monoclonal antibody (see above) to ensure that there was no CEA expressed in the liver parenchyma. CTT do not express the CEA epitope recognized by T84.66 in fixed tissues [12, 17], thus staining for CEA was not performed. A polyclonal full-length human CEAR antibody [21, 22] was used for staining.
Approval for the study was obtained from Wayne State University Institutional Review Board. Informed consent was obtained from the human participants where appropriate.
Statistical Analysis
Differences in proportions were compared in 2 × 2 contingency tables and analyzed by Fisher’s exact test with Yates’ correction. Differences in means were analyzed by Student’s t-test and linear correlation by logistic regression analysis by the method of least squares. Probability values of P < 0.05 were considered significant.
Results
Sequence Changes in the PELPK Region
We evaluated the CTT for potential nucleotide sequence changes in the PELPK region, compared them to the known human sequence, and to that of sequences obtained in the CM. Table 1 shows a cluster multiple sequence alignment of genomic human amino acids (HCEA-A1) beginning with the PELPK sequence of CEA in comparison with sequences obtained from representative nonhuman primates. Interestingly, only one (in CTT) or two (in CM) amino acids differ from those of humans. This suggests that the sequence of PELPK regions of the CEA is conserved throughout humans and Callitrichidae. Representative somatic amino acid sequences in CTT and CM are shown in Table 2. In CTT 63% (12/19) showed PELPK substitutions while only 29% (5/17) of CM showed these changes (P < 0.05). Of these, 71% (5/7) of cancer-bearing CTT showed PELPK changes compared with 58% (7/12) of CTT without CRC (P > 0.05). Mutations in the CEA PELPK pentapeptide may affect CEA uptake via hepatic binding sites of Kupffer cells and may therefore lead to decreased CEA uptake in CTT. Because CM is not endangered, further research could be considered in this animal species.
Table 1.
Genomic amino acid sequences for primates in the Pro-Glu-Leu-Pro-Lys (PELPK) region of the carcinoembryonic antigen (CEA) gene
| Primates | Sequences |
|---|---|
| HCEA-A1 | PELPKPSISSNNSKPVEDKDAVAFTCEPETQDA |
| Common Chimpanzee (old world) | PELPKPFIFSINFKPVEDKDAGAFTCEPETQDA |
| Rhesus Macaque (old world) | PELPKPSISSNNSKPVEDKDAGAFTCEPETQDA |
| Tarsier (new world) | PELPKPSISSNNSKPVEDKDAVAFTCEPETQDA |
| Mangaby (old world) | RELPKPFIFSFNFKPVEDKDAGAFTCEPETQDA |
| Red Howler (new world) | RELPKPSIFSFNSKPVEDKDAGAFTCEPETQDA |
| Black Howler (new world) | RELPKPSIFSFNSKPVEDKDAGAFTCEPETQDA |
| Squirrel (new world) | RELPKPSIFSFNSKPVEDKDAGAFTCEPETQDA |
| Titi (new world) | PELPKPSIFSFNSKPVEDKDAGAFTCEPETQDA |
| Common Marmoset (new world) | PELPKPSISSINSKPVEDKDAGAFTCEPETQDA |
| Cotton Top Tamarin (new world) | PELPKPSISSNNSKPVEDKDAGAFTCEPETQDA |
Genomic DNA from blood samples of Old- and New World primates are compared. HCEA-A1 is the normal human sequence derived from a human colorectal cancer cell line (MIP-101). Changes from the human sequences in the primates are shown in bold
Table 2.
Representative somatic sequences from the Pro-Glu-Leu-Pro-Lys (PELPK) region of carcinoembryonic antigen (CEA) in cotton top tamarin (CTT) and common marmoset (CM)
| Primates | Sequences |
|---|---|
| CM-WT | PELPKPSISSINSKPVEDKDAGAFTCEPETQDA |
| CTT-WT | PELPKPSISSNNSKPVEDKDAGAFTCEPETQDA |
| M1-A1 (CTT) | PEVPKPSISSNNSKPGGDKDAGAFTWEPETQDA |
| M2-A1 (CTT) | PEVSKPSISSNNSKPGGDKDAGAFTWEPETQDA |
| M3-A1 (CTT) | PEVSKPSISSNNSKPVGDKDAGAFTWEPETQDA |
| M4-A1 (CTT) | PEVSKPSISSNNSKPVGDKDAGAFTWEPETQDA |
| M5-A1 (CTT) | PEVSKPYISSNNSNPVENKDAGAFTWEPETQDA |
| M6-A1 (CTT) | PEVSKPFIFSNNSKPGGDKDAGAFTWEPETQDA |
| M8-A1 (CTT) | PEVSKPFIFRNNSKPGGDKDAGAFTWEPGTQDA |
| M10-A1 (CTT) | PELPKPFIFSNNFKPVEDKDAVAFTCEPETQDT |
| M14-A1 (CTT) | PELPKPFIFSNNFKPVEDKDAVAFTCEPETQDA |
| M15-A1 (CTT) | PELPKPFIFSNNFKPVEDKDAVAFTCEPETQDA |
| M16-A1 (CTT) | PELPKPSILSNNSKPVEDKDAVAFTCEPETQDA |
| M17-A1 (CTT) | PELPKPSIPSNNSNPVEDKDAVGLTCEPDTQNT |
| M19-A1 (CM) | PELPKPSISSYNSKPVEDKDAGAFTCEPETQDA |
| M20-A1 (CM) | PELPKPSISSYNSKPVEDKDAGAFTCEPETQDA |
| M21-A1 (CM) | PELPKPFISSYNFKPVEDKDAGAFTCEPETQDA |
| M24-A1 (CM) | PELPKPSIFSNNSKPVEDKDAVAFTCEPETQDA |
| M27-A1 (CM) | PELPKPSISSNNSNPVEDKDAVAFTCEPETQDA |
| M31-A1 (CM) | PEVSKPFIFSNNSKPVGDKDAGAFTCEPETQDA |
| M40-A1 (CM) | PGVPKPFIFRINFKPVGDKDAGAFTCEPETQDA |
Sequence changes from those of wild type (germ line) are shown in bold
Reduced Expression of CEA-Receptor
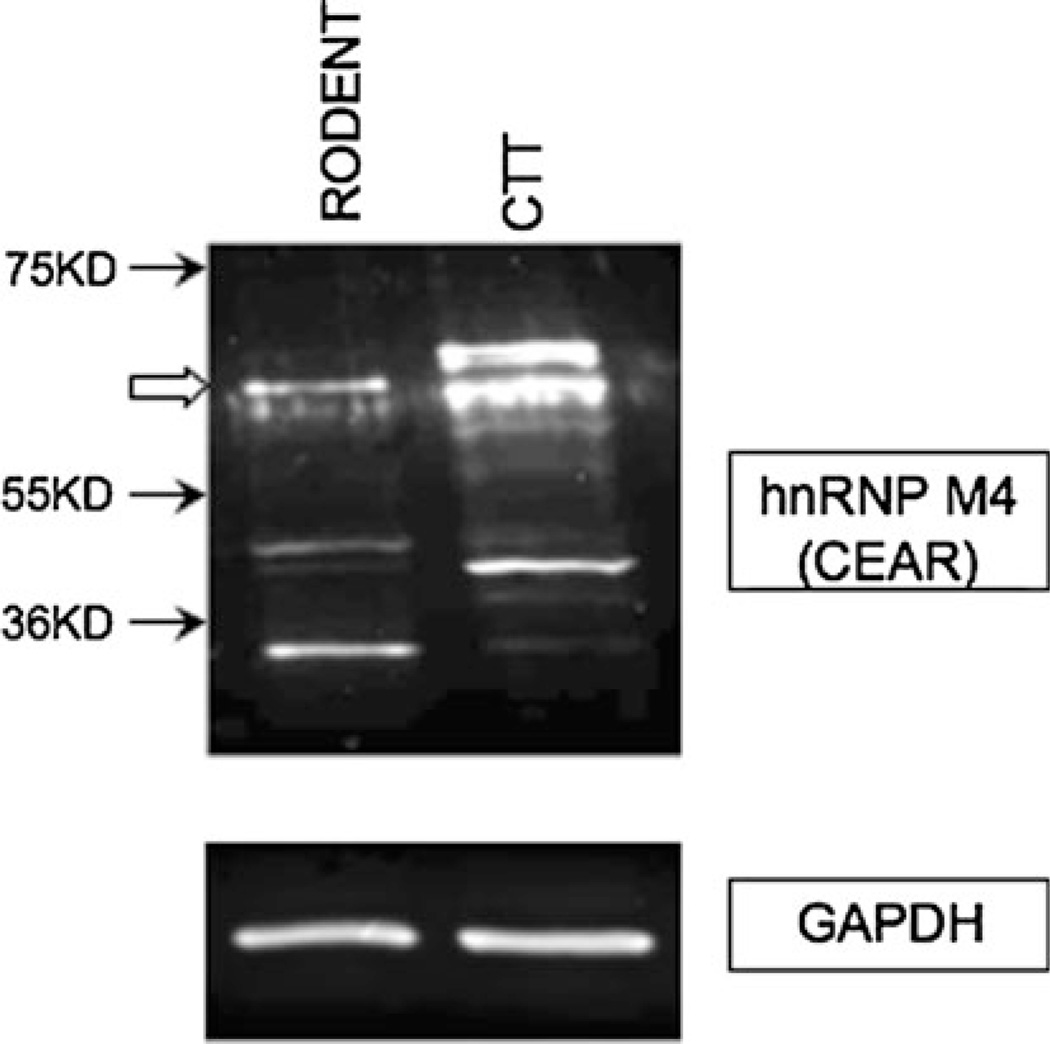
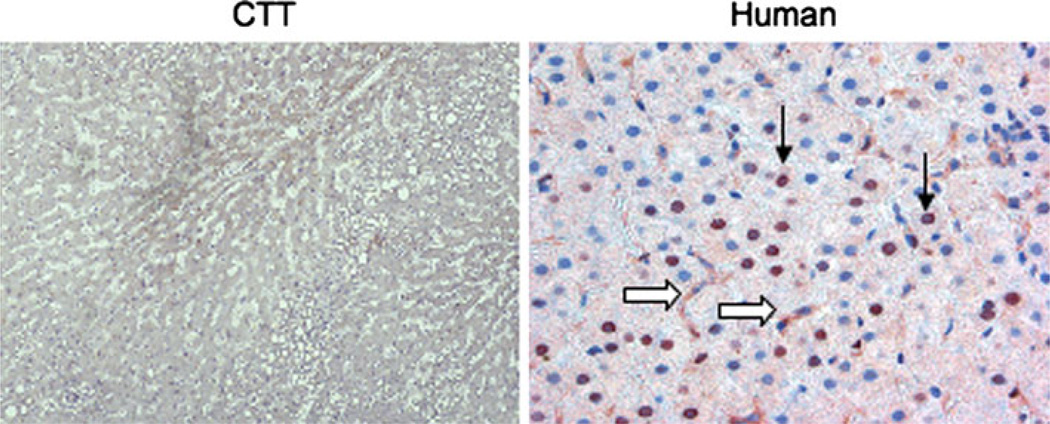
PELPK mutations are unlikely entirely accountable for the lack of liver involvement in the remaining 98.8% of CTT with cancer. Another possibility is decreased expression of the CEA-receptor (CEAR). We performed Western blotting with anti-hnRNP M4 (heterogeneous nuclear RNA binding protein M4 in human) which is an ortholog of the rat Kupffer cell CEAR [22]. The presence of specific bands of CEAR in CTT liver was observed (Fig. 1). For expression studies, CTT liver tissue sections were stained and compared to human samples. CEAR was found mainly in the nuclei of hepatocytes with some cytoplasmic staining in both CTT and human (Fig. 2). However, the presence of CEAR was significantly greater in 28 human livers compared to six CTT liver sections in both the hepatocytes and Kupffer cell (77 vs. 42%; 71 vs. 33%, respectively; P < 0.05). In humans, Kupffer cell and bile duct cell cytoplasmic staining was much stronger than that of hepatocytes with greater proportions staining positive (84, 56 & 16%, respectively; P < 0.05 by Fisher’s exact test). This suggests that a lower CEAR expression may lead to reduced hepatic uptake of CEA in the CTT liver compared to human.
Fig. 1.
Western blotting result shows the presence of specific bands of CEAR (CEA uptake medicated by a specific receptor) in cotton top tamarin (CTT) liver. hnRNP M4 (heterogeneous nuclear RNA binding protein M4) is a receptor for carcinoembryonic antigen (CEA) in Kupffer cells. GAPDH serves as loading control. Open arrow indicates a specific band
Fig. 2.
Distribution of CEAR (carcinoembryonic antigen (CEA) uptake medicated by a specific receptor) in cotton top tamarin (CTT) and human livers. Staining performed with anti-full-length human CEAR antibody. CTT on left (in low power) and human on right (in high power). CEAR was found mainly in the nuclei of hepatocytes (arrows) with some cytoplasmic staining in both CTT and human. However, CEAR expression was significantly greater in humans liver compared to CTT liver sections, in both hepatocytes and Kupffer cells (open arrows)
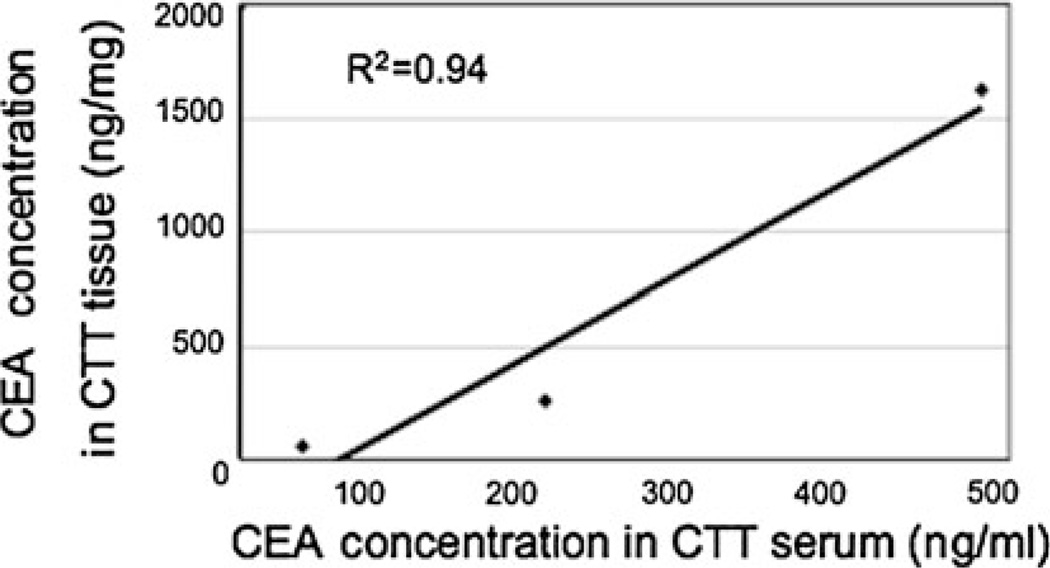
Because PELPK and CEAR changes in the CTT may result in decreased CEA uptake and subsequent secretion in bile, it could be anticipated that CEA concentrations in the blood would be higher in CTT than in humans. We therefore studied CEA blood levels in CTT and CM, and compared them to humans (Table 3). CEA concentrations in CTT were much higher than in humans with significant colorectal neoplasia (adenomas and cancers) including one CRC patient. Low CEA levels in the human samples may be attributed to early stage lesions in this group. CTT samples are from both normal and cancer-bearing animals. Stability, as measured by repeat assays at more than a 5-year interval, showed reproducibility in both human blood (r = 0.997; n = 12) and CTT tissues (r = 0.891, n = 5; data not shown). CTT CEA levels in cancerous versus normal mucosa were not significantly different (700.8 ± 601.6 and 632.1 ± 632.1 ng/ml by volume or 417.8 ± 455.7 and 385.8 ± 236.9 ng/mg by protein, respectively), unlike the situation in humans. A lack of correlation between human tissue CEA contents and blood levels is well described [25]. However, unlike the human situation, blood and tissue levels of CEA in the CTT tend to correlate (Fig. 3; r2 = 0.94; n = 3). Taken together, these data suggest that elevated plasma level of CEA in CTT may reflect reduced hepatic uptake of this protein.
Table 3.
The blood CEA values in humans, CTT and CM
| Species | Human | CTT | CM | ||
|---|---|---|---|---|---|
| Normal | Significant colorectal neoplasiaa |
Othersb | |||
| Number | 6 | 11 | 8 | 5 | 5 |
| Mean CEA (ng/ml) | 1.97 | 1.93 | 2.40 | 191.5* | 0.14 |
| Standard deviation | 0.84 | 0.86 | 2.15 | 79.9c | 0.31 |
CEA carcinoembryonic antigen, CTT cotton top tamarin, CM common marmoset
Including one colorectal cancer (CRC) patient
Including family history of CRC and insignificant adenoma; normal level CEA is <2.5 ng/ml
Means significantly different between CTT and CM, P < 0.006
P < 0.05
Fig. 3.
Correlation between blood and tissue levels of carcinoembryonic antigen (CEA) in the cotton top tamarin (CTT). Linearity of CEA blood and tissue levels in CTT is demonstrated. This suggests reduced hepatic uptake of CEA as a potential explanation
Differences in CEA Glycosylation
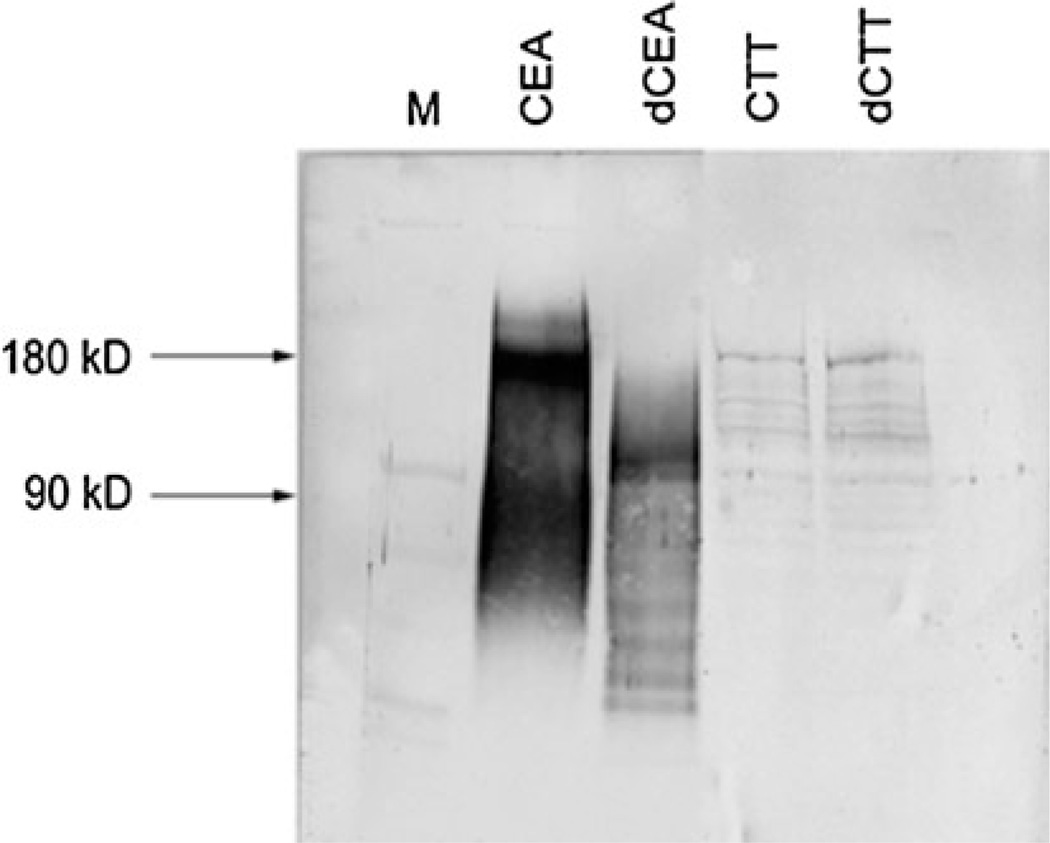
The alteration of a post-transcriptional glycosylation of ICAMs may contribute to a more invasive or metastatic phenotype in CRC [26]. Like other members of the Ig superfamily, CEA is heavily glycosylated, and the pattern is altered in CRC [27]. We therefore compared CEA glycosylation in the CTT and human. CTT CEA showed a low level of glycosylation compared to human CEA (Fig. 4). In CTT CEA the protein core is ~90 kDa, as seen in human CEA [20]; but unlike human, there is no marked increase in relative mobility by deglycosylation. Decreased glycosylation is associated with decreased metastatic potential of human colon cancer cells [28]. Therefore, reduced CTT CEA glycosylation may further explain the rarity of hepatic metastasis.
Fig. 4.
Representative deglycosylation result of carcinoembryonic antigen (CEA) in human and cotton top tamarin (CTT). Deglycosylation was achieved by treatment with N-glycosidase. Western blotting was performed with T84.66 monoclonal antibody. A molecular weight ladder is shown in the first lane (M) for comparison. Human CEA is shown as native product in lane 2 and exhibits increased mobility effect by deglycosylation (lane 3; dCEA). No increased mobility is seen on glycosylation treatment in CTT samples (lane 5; dCTT) compare to control (lane 4)
CEACAM1 Expression in Liver Tissues
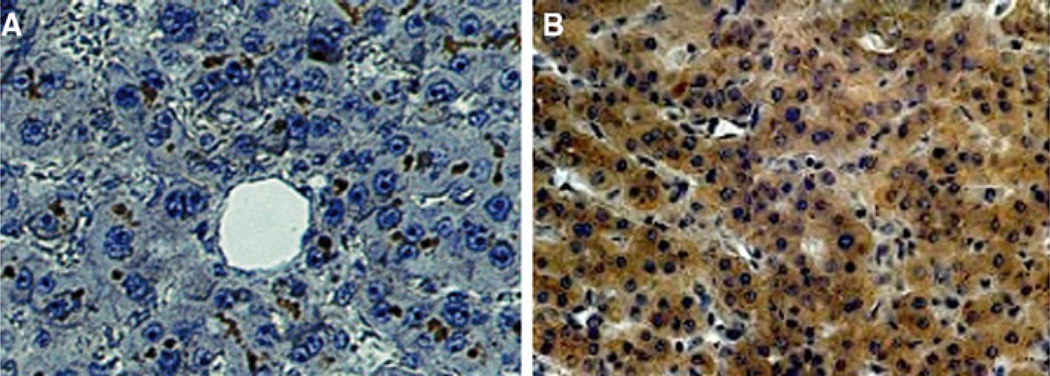
CEACAM1 is another member of the Ig superfamily shared by Callitrichidae and humans playing an important role in angiogenesis [14, 15]. We hypothesize that its functional and structural roles may be important in metastasis. We therefore immunostained liver sections for CEACAM1; CTT and CM livers were devoid of CEACAM1 with positive expression in gallbladder mucosa (data not shown). CEACAM1 was expressed in normal human livers, mainly in the hepatocyte biliary canaliculus (Fig. 5a). In human livers with metastatic disease, the peritumoral regions showed an apparent translocation of the CEACAM1 from the hepatocyte biliary canaliculus to the cytoplasm of hepatocytes (Fig. 5b). With respect to the hepatocyte cytoplasm, there were no significant differences in any of the diagnostic non-metastatic categories (Table 4). In contrast, CEACAM1 expression in cholangiocytes and in hepatocyte canaliculi was significantly reduced in patients with metastases as compared to those with chronic hepatitis or cirrhosis (P < 0.02; Table 4). CEACAM1 in Kupffer cells was seen only in cirrhosis and chronic hepatitis patients with minimal staining (9 and 6%, respectively). The possible translocation of CEACAM1 from the hepatocyte biliary canaliculus where it is a purely structural entity to the cytoplasm of hepatocytes where it is a possible functional entity may represent the provision of a fertile field for hepatic metastasis as CEACAM1 is a mediator of VEGF [15]. Thus, absence of CEACAM1 expression in CTT abrogates a fertile field for cancer cell growth.
Fig. 5.
Distribution of CEACAM1 in human liver. There is no CEACAM1 expression in cotton top tamarin (CTT) and common marmoset (CM) despite expression of CEACAM1 in gallbladder mucosa. a Normal human liver stained with CEACAM1 monoclonal antibody, 4D1/C2. Photomicrograph shows dark staining mainly in hepatocyte biliary canaliculi. b Distribution of CEACAM1 in human liver with metastases. Photomicrograph of normal area of a metastasis-bearing human liver, showing dark staining distributed mainly within the cytoplasm of hepatocytes, with no canalicular staining visible
Table 4.
Percentage of positive staining with CEACAM1 specific monoclonal antibody, 4D1/C2, in human liver
| Category (n) | Hepatocyte | Cholangiocyte | |
|---|---|---|---|
| Cytoplasm | Canaliculus | ||
| Cirrhosis (12) | 83 | 91.7 | 75 |
| Chronic hepatitis (14) | 62.5 | 93.8 | 67 |
| Metastatic liver (6) | 50 | 33.3a | 16b |
Single normal human liver sample was used as the control
OR26, CI 1.84–367.9, P = 0.014
OR20, CI 1.65–241.87, P = 0.01
These findings are summarized in Table 5. The differences seen between CTT, CM and human subjects may suggest potential pathways used by the CTT to avoid hepatic metastasis.
Table 5.
Summary of the findings in this study
| Result | CTT | CM | Human |
|---|---|---|---|
| PELPK change | ++++ | ++ | − |
| CEAR expression | ± | ± | ++++ |
| CEA glycosylation | ± | ± | ++++ |
| CEACAM1 expression | − | − | ++++ |
CTT cotton top tamarin, CM common marmoset
Discussion
In this study, we suggest a molecular basis for the paucity of hepatic metastases in the CTT that is supported by genomic, clinical and immunologic data. Because CEA appears to be important for the uptake and growth of colonic tumor cells in the liver, the described PELPK substitutions may be the mechanism partially accounting for the paucity of liver metastasis in the CTT. PELPK substitutions are found in 63% of CTT and 29% in CM. In CTT these changes are similar between cancer and non-cancer animals (71 and 58%, respectively, P > 0.05). Serine (S) is replaced by phenylalanine (F) just after the PELPK sequence and the second proline (P) is replaced by serine (S) as previously observed in human subjects [18]. Because there is an intron that breaks the codon for the first P of the PELPK sequence, there could be inherent regional instability of the gene creating mutational hotspots in the CEA binding sequence region in both man and monkeys. CRC patients with PELPK mutations showed high CEA concentration in the serum [18] suggesting decreased CEA uptake into the liver. Similarly, CTT PELPK changes may also result in decreased hepatic uptake with resultant higher CEA serum levels than those found in normal human controls. PELPK substitutions may reduce hepatic uptake of CEA expressing tumor cells in the CTT, resulting in reduced implantation, and maintain survival by altering the liver inflammatory response to elevated CEA levels [10].
The linear relationship between CEA CTT tissue and blood levels (Fig. 3) suggests that the PELPK-associated reduced hepatic uptake of cancer cells results in the lack of liver tumors. Identical CEA stability in the CTT and humans exclude this as a cause for differing concentration. Our previous work has shown that the CTT DNA encoding the carboxyl-terminal domain of the CEA family member molecules shows homology with the human suggesting that this moiety is relatively well-conserved in this primate through to the human [29]. Higher CEA tissue levels may partially explain the increased serum levels seen in the CTT but the major contribution is likely to be reduced hepatic uptake and clearance of CEA. Low CEAR expression levels in CTT may further explain the ability of these animals to evade metastases since they have both a mutated CEA uptake motif and a reduced CEA ligand binding capacity.
In our deglycosylation studies, CTT and CM CEA showed little glycosylation as compared with human CEA (Fig. 4). This lack of post-translation modification of CTT CEA may also contribute to the decreased incidence of liver metastases. These differences in the CTT that may potentially inhibit metastasis might permit the design of specific drugs acting as CEA decoys altering the hepatic environment and blocking hepatic uptake of cancer cells. Gadolinium has been shown to effectively block Kupffer cell function in rats [30], and gadolinium blockade allows us to study human Kupffer cell blockade and CEA uptake kinetics.
CEACAM1 is localized in endothelial cells and in the basement membrane of tumor capillaries [15]. Localization of CEACAM1 in capillaries belies its role in angiogenesis. However, since matricial vascular mimicry may apply to primary tumors as well as metastasis [31], distribution in any one location may be important. We postulate that if the protein expression is structural in nature (canalicular membrane) it cannot act as an angiogenesis factor, however, if solubilised it can function as an angiogenesis growth factor. Previous work using CEACAM1 in human showed a similar expression pattern to what we describe in our observations [32, 33]. Our results showed no expression of CEACAM1 in CTT and CM livers despite expression in gallbladder mucosa. In a study of rats with liver disease, increased secretion of a CEACAM1 homolog was demonstrated in the serum and urine, but not in the cholangiocyte [34], suggesting a translocation/disruption of CEACAM1 from its usual structural hepatocyte bile canalicular-bound location. This hepatic distribution is similar to that found in our patients with metastases. In the case of these patients, the apparent change of CEACAM1 distribution from the hepatocyte biliary canaliculus to the cytoplasm of hepatocytes may have allowed the now putatively soluble CEACAM1 to assume a VEGF function, providing a fertile field for hepatic metastasis growth. Whether the mechanism is a local manifestation of a paracrine effect exerted by metastatic cells in the liver or a more generalized systemic paraneoplastic effect of the primary tumor remains to be established. This notwithstanding, using another less specific monoclonal antibody that cross-reacts with CEA and CEACAM1 (T84.1; [17]), we found that the Callitrichidae express CEACAM1 in cancerous and non-cancerous tissues (data not shown). This suggests that this antigen and its epitope exists in the CTT but is absent from the liver parenchyma. The absence of CEACAM1 from liver parenchyma may present an inhospitable environment for the cancer cell related to its angiogenic effector.
These attributes may confer anti-metastatic properties and further enhance the anti-metastatic array exhibited by the CTT, which could be further studied in the CM and then applied to the human. These data are presented more to reflect testable hypotheses rather than causal events and the latter remain to be demonstrated in future studies.
We therefore hereby propose plausible and multiple mechanisms by which CTT evades hepatic metastasis. CTT is liable to decrease hepatic uptake both by functional (missense) mutations on PELPK sequences that are important for liver uptake of CEA, as well as by decreased CEA glycosylation. A third potentially important pathway is reduced CEAR expression in the CTT, which may further lead to reduced CEA uptake. The fourth is the absence of hepatocyte CEACAM1 possibly leading to alteration of the internal mileau of the liver excluding molecules that could act as a angiogenesis VEGF effector, thus creating an inhospitable environment for blood-borne metastasis. Providentially, these pathways are feasible targets for prevention of hepatic metastasis in man.
Acknowledgments
The authors would like to thank Violeta Iordanova, Fabrizio Di Noto M.D., Jason Hallman, B.S., and Prabhat Seghal M.D. for their able assistance. We are grateful for Lois Nakayama (Tosoh Medics) for performing CEA analysis and Dr. Karel Kithier’s contribution to the discovery of CTT CEA. We are extremely grateful to Drs. Linda E. Greenbaum, David Kaplan and Martin Heyworth for their internal review of this manuscript and to Dr. Kaplan for providing research material. This article is dedicated to the memory of Dr. Neal K. Clapp, a devoted veterinarian who deeply cared about the welfare of his primate charges and pioneered the adoption of the CTT as a model for human inflammatory bowel disease and colorectal cancer. He was one of the original authors and made this entire work possible. This work was supported in part by the National Cancer Institute (CA 74941), NIH/NCRR (PS 1RR000168), the Kaiser Permanente Research Foundation and the Health Future Foundation (Creighton University).
Footnotes
Disclosure The authors declare no conflict of interest.
Contributor Information
Martin Tobi, Email: martin.tobi@va.gov, Department of Medicine and Pathology, VAMC and Wayne State University, Detroit, MI, USA; Gastroenterology Division, University of Pennsylvania, Philadelphia, PA, USA.
Mijin Kim, Gastroenterology Division, University of Pennsylvania, Philadelphia, PA, USA.
Regis Zimmer, Department of Surgery, Boston University, Boston, MA, USA.
James Hatfield, Department of Medicine and Pathology, VAMC and Wayne State University, Detroit, MI, USA.
Michael Kam, Department of Medicine and Pathology, VAMC and Wayne State University, Detroit, MI, USA.
Nabiha Khoury, Department of Medicine and Pathology, VAMC and Wayne State University, Detroit, MI, USA.
Angela Carville, New England Primate Center, Boston, MA, USA.
Michael J. Lawson, Kaiser Permanente Medical Center, Sacramento, CA, USA
William P. Schiemann, Department of Pharmacology, University of Colorado, Aurora, CO, USA
Peter Thomas, Department of Surgery, Boston University, Boston, MA, USA; Department of Surgery, Creighton University, Omaha, NE, USA.
References
- 1.Sporn MB. The war on cancer. Lancet. 1996;347:1377–1381. doi: 10.1016/s0140-6736(96)91015-6. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Robertson JHP, Sarkar S, Yang SY, Seifalian AM, Winslet MC. In vivo models for early development of colorectal liver metastasis. Int J Exp Path. 2008;89:1–12. doi: 10.1111/j.1365-2613.2007.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapp NK, Nardi RV, Tobi M. Future directions for colon disease research using cotton-top tamarin. In: Clapp NK, editor. A primate model for the study of colitis and colonic carcinoma: the cotton-top tamarin. Boca Raton: CRC Press; 1993. pp. 319–324. [Google Scholar]
- 5.Tobi M, Chintalapani S, Kithier K, Clapp N. Gastrointestinal tract antigenic profile of cotton-top tamarin, Saguinus oedipus, is similar to that of humans with inflammatory bowel disease. Dig Dis Sci. 2000;45:2290–2297. doi: 10.1023/a:1005622521294. [DOI] [PubMed] [Google Scholar]
- 6.Lushbaugh C, Humason G, Clapp N. Histology of colon cancer in Saguinus oedipus. Dig Dis Sci. 1985;12(Suppl):119S–125S. doi: 10.1007/BF01296990. [DOI] [PubMed] [Google Scholar]
- 7.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 8.Williams AF, Barclay AN. The immunoglobulin superfamily-domains for cell surface recognition. Ann Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 9.Thomas P, Gangopadhyay A, Steele G, Jr, et al. The effect of transfection of the CEA gene on the metastatic behavior of the human colorectal cancer cell line MIP-101. Cancer Lett. 1995;92:59–66. doi: 10.1016/0304-3835(95)03764-n. [DOI] [PubMed] [Google Scholar]
- 10.Gangopadhyay A, Bajenova O, Kelly TM, Thomas P. Carcinoembryonic antigen induces cytokine expression in Kupffer cells: implications for hepatic metastasis from colorectal cancer. Cancer Res. 1996;56:4805–4810. [PubMed] [Google Scholar]
- 11.Gangopadhyay A, Thomas P. Processing of carcinoembryonic antigen by Kupffer cells: recognition of a penta-peptide sequence. Arch Biochem Biophys. 1996;334:151–157. doi: 10.1006/abbi.1996.0440. [DOI] [PubMed] [Google Scholar]
- 12.Tobi M, Chintalapani S, Kithier K, Clapp N. Carcinoembryonic antigen family of adhesion molecules in the cotton top tamarin (Saguinus oedipus) Cancer Lett. 2000;157:45–50. doi: 10.1016/s0304-3835(00)00482-1. [DOI] [PubMed] [Google Scholar]
- 13.Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagener C, Ergün S. Angiogenic properties of the carcinoembryonic antigen-related cell adhesion molecule 1. Exp Cell Res. 2000;261:19–24. doi: 10.1006/excr.2000.5038. [DOI] [PubMed] [Google Scholar]
- 15.Ergün S, Kilik N, Ziegeler G, et al. CEA-related cell adhesion molecule 1; a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- 16.Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellular matrix. Lab Invest. 1983;49:636–649. [PubMed] [Google Scholar]
- 17.Tobi M, Memon M, Kithier K, Clapp N. A putative CEA moiety is shared by the cotton-top tamarin (Saguinus oedipus) and humans. Cancer Lett. 1994;77:7–13. doi: 10.1016/0304-3835(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 18.Zimmer R, Thomas P. Mutations in the carcinoembryonic antigen gene in colorectal cancer patients: implication on liver metastasis. Cancer Res. 2001;61:2822–2826. [PubMed] [Google Scholar]
- 19.Börmer OP, Thrane-Steen K. Epitope group specificity of six immunoassays for carcinoembryonic antigen. Tumour Biol. 1991;12:9–15. doi: 10.1159/000217682. [DOI] [PubMed] [Google Scholar]
- 20.Tobi M, Maliakkal B, Zitron I, et al. Adenoma-derived antibody, Adnab-9 recognized a membrane-bound glycoprotein in colonic tissue and effluent material from patients with colorectal neoplasia. Cancer Lett. 1992;67:61–69. doi: 10.1016/0304-3835(92)90009-k. [DOI] [PubMed] [Google Scholar]
- 21.Bajenova O, Stolper E, Gapon S, Sundina N, Zimmer R, Thomas P. Surface expression of heterogeneous nuclear RNA binding protein M4 on Kupffer cell relates to its function as a carcinoembryonic antigen receptor. Exp Cell Res. 2003;291:228–241. doi: 10.1016/s0014-4827(03)00373-2. [DOI] [PubMed] [Google Scholar]
- 22.Bajenova OV, Zimmer R, Stolper E, Salisbury-Rowswell J, Nanji A, Thomas P. Heterogeneous RNA-binding protein M4 is a receptor for carcinoembryonic antigen in Kupffer cells. J Biol Chem. 2001;276:31067–31073. doi: 10.1074/jbc.M104093200. [DOI] [PubMed] [Google Scholar]
- 23.Hsu SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- 24.Tobi M, Chintalapani S, Kithier K, Clapp N. An antigenic profile in cotton-top tamarins (Saguinus oedipus): a model for human inflammatory bowel disease and colorectal cancer. In: Clapp N, editor. The cotton-top tamarin: a primate model for the study of colitis and colonic carcinoma. Boca Raton: CRC Press; 1993. pp. 113–125. [Google Scholar]
- 25.Tobi M, O’Kieffe D, Trujillo N, Nochomovitz LE, Steinberg WM. Detection of carcinoembryonic antigen in colonic effluent by specific anti-CEA monoclonal antibodies. Cancer Lett. 1992;67:47–54. doi: 10.1016/0304-3835(92)90007-i. [DOI] [PubMed] [Google Scholar]
- 26.von Lampe B, Stallmach A, Riecken EO. Altered glycosylation of integrin adhesion molecules in colorectal cancer cells and decreased adhesion to the extracellular matrix. Gut. 1993;34:829–836. doi: 10.1136/gut.34.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia M, Seigner C, Bastid C, Choux R, Payan MJ, Reggio H. Carcinoembryonic antigen has a different molecular weight in normal colon and in cancer cells due to N-glycosylation differences. Cancer Res. 1991;51:5679–5686. [PubMed] [Google Scholar]
- 28.Bresalier RS, Byrd JC, Brodt P, Ogata S, Itzkowitz SH, Yunker CK. Liver metastasis and adhesion to the sinusoidal endothelium by human colon cancer cells is related to mucin carbohydrate chain length. Int J Cancer. 1998;76:556–562. doi: 10.1002/(sici)1097-0215(19980518)76:4<556::aid-ijc19>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Naghibalhossaini F, Yoder AD, Tobi M, Stanners CP. Evolution of a tumorigenic property conferred by glycophosphatidyl-inositol membrane anchors of carcinoembryonic antigen gene family members during the primate radiation. Mol Biol Cell. 2007;18:1366–1374. doi: 10.1091/mbc.E06-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roland CR, Naziruddin B, Mohanakuma T, Flye MW. Gadolinium blocks rat Kupffer cell calcium channels: relevance to calcium-dependent prostaglandin E2 synthesis and septic mortality. Hepatology. 1999;29:756–765. doi: 10.1002/hep.510290345. [DOI] [PubMed] [Google Scholar]
- 31.Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007;131:1776–1781. doi: 10.5858/2007-131-1776-apsovm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prall F, Nollau P, Neumaier M, et al. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem. 1996;44:35–41. doi: 10.1177/44.1.8543780. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka K, Hinoda Y, Takahashi H, Sakamoto H, Nakajima Y, Imai K. Decreased expression of biliary glycoprotein in hepatocellular carcinomas. Int J Cancer. 1997;74:15–19. doi: 10.1002/(sici)1097-0215(19970220)74:1<15::aid-ijc3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Lucka L, Sel S, Danker K, Horstkorte R, Reutter W. Carcinoembryonic antigen-related cell-cell adhesion molecule C-CAM is greatly increased in serum and urine of rats with liver disease. FEBS Lett. 1998;438:37–40. doi: 10.1016/s0014-5793(98)01265-4. [DOI] [PubMed] [Google Scholar]