Abstract
As modern medicine advances, various methodologies are being explored and developed in order to treat severe osteochondral defects in joints. However, it is still very challenging to cure the osteochondral defects due to their poor inherent regenerative capacity, complex stratified architecture, and disparate biomechanical properties. The objective of this study is to create novel three-dimensional (3D) printed osteochondral scaffolds with both excellent interfacial mechanical properties and biocompatibility for facilitating human bone marrow mesenchymal stem cell (MSC) growth and chondrogenic differentiation. For this purpose, we designed and 3D printed a series of innovative bi-phasic 3D models that mimic the osteochondral region of articulate joints. Our mechanical testing results showed that our bi-phasic scaffolds with key structures have enhanced mechanical characteristics in compression (a maximum Young's modulus of 31 MPa) and shear (a maximum fracture strength of 5768 N/mm2) when compared with homogenous designs. These results are also correlated with numerical simulation. In order to improve their biocompatibility, the scaffolds' surfaces were further modified with acetylated collagen (one of the main components in osteochondral extracellular matrix). MSC proliferation results demonstrated that incorporation of a collagen, along with biomimetically designed micro-features, can greatly enhance MSC growth after 5 days in vitro. Two weeks' chondrogenic differentiation results showed that our novel scaffolds (dubbed “key” scaffolds), both with and without surface collagen modification, displayed enhanced chondrogenesis (e.g., 130%, 114%, and 236% increases in glycosaminoglycan, type II collagen deposition, and total protein content on collagen-modified key scaffolds when compared with homogeneous controls).
Introduction
Nowadays, osteochondral defects resulting from trauma, congenital defects, and/or pathological disorders present a crucial clinical problem.1,2 More than 6 million Americans visit the hospital each year for various knee, wrist, and ankle-related injuries or complications.3 Typical osteochondral defects penetrate the entire thickness of articular cartilage, beyond the calcified zone, and into the subchondral bone. Osteochondral tissue is a nanostructured tissue that is notoriously difficult to regenerate due to its poor regenerative capacity, complex stratified architecture, and disparate biomechanical properties.1,2,4,5 Although various biomaterials and tissue engineering approaches to treat osteochondral defects have been investigated, it is still very challenging to replicate both the robust integration of cartilage and subchondral bone and the complex stratified tissue structure. No currently available treatment option provides a perfect solution for osteochondral regeneration.
It has been known that three-dimensional (3D) scaffold architecture and geometric cues play a major role in dictating cell behavior and tissue regeneration.6 For current cartilage and osteochondral studies, conventional scaffold fabrication methods such as solvent casting and particle leaching,7–9 electrospinning,10–12 and freeze drying13–15 have been widely used to fabricate 3D porous osteochondral scaffolds. These scaffolds have been shown to influence cell functions and improve cartilage, osteochondral, and bone regeneration. However, such techniques offer limited control over scaffold geometry, pore size and distribution, pore interconnectivity, and internal channel construction. Random, spontaneously generated and disconnected pores significantly decrease nutrient transportation, cell migration, and cell survival, especially in the center of a thick tissue scaffold. As modern medicine advances, novel methodologies are being explored and developed in order to solve and improve current cartilage and osteochondral problems.16–18 In particular, as an emerging complex tissue manufacturing technique, 3D printing offers great precision to control the internal architecture of a scaffold and print complicated structures close in architecture to native tissue.19 More importantly, based on computer-aided design (CAD) models, 3D printers can easily fabricate a predesigned patient-specific tissue construct in a layer-by-layer fashion.20–24 Shim et al. printed a 3D hybrid scaffold via “solid freeform fabrication.” A structurally sound polymer was deposited simultaneously with a cell-laden hydrogel. The printed scaffold served as a structural support, while the printed soft hydrogel served to encapsulate and evenly distribute cells throughout the printed construct.17 Recently, Cui et al. successfully Inkjet printed a poly(ethylene glycol) dimethacrylate solution containing chondrocytes into a defect formed in an osteochondral plug.20 They observed greater proteoglycan deposition in the interface of implant and native tissue. In addition, Fedorovich et al. used 3D fiber deposition to print cell-laden, heterogeneous hydrogel constructs for potential use as osteochondral grafts. By changing fiber spacing or angle of fiber deposition, it was possible to yield scaffolds of varying porosity and elastic modulus and encapsulating fluorescently labeled human chondrocytes and osteogenic progenitors in alginate hydrogel.21 Recently, Lee et al. also successfully 3D printed custom scaffolds mimicking human mandibular condyle using polycaprolactone and chitosan and modified inert 3D printed materials with bioactive apatite coating for osteochondral tissue regeneration.25 Bone marrow stromal cells showed good viability in the scaffolds, and the apatite coating further enhanced cellular spreading and proliferation. These current attempts have shown great promise of 3D bioprinting for tissue regeneration. Since 3D bioprinting osteochondral tissue is still a field in its infancy, it is highly desirable to achieve advanced sophistication in bioprinting design and implementation of appropriate osteochondral scaffold micro architecture.
The objective of this work was to create novel 3D printed osteochondral scaffolds with both excellent interfacial mechanical properties and biocompatibility for facilitating human bone marrow mesenchymal stem cell (MSC) growth and chondrogenic differentiation. Previous work exploring osteochondral regeneration has yielded scaffolds that are weak at the interface between the cartilage and bone regions. Often, scaffolds are fabricated in two or three layers separately and then joined together with a glue or suture.26,27 With this in mind, our novel bi-phasic scaffolds were also designed with novel internal “key” features (the central, tubular-shaped structures in the center of the “key” scaffolds), intended to increase the overall mechanical strength of the constructs and prevent failure at this interface. In addition, we utilized a numerical simulation method to assist analysis and evaluation of our osteochondral scaffold. Compressive and shear mechanical testing, MSC proliferation, and chondrogenic differentiation were evaluated in vitro.
Materials and Methods
Three-dimensional osteochondral scaffold design and fabrication
All 3D osteochondral scaffolds were fabricated using a PrinterBot 3D printing system (Fig. 1), modified with a 347 μm diameter nozzle, and a spool of 1.75 mm diameter biocompatible poly lactic acid (PLA) polymer. Our 3D printer consists of a heated printing bed (heated to 60°C), a heated printing tool (heated headset to 185°C and extrusion motor) that is capable of 3D axial movement, and a computer/controller using the Pronterface control software package. A series of CAD drawings converted to Sterelithography (stl) format (to be described next) were used in conjunction with Slic3r stl slicing software and the 3D printer to create predesigned 3D structures. The printing tool draws a PLA filament and forces it through a heated extruder nozzle, which melts and deposits polymer on the printing surface in a thin layer. The machine then prints multiple thin layers on top of the previously deposited layers to create various designed, fully 3D osteochondral constructs.
FIG. 1.
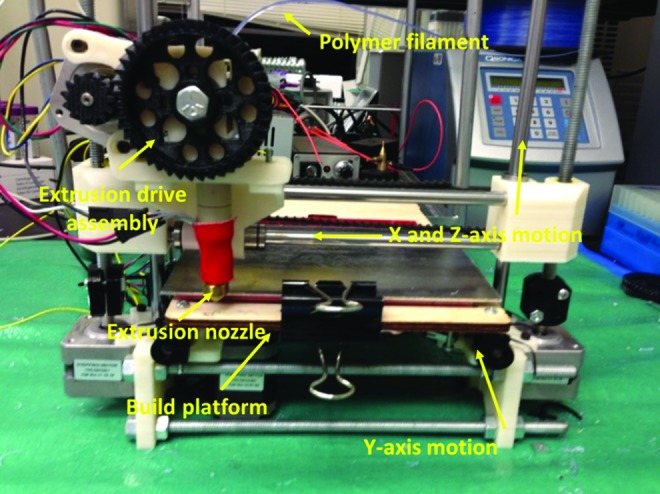
A picture of our three-dimensional (3D) printer setup. Color images available online at www.liebertpub.com/tea
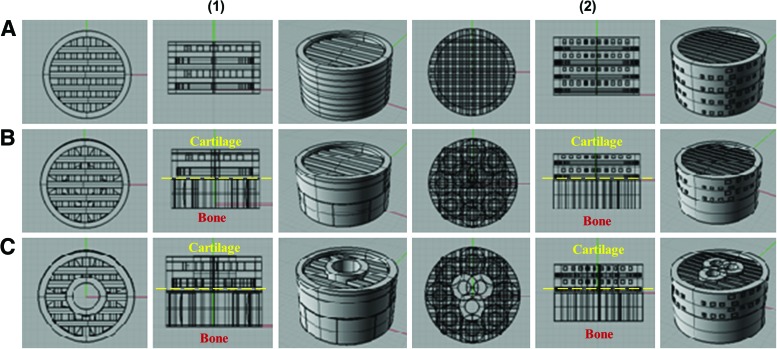
In our study, a total of six experimental osteochondral scaffold groups were designed (Fig. 2) using the Rhinoceros 3D modeling package. All of the 3D CAD models were converted into a gcode instruction file in Slic3r, and then used to instruct the printer through Pronterface. The first group was a homogenous cross-hatched structure. The second was a bi-phasic structure consisting of a cross-hatched pattern and an intersecting ring structure. The hatch pattern, which has already been widely used for 3D printed joint repair,28 was chosen for both its proven performance as a biomimetic micro pattern and as a biomimetic analog to the alignment of extracellular matrix (ECM) and chondrocytes in articulate cartilage. The ring structure for the bone layer was chosen as a means for designing randomly oriented, interconnected pores, which mimics the porous structure of subchondral bone. Finally, a bi-phasic key model, with an internal structural feature traversing the length of the scaffold, was designed and printed. All scaffolds described were designed as cylindrical plugs, 14 mm in diameter and 8 mm in height. All of the models described earlier have both small and large pore features (500 and 1000 μm, respectively) based on the printing limitations of the setup at the time. For shear testing, we also designed an additional intermediate pore size (750 μm) for the three models. These pore sizes and features, when printed and analyzed under scanning electron microscopy (SEM), were virtually the same in size and shape as those of the 3D models originally designed. In addition, a full knee model with anatomical shape was designed and printed.
FIG. 2.
Computer-aided design (CAD) images of our (1) large and (2) small pore model and (A) homogeneous, (B) bi-phasic, and (C) bi-phasic key featured designs. For bi- and key scaffolds, the bone layer is on the bottom (based on the orientation of this figure) and the cartilage layer is on the top. Color images available online at www.liebertpub.com/tea
Moreover, we applied a collagen modification on one osteochondral scaffold (i.e., bi-phasic key osteochondral scaffold with small pores, expected to have optimized properties for our study) to further improve their cytocompatibility properties. A protocol for chemically functionalized attachment known as acetylation29 was used. Briefly, the scaffolds were immersed in an ethylenediamine/n-propanol (1:9 ratio) solution at 60°C for 5 min. They were then extensively washed with deionized water and dried at 35°C. The aminolysed scaffolds were then immersed in a 1% gluteraldehyde solution at room temperature for 3 h to transfer the NH2 groups into CHO groups on the surface. After washing extensively, the scaffolds were immersed in 0.1% acetylated collagen at 4°C for 24 h. The process itself yields a series of layered chemical attachments, finally resulting in a collagen type I surface modification. As illustrated in Figure 3, we have an ester linkage between the PLA and the ethylenediamine, a Schiff's base linkage between the ethylenediamine and the gluteraldehyde, and a further Schiff's base linkage between the gluteraldehyde and the collagen. All printed scaffolds were sterilized under UV light for 15 min on either side before cell study.
FIG. 3.
Schematic illustration of acetylated collagen linked on 3D printed bi-phasic key scaffold. Color images available online at www.liebertpub.com/tea
Mechanical testing, modeling, and scaffold characterization
All mechanical testing, including compressive and shear testing, was conducted using a uniaxial testing system (ATS systems). For compression testing, a flat 2 cm diameter platen was attached to a 500 N load cell. The platen was then advanced into the scaffolds, oriented uniaxially with the bone layer on the bottom and the cartilage layer interacting with the platen, at a 0.02 cm/min strain rate. Data were taken using LabView, and then analyzed in Microsoft Excel. Load and displacement were used to plot the stress/strain curves and then, Young's modulus was calculated from the linear elastic region. For shear testing, the same setup and conditions were used, with the exception of the platen being replaced with a 5° wedge (from centerline, 10° total) and the scaffold rotated 90°. The interface between the bone and cartilage layers was aligned parallel to the wedge, and the wedge was advanced into the interface line for bi-phasic and key scaffolds. For homogeneous models, the wedge was advanced into the scaffold at half of the scaffolds' height, which is consistent to the dimensions and orientations of the other two models. Force was plotted against displacement, and the area under the curve was taken to provide the shear fracture strength in N/mm2.
Based on the obtained experimental data, a computational model was composed to estimate and correlate the properties of various micro structures with different porosities. In addition, a Zeiss SigmaVP SEM was used to image the surfaces of acetylated collagen constructs and controls (unmodified scaffolds). Scaffolds were coated with an ∼4–8 nm of gold nanoparticles and then isolated on carbon tape dots to facilitate imaging.
We conducted a contact angle analysis on pure PLA and collagen type I modified PLA to evaluate scaffold surface wettability before and after collagen coating. One-millimeter thick PLA scaffolds were 3D printed with the same footprint as the designed scaffolds. The scaffolds were then either left plain or modified with collagen type I using the acetylation process already described. A Kriss DSA25 drop shape analysis machine was used to characterize samples (n=10 each group) using an 8 μL droplet for each. Screen captures were taken from recorded video, right after the droplet was placed on the sample, and the prepackaged DSA software was used to set the material/drop baseline, compute the drop curvature, and calculate the obtuse angle between tangent lines, from the points of intersection between the baseline and drop shape arc.
In vitro MSC adhesion, proliferation, and confocal imaging on the 3D printed osteochondral scaffolds
Primary human bone marrow MSCs were derived from healthy consenting donors from the Texas A&M Health Science Center and thoroughly characterized.30 They were used to evaluate the cytocompatibility properties of the 3D printed scaffolds. MSCs (passage #3–6) were cultured in a standard MSC growth media comprising alpha minimum essential medium supplemented with 16.5% fetal bovine serum, 1% (v/v) l-glutamine, and 1% penicillin:streptomycin solution and cultured under standard cell culture conditions (37°C, a humidified, 5% CO2/95% air environment). They were subsequently lifted from cell culture flasks using trypsin-EDTA for an in vitro proliferation study.
A 4-h MSC adhesion study was conducted on six types of osteochondral scaffolds and one collagen-modified bi-phasic key scaffold in 24-well plates, with cells seeded at 10,000 cells per scaffold. After 4 h, all scaffolds were rinsed using phosphate-buffered saline (PBS) to remove nonadherent cells and those remaining were counted via a Thermoscientific photometric cell counting reagent (MTS assay). Results were read using a Thermo Scientific Multiskan GO microplate reader at a setting of 490 nm wavelength light. Similarly, a 1, 3, and 5 day proliferation study was conducted on the same scaffold groups with 100,000 cells per scaffold. Media were changed every other day, and cells were lifted for analysis via MTS assay.
Confocal microscopy was used to characterize MSC growth and spreading morphology on the various 3D printed scaffolds. In order to facilitate confocal imaging, thinner scaffolds with representative 3D homogenous, bi-phasic, and key design features were printed. MSCs were seeded at 50,000 cells per construct and cultured for 1 and 3 days. At each time point, scaffolds were washed twice with PBS, fixed with 10% formalin, and soaked in 0.1% Triton X-100. After further PBS washings, the remaining cells were stained with a Rhodamine red fluorescent dye (to stain the cells' cytoskeleton) for 1 h and then a DAPI blue fluorescent dye (to stain the cells' nuclei) for 15 min. The double-stained samples were imaged on a Zeiss LSM 710 confocal microscope system. Cell images were taken as two-dimensional (2D) and multi-focal plane 3D reconstructed images, to show both MSC spreading morphology and 3D migration and distribution.
In vitro MSC chondrogenic differentiation on the 3D printed osteochondral scaffolds
A 2 week differentiation study was also conducted on scaffolds with optimal pore density decided by MSC proliferation (i.e., small pore features). New scaffolds were fabricated, of the same physical specifications with small pores (control, bi-phasic, bi-phasic key scaffolds, and bi-phasic key scaffolds with collagen). MSCs were seeded at 150,000 cells per scaffold and cultured in the chondrogenic media, including the MSC growth media with the addition of 100 nM dexamethasone, 40 μg/mL proline, 100 μg/mL sodium pyruvate, 50 μg/mL l-ascorbic acid 2-phosphate, and 1% ITS+. Samples were then taken at 1 and 2 weeks and digested in a papain-based enzymatic digestion solution for 24 h at 60°C. Aliquots for appropriate assays were then taken from the bulk solutions. The following standard chondrogenic biochemistry assays were used to evaluate MSC chondrogenic differentiation in our 3D printed scaffolds.
Glycosaminoglycan content
Glycosaminoglycan (GAG), a key component of cartilage matrix, was measured using a standard GAG assay kit (Accurate Chemical & Scientific Corp, Westbury, NY) according to the manufacturer's instructions. Briefly, a predetermined volume of sample and buffer solution was added to a microcentrifuge tube with 500 μL of dye reagent and mixed for 30 min. The GAG-dye complex was centrifuged for 10 min at 10,000 g until a pellet was visible. The supernatant was decanted, and all residual fluid was blotted dry. Next, 600 μL of dissociation reagent was added to the tubes and shaken for 30 min; 100 μL of each solution was placed into a 96-well plate and analyzed in triplicate. Absorbance was read at 656 nm and correlated to a standard curve of known standards.
Type II collagen synthesis
Human type II collagen was evaluated via a type II collagen ELISA assay (Fisher Scientific, Pittsburgh, PA). Briefly, control and sample aliquots were added to a precoated 96-well plate and incubated. Unbound sample was washed, and a horseradish peroxidase-labeled collagen II antibody was added, incubated, and washed. After washing, tetramethylbenzidine was added and produced a blue color. The reaction was stopped by the addition of an acidic stop solution and read at 450 nm.
Total protein synthesis
Total protein was evaluated using a Micro BCA assay (Thermo Scientific, Rockford, IL). A working reagent (WR) was prepared using the following formula:
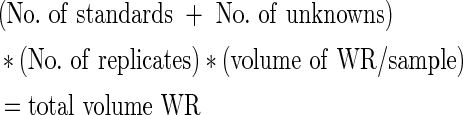 |
One milliliter of each standard and sample was pipette into appropriate test tubes, and 1 mL of the WR was added. Tubes were covered from light and incubated at 60°C in a water bath for 1 h. All tubes were cooled to room temperature and measured in a photometric plate reader at 562 nm. An uncultured collagen-modified scaffold control was also digested and tested for total protein content. This measurement was then subtracted from the total protein analysis in weeks 1 and 2.
Statistics
All experimental data were compiled as mean±standard error mean for each property measured. Numerical data were analyzed via one-way ANOVA and Student's t-test to determine differences among the groups. Statistical significance was considered at p<0.05.
Results
Characterization of 3D printed osteochondral scaffolds
Figures 2 and 4 show our novel cylindrical osteochondral construct design and printed scaffolds, with a different internal structure. These homogenous (Fig. 2A) and bi-phasic scaffolds (Fig. 2B) were designed to establish both a control group and a more traditionally designed osteochondral scaffold for comparison of key featured design (Fig. 2C). The homogenous model is a uniformly patterned structure, mimicking only one type of tissue. The bi-phasic scaffold is more similar to traditional osteochondral scaffold designs,8,31 containing both a cartilage and bone layer and no other materials or features. This key feature was designed to traverse the entire length of the scaffold, and penetrates both the cartilage and bone layer. It was intended to increase overall mechanical strength and to prevent failure of the device at the bi-phasic interface between the bone and cartilage layers. Physical characteristic data of all printed scaffolds were computed from 3D models of all the scaffold groups (Table 1). It can be seen that the total surface area of the construct increases from a homogenous design to a bi-phasic design, and again when a key feature is added. Furthermore, the total surface area of the construct increases again when the feature size is decreased from large to small pores. However, the surface area to volume ratio of the construct follows the opposite trend as described earlier. This is due to the fact that, with a decrease in feature size, more features can be added to the construct, thus increasing the overall volume, and this is not a reflection of the surface to volume ratio of a given feature.
FIG. 4.
Images of 3D printed scaffolds with different internal geometry and pore density in mesenchymal stem cell (MSC) growth media. Small (top) and large (bottom) pore models, and (from left to right) homogeneous, bi-phasic, and bi-phasic key feature scaffolds. Color images available online at www.liebertpub.com/tea
Table 1.
Physical Data for Different Three-Dimensional Osteochondral Constructs and Pore Sizes
| 3D scaffolds | Smallest feature (mm) | Pore density (pores/mm3) | Total surface area (mm2) | Bulk volume (mm3) | SA/V ratio |
|---|---|---|---|---|---|
| Large | |||||
| Homogeneous | 1 | 0.5 | 1850.6 | 616.3 | 3.002 |
| Bi-phasic | 1–4 | 0.5 bone | 2094.4 | 716.2 | 2.924 |
| 0.001 cartilage | |||||
| Bi-phasic key | 1–4 | 0.5 bone | 2150.7 | 749.8 | 2.868 |
| 0.00 cartilage | |||||
| Intermediate | |||||
| Homogeneous | 0.71 | 0.53 | 2368.6 | 576.3 | 4.109 |
| Bi-phasic | 0.71–1.7 | 0.53 bone | 2700.8 | 863.9 | 3.126 |
| 0.003 cartilage | |||||
| Bi-phasic key | 0.71–1.7 | 0.53 bone | 2724.6 | 904.3 | 3.012 |
| 0.003 cartilage | |||||
| Small | |||||
| Homogeneous | 0.5 | 5.3 | 2817.7 | 571.1 | 4.933 |
| Bi-phasic | 0.5–2 | 5.3 bone | 2854.0 | 863.6 | 3.304 |
| 0.005 cartilage | |||||
| Bi-phasic key | 0.5–2 | 5.3 bone | 2921.7 | 947.4 | 3.083 |
| 0.005 cartilage | |||||
3D, three dimensional; SA/V, surface area to volume ratio.
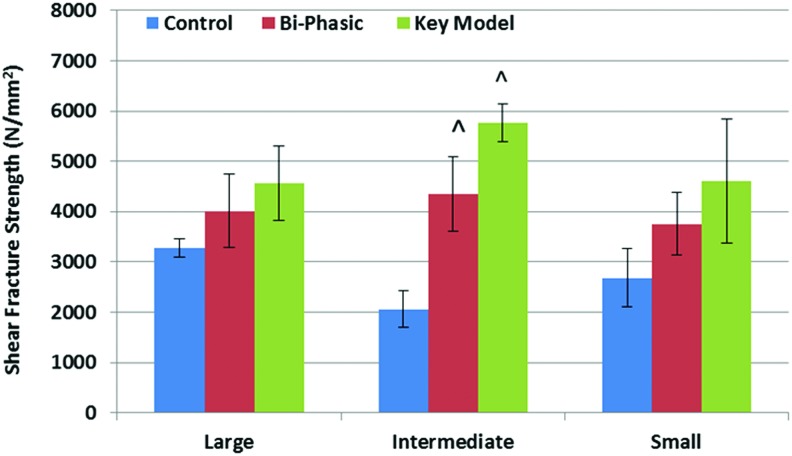
Mechanical compression tests were also conducted on the six different scaffold construct designs (Fig. 5). All of the scaffolds showed excellent mechanical properties similar to or exceeding cartilage (0.75–1 MPa) and subchondral bone (30–50 Mpa)32–36 in human osteochondral tissue. Under compressive loading, the bi-phasic key models with both small and large features have the highest modulus when compared with the homogeneous controls and the bi-phasic models, with increases of 27% and 19% (control and bi-phasic models) among the large pore scaffolds, and with increases of 20% and 64% among the small pore models. The bi-phasic scaffolds with large features performed better than the similar constructs with small features. Shear testing was conducted on our bi-phasic key scaffold, bi-phasic scaffold, and homogeneous controls, with three varying pore sizes, for a total of nine scaffolds (Fig. 6). In all cases, the scaffolds showed a trend in the force per unit area that it took to cleave the scaffolds apart, increasing from the homogeneous control to the bi-phasic model to our novel key model. Specifically, the inclusion of our key feature can greatly increase scaffold performance in shear (e.g., the shear fracture strength of key scaffold with an intermediate-sized feature can increase 180% over the respective homogeneous controls).
FIG. 5.
Compressive Young's modulus data for 3D printed scaffolds. Data are±standard error of the mean, n=5; *p<0.05 when compared with all homogenous and bi-phasic scaffolds; **p<0.05 when compared with all other scaffolds with small features; and #p<0.05 when compared with all other scaffolds. Color images available online at www.liebertpub.com/tea
FIG. 6.
Shear fracture strength of 3D printed scaffolds, performed under wedge fracture shear testing. Data are±standard error of the mean, n=5; ^p<0.01 when compared with controls with intermediate pores. Color images available online at www.liebertpub.com/tea
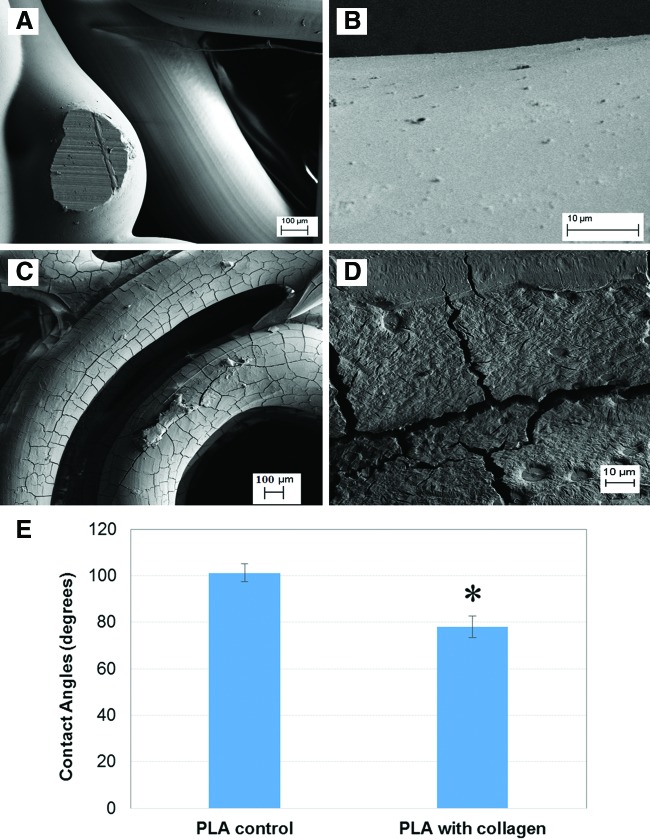
Moreover, the SEM surface morphology of the 3D printed scaffolds with and without collagen type I modification was shown in Figure 7A–D. The modified scaffolds exhibited a collagen texturing when compared with unmodified controls. In addition, contact angle analysis (Fig. 7E) showed that collagen type I modified PLA exhibited a 30% lower contact angle than plain PLA, indicating that this modification can contribute to creating a more hydrophilic surface for improved cellular activity.
FIG. 7.
Scanning electron microscopy images of (A, B) unmodified and (C, D) acetylated collagen-modified 3D printed osteochondral scaffolds. (E) Contact angle analysis of pure poly lactic acid (PLA) and PLA with acetylated collagen type I, showing decreased contact angle on the collagen-modified PLA. Data are±standard error of the mean, n=10; *p<0.05 when compared with control. Color images available online at www.liebertpub.com/tea
Computational modeling
In this work, we also adopted an exponential relationship for predicting Young's modulus (E) of the homogenous structure, the bi-phasic structure, and the bi-phasic key featured structure. This relationship can be expressed as E=E0exp(−Bp), where E0 stands for the Young's modulus of the parent solid, p refers to the total porosity volume fraction, and B is a geometric parameter. Specifically, p is defined as 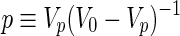 , where V0 is the volume of the parent solid, and Vp is the volume of the pores. It should be noted that if Vp=0, that is, there is no pore in the structure, then p=0, that is, the porous structure returns to its parent solid. However, if Vp approaches V0, that is, there are numerous pores in the structure, then p approaches infinity and the Young's modulus E is vanishing. To determine the value of the geometric parameter B, the average value of the Young's modulus of a given porous structure obtained from experimental measurements is used. For the homogeneous structure, the average values of the Young's modulus with the large feature (p=0.5) and with the small feature (p=5.3) are 24.212 and 21.432 MPa, respectively. Then, the geometry parameter is obtained as Bh=0.025. Similarly, the geometry parameters for bi-phasic structure and the structure with a key feature are obtained as Bb=0.079 and Bk=0.039, respectively.
, where V0 is the volume of the parent solid, and Vp is the volume of the pores. It should be noted that if Vp=0, that is, there is no pore in the structure, then p=0, that is, the porous structure returns to its parent solid. However, if Vp approaches V0, that is, there are numerous pores in the structure, then p approaches infinity and the Young's modulus E is vanishing. To determine the value of the geometric parameter B, the average value of the Young's modulus of a given porous structure obtained from experimental measurements is used. For the homogeneous structure, the average values of the Young's modulus with the large feature (p=0.5) and with the small feature (p=5.3) are 24.212 and 21.432 MPa, respectively. Then, the geometry parameter is obtained as Bh=0.025. Similarly, the geometry parameters for bi-phasic structure and the structure with a key feature are obtained as Bb=0.079 and Bk=0.039, respectively.
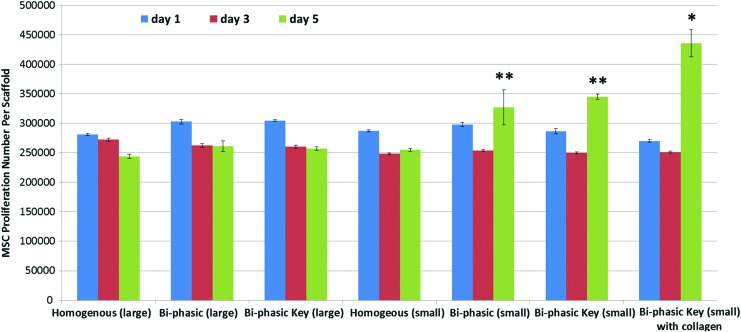
Improved MSC proliferation and chondrogenic differentiation in vitro
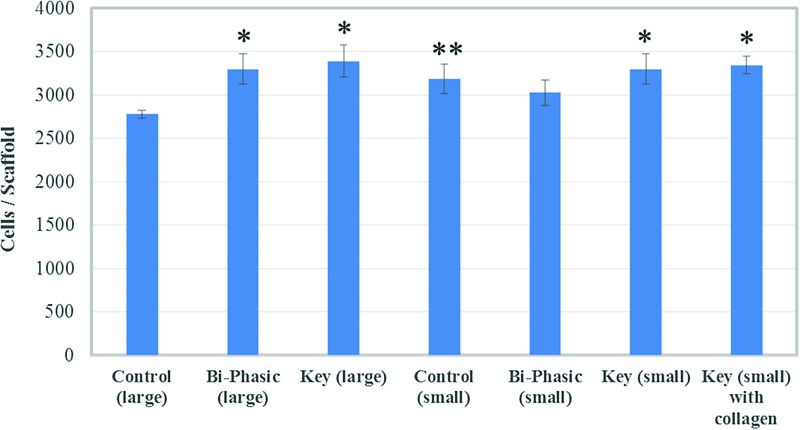
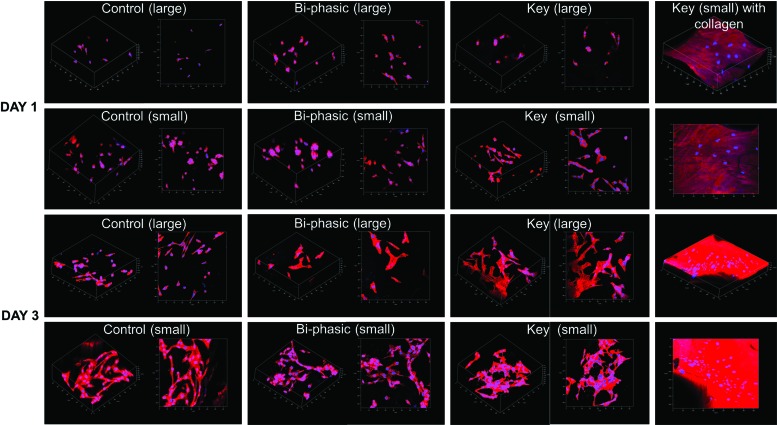
MSC 4 h adhesion results (Fig. 8) showed good cellular attachment on all of the scaffolds. Specifically, scaffolds with the key structure performed much better than the control with large pore features. The 5 day proliferation study (Fig. 9) showed that the bi-phasic scaffolds with small pore features can significantly promote MSC proliferation. In particular, the scaffolds with acetylated collagen outperformed all other groups, which show that chemical surface modification can greatly increase MSC proliferation in vitro. It should be noted that all of the scaffolds experienced a decrease in cellular activity from day 1 to 3. This may be due to the fact that our scaffolds had large internal features and surface areas, and MSCs may have temporarily ceased proliferative activity when migrating through a given construct.37–39 Figure 10 shows the 3 day MSC growth and spreading morphology on all printed scaffolds. The small featured scaffolds and the scaffolds with key structure specifically had improved cell spreading morphology after 3 days. It was difficult to examine cellular morphology on the collagen-modified scaffolds, as the Rhodamine dye bound to the collagen, but the stained cells nuclei could be observed in a dense distribution.
FIG. 8.
MSC adhesion on different 3D printed scaffolds. Data are±standard error of the mean, n=9; *p<0.05 when compared with control with large features and bi-phasic scaffold with small features. **p<0.05 when compared with control with large features. Color images available online at www.liebertpub.com/tea
FIG. 9.
MSC proliferation in a variety of 3D printed PLA scaffolds with different internal structure and surface modification. Data are±standard error of the mean, n=9; *p<0.05 when compared with all other scaffolds and **p<0.05 when compared with all scaffolds with large features and homogenous controls with small features at day 5. Color images available online at www.liebertpub.com/tea
FIG. 10.
Confocal microscopy images of MSC growing on 3D printed scaffolds after 1 and 3 days of culture. Improved cell growth and spreading morphology are observed on smaller featured scaffolds. Color images available online at www.liebertpub.com/tea
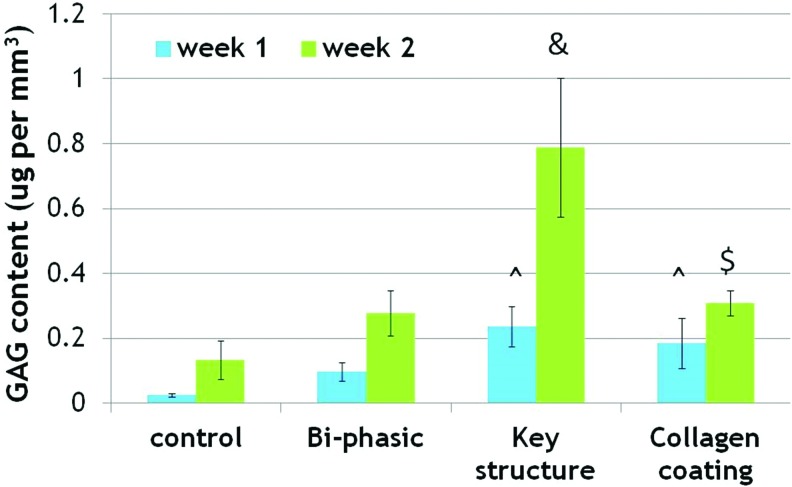
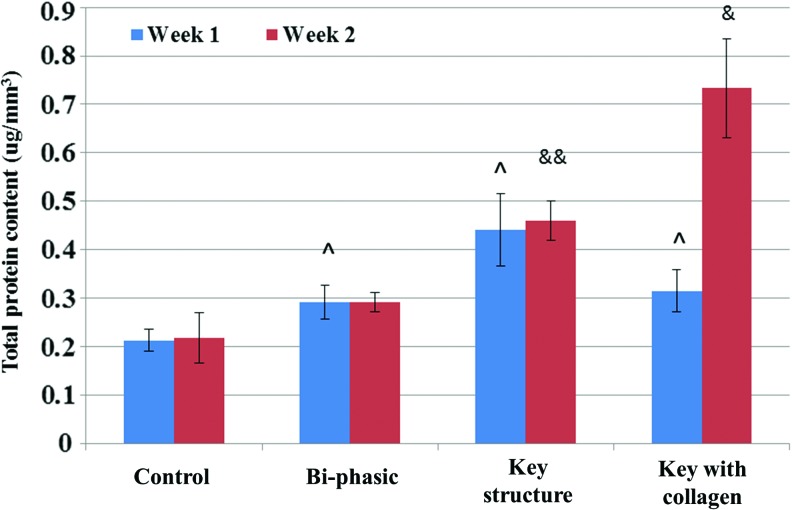
For 2 week chondrogenic differentiation, each sample was analyzed for GAG, total protein, and collagen type II synthesis. Results of the GAG assay showed that the most GAG deposition was present on the key and collagen-modified key scaffolds after 1 and 2 weeks (Fig. 11). More interestingly, all samples showed an increase, with the far greatest increase on the key scaffold. After 2 weeks, GAG deposition on the unmodified and modified key scaffolds improved dramatically, by 489% and 130% when compared with homogeneous control scaffolds. In addition, Figure 12 shows that all bi-phasic and bi-phasic key scaffolds with and without collagen had greatly enhanced type II collagen deposition when compared with controls. Collagen-modified samples specifically showed an increase of 56% type II collagen deposition after 1 week and then an increase of 114% after 2 weeks when compared with the control scaffolds. Figure 13 showed increased total protein content on bi-phasic, key scaffolds with/without collagen modification after 1 week when compared with controls, with the most total protein present on the key model. Total protein content was greatly enhanced on the key model scaffolds with and without collagen. At 2 weeks, the largest protein content was observed on the collagen-modified key scaffolds, which increased 236% when compared with the homogenous control scaffolds.
FIG. 11.
Glycosaminoglycan (GAG) synthesis in various 3D printed osteochondral scaffolds. Data are±standard error of the mean, n=9; &p<0.05 when compared with all other scaffolds and $p<0.05 when compared with controls after 2 weeks; and ^p<0.05 when compared with controls and bi-phasic scaffolds after 1 week. Color images available online at www.liebertpub.com/tea
FIG. 12.
Collagen type II synthesis on 3D printed scaffolds. All groups showed improvement after 2 weeks of chondrogenic differentiation. Data are±standard error of the mean, n=9; *p<0.05 when compared with all other scaffolds at week 1 and ^ p<0.05 when compared with all other scaffolds at week 2. Color images available online at www.liebertpub.com/tea
FIG. 13.
Total protein synthesis. Data are±standard error of the mean, n=9; ^p<0.05 when compared with controls, &p<0.05 when compared with all other scaffolds and &&p<0.05 when compared with bi-phasic and controls after 2 weeks. Color images available online at www.liebertpub.com/tea
Knee concept design and fabrication
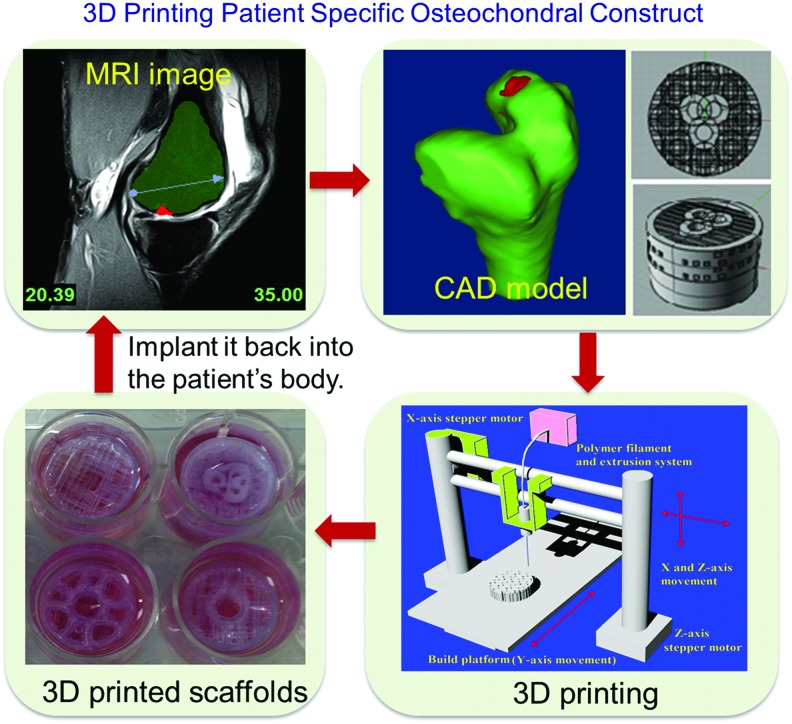
Figure 14 shows a flow chart demonstrating the process of applying our designed bi-phasic scaffolds in future clinical osteochondral regeneration. Based on noninvasive MRI images of patients' osteochondral defects, we can inform our CAD design, allowing the designed scaffold to integrate perfectly into the defect site. This approach would also be ideal for patient-specific shape requirements of critical-sized osteochondral implants. In addition to samples printed for the earlier cellular study and mechanical testing, a large construct, mimicking the structure of a human knee with internal bi-phasic and key features, was also designed (Fig. 15). This model also had superficial pores on the surface, to allow fluid perfusion in a theoretical in vivo scenario. When exposed to fluid, there was an ease of flow through the full construct, showing that the internal architecture was interconnected.
FIG. 14.
A flow chart illustrating 3D printing patient-specific scaffolds with designed internal structures for osteochondral defect treatment. Color images available online at www.liebertpub.com/tea
FIG. 15.
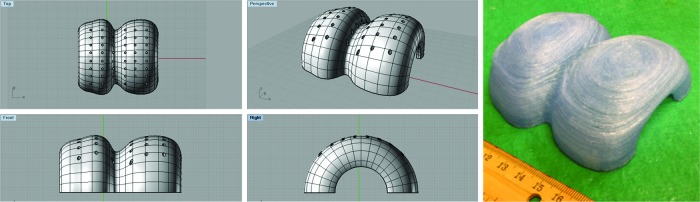
A full knee cartilage layer CAD model and the respective 3D printed full knee construct. Color images available online at www.liebertpub.com/tea
Discussion
Recently, 3D printing and rapid prototyping processes have been used to create tissue scaffolds that are 3D with user-defined micro architectures.40–44 This ensures not only that the scaffold has fully interconnected pores, but also a great deal of more complex, predesigned architectures patterns and structures. A critical 3D scaffold design criteria for osteochondral tissues is that scaffolds should have suitable mechanical properties at the interface which can tolerate the high loading requirement of human joints. In addition, highly interconnected pores, specifically at the nano to micro-scale, are also very important.45,46 This complicated, hierarchical structure is one that is difficult to achieve, and then repeat throughout a larger scaffold structure in even very advanced scaffold fabrication techniques. With the application of 3D printing, there is an allowance not only for the creation of delicate and intricate structures from biocompatible materials, but also for the potential to create highly ordered structures that could conceivably match any desired architecture.47 This later advantage is one that also makes 3D printing attractive for other types of targeted tissue 3D scaffolds. Later, we will discuss our 3D printed osteochondral scaffolds in detail.
Creation of novel 3D printed osteochondral scaffolds with improved mechanical properties
As mentioned in the Results section, the small featured key scaffold had the largest surface area. From these data, it is postulated that the small featured key designed construct would yield the greatest cell growth, maybe because cells would have increased surface area, within the same volume, to attach to, as well as providing a more interconnected network of surfaces for cells to migrate throughout. This is relevant for the relatively short duration of our in vitro experimentation, because initial adhesion and MSC proliferation/migration, which happen soon after cellular introduction into the system, are most affected by the available surface topography.48,49
Traditional approaches to osteochondral scaffold engineering often fail at the interface,50,51 necessitating a construct with robust integration between cartilage and bone.52–54 Our shear fracture results demonstrate that in addition to providing an advanced 3D fabrication technique which yields a scaffold with complex internal structure, 3D printing has the ability to achieve predesigned structures which may provide a scaffold with better interfacial integration (Fig. 6). Generally, in the human knee, it takes between 1300 and 1800 newtons of compressive force to shear the cartilage and bone interface of the femoral condyle.55 This compressive force is, in the moment of failure, translated into a shear force across the interface that is strong enough to dislodge cartilage from subchondral bone at a 35° flexing of the knee, and at 45° of medial torsion. This ends up translating to shear force energies (in the orthogonal dimension) of as much as 50 Nm56 and shear fracture strength of anywhere (for wet bone and tissue) from 30 to 100 N/mm2.57 Our key model scaffolds achieved shear fracture strength above 4000 N/mm2 and in the best case 5768 N/mm2, demonstrating their excellent and physiologically acceptable performance. The mechanical compression results also demonstrated that the inclusion of the key feature can greatly increase the compressive Young's modulus when compared with other control designs (Fig. 5). As we decreased the pore size of our constructs, we were able to increase the number of pores in the scaffold, and thus increase the overall porosity. The large pore scaffolds showed slightly higher moduli than small pore scaffolds. This may be due to the fact that, as the porosity of a material increases, its overall compressive modulus decreases.58,59 This increase in bulk porosity of the small featured scaffolds may be responsible for the decrease in mechanical performance.
Computational modeling for 3D printing design optimization
The computational process has great potential for optimized 3D printing design, as it is easier and faster to perform than experimental measurement. Generally, Poisson's ratio varies in a small range, so for our purposes we assume it as a constant. The mechanical properties of the newly designed porous structure were calculated through a relationship between Young's modulus and porosity, which has been widely discussed. For instance, Rossi60 modified Hashin's equation so that Young's modulus is a function of low concentration of spherical pores, that is, E=E0(1−Bp). Based on this, Rice61 proposed an exponential function that can be applied for a wide range of pore character. Later, this empirical formula was successfully applied to predict the mechanical properties of porous hydroxyapatite bioceramic.62 In our study, in accordance with experimental data, we developed a theoretical model. This model simulated designed osteochondral scaffolds, in both mechanical compression and shear, but not simulating failure. It will play a role for future studies involving a large amount of sample specimens with different porosity for different structure, including homogeneous structure, bi-phasic structure, and the structure with a key feature, so that scaffolds can be more readily optimized.
Improved MSC functions on 3D printed osteochondral scaffolds
Our MSC adhesion and proliferation results demonstrated that both small pore feature and acetylated collagen modification can greatly promote MSC attachment and growth as shown in Figures 8 and 9. In addition, our confocal images reveal good cell growth and spreading morphology on our 3D printed scaffolds. Especially on the scaffolds with smaller features and key structures, cells exhibited a well spreading morphology with expanding, multidirectional filopodia, indicative of excellent cell behavior.63–65 Research has already shown that 3D printed constructs can be easily enhanced with bioactive coatings postfabrication, for improved cell functions. For instance, Poldervaart et al. 3D printed bone constructs containing gelatin encapsulated bone morphogenetic protein-2 (BMP-2).66 They further enhanced the scaffolds with a calcium phosphate coating. The scaffold showed promising results when seeded with goat multipotent stromal cells. Cells displayed enhanced osteogenic activity after 3 weeks, and bone formation was observed after an in vivo study where constructs were subcutaneously implanted in rats and mice. While these results are promising, the effects and use of more exotic modification have not been extensively explored for 3D printed osteochondral constructs. The collagen surface modification method used in our study can be used to improve the hydrophilicity and cytocompatibility properties of 3D printed osteochondral construct and has the potential for further conjugating various bioactive factors (such as peptide and growth factors) with 3D printed scaffolds.
Our key feature scaffolds exhibited excellent performance in improving chondrogenic differentiation of MSCs in vitro when compared with controls. This is likely due to the fact that the key scaffolds have a greater surface area, while maintaining a comparable surface area to volume ratio to the bi-phasic model (Table 1). Our collagen on the scaffolds further enhanced some cartilage ECM protein deposition but not GAG. It has been well established that chemical factors can attribute to differential fates of MSCs.67–69 GAG deposition is especially sensitive to matrix environments. It is possible that scaffolds modified with collagen type I may interfere with the GAG synthesis potential of seeded MSCs. Furthermore, production of specific ECM components of differentiated or partially differentiated stem cells can gain dominance over the fate of the cellular community, and suppresses other protein/material production.68,70 Farrell et al. conducted an osteogenic study with MSCs cultured in 2D and 3D on novel collagen-GAG composite scaffolds.71 They showed that, when cultured in osteogenic media, scaffolds with incorporated GAG expressed a higher amount of GAG deposition. Work in the field of MSC differentiation suggests that tissue-specific materials, when present in a scaffold, elicit the formation of those materials and others specific to the tissue ECM that they reside in. We believe that this is why, when subjected to chondrogenic differentiation, collagen type I scaffolds underperformed in GAG material deposition. Collagen type I is the key collagen species present in subchondral bone ECM, hence its original selection. It was originally incorporated into our osteochondral scaffolds to add a biomimetic and cell-adhesive surface coating, and to demonstrate the ease and ability to modify 3D printed scaffolds postfabrication. There are, however, many other bioactive materials, natural polymers, and nanobiomaterials that could be implemented (i.e., GAG and collagen type II for the cartilage layer, nanocrystalline hydroxyapatite (nHA) for the bone layer, transforming growth factor-β1 [TGF-β1] or BMP-2-loaded nanospheres/functionalized nanotubes, etc.). Future iterations of our scaffolds could easily incorporate other surface modifications, and even have localized surface modification for spatiotemporal osteochondral differentiation (e.g., collagen type I in the bone layer and collagen type II in the cartilage layer). As previously discussed, collagen type II and total protein synthesis differed from the trends observed for GAG deposition. A more ECM-like substrate and culture environment generally increases MSC proliferative and predifferentiation activities.72 Given the already established enhanced proliferation on collagen-modified scaffolds, it can be postulated that the collagen surface modification greatly promoted MSC growth, which may contribute to subsequently high total protein synthesis.
Conclusion
In this study, we have designed and fabricated a series of novel biocompatible 3D printed scaffolds for osteochondral tissue repair. These scaffolds sought to not only recreate the cartilage and bone layers of the osteochondral region, but also, ultimately, incorporate special mechanical reinforcement elements, dubbed “key” features, which were intended to increase the mechanical strength and integration of the two distinct tissue zones. Mechanical testing showed that key scaffolds performed better in both compression and shear when compared with homogeneous controls consistently across a variety of different pore sizes. These results were then further supported by computational analysis of scaffold mechanical properties. This implies that our constructs would perform better under natural mechanical loading at the osteochondral interface in situ. In addition, we further conjugated biocompatible collagen onto the optimal 3D printed scaffolds. The cellular results show that the key designed 3D printed scaffold with collagen can greatly enhance MSC growth, and expressed more chondrogenic synthesis of GAG, type II collagen, and total protein content than controls. In summary, this study demonstrated that the key design with small features and a biomimetic collagen coating is the most effective scaffold among other designs, making it promising for future osteochondral regeneration applications.
Acknowledgments
This work was supported by Award Number UL1TR000075 from the NIH National Center for Advancing Translational Sciences and by the George Washington Institute for Biomedical Engineering (GWIBE).
Disclosure Statement
No competing financial interests exist.
References
- 1.Castro N.J., Hacking S.A., and Zhang L.G.Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann Biomed Eng 40,1628, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L., Hu J., and Kyriacos A.Athanasiou. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng 37,1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. A National Public Health Agenda for Osteoarthritis 2010, Available at: www.cdc.gov/arthritis/docs/oaagenda.pdf
- 4.Zhang L., and Webster T.J.Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4,66, 2009 [Google Scholar]
- 5.Levine B., and Kanat I.O.Subchondral bone cysts, osteochondritis dissecans, and Legg-Calve-Perthes disease: a correlation and proposal of their possible common etiology and pathogenesis. J Foot Surg 27,75, 1988 [PubMed] [Google Scholar]
- 6.Kilian K.A., Bugarija B., Lahn B.T., and Mrksich M.Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A 107,4872, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikos A.G., Thorsen A.J., Czerwonka L.A., Bao Y., Langer R., Winslow D.N., and Vacanti J.P.Preparation and characterization of poly(L-lactic acid) foams. Polymer 35,1068, 1994 [Google Scholar]
- 8.Jiang C.C., Chiang H., Liao C.J., Lin Y.J., Kuo T.F., Shieh C.S., Huang Y.Y., and Tuan R.S.Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res 25,1277, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Castro N.J., O'Brien C., and Zhang L.G.Biomimetic biphasic 3D nanocomposite scaffold for osteochondral regeneration. AIChE J 60,432, 2014 [Google Scholar]
- 10.Sill T.J., and von Recum H.A.Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29,1989, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Holmes B., Castro N.J., Zhang L.G., and Zussman E.Electrospun fibrous scaffolds for bone and cartilage tissue generation: recent progress and future developments. Tissue Eng Part B Rev 18,478, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Holmes B., Castro N.J., Li J., Keidar M., and Zhang L.G.Enhanced human bone marrow mesenchymal stem cell functions in novel 3D cartilage scaffolds with hydrogen treated multi-walled carbon nanotubes. Nanotechnology 24,365102, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Whang K., Thomas C.H., Healy K.E., and Nuber G.A novel method to fabricate bioabsorbable scaffolds. Polymer 36,837, 1995 [Google Scholar]
- 14.Wang M., Cheng X., Zhu W., Holmes B., Keidar M., and Zhang L.G.Design of biomimetic and bioactive cold plasma-modified nanostructured scaffolds for enhanced osteogenic differentiation of bone marrow-derived mesenchymal stem cells. Tissue Eng Part A 20,1060, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Im O., Li J., Wang M., Zhang L.G., and Keidar M.Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int J Nanomedicine 7,2087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Childs A., Hemraz U.D., Castro N.J., Fenniri H., and Zhang L.G.Novel biologically-inspired rosette nanotube PLLA scaffolds for improving human mesenchymal stem cell chondrogenic differentiation. Biomed Mater 8,065003, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Shim J.H., Kim J.Y., Park M., Park J., and Cho D.W.Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 3,034102, 2011 [DOI] [PubMed] [Google Scholar]
- 18.O'Shea T.M., and Miao X.Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B Rev 14,447, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Derby B.Printing and prototyping of tissues and scaffolds. Science 338,921, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Cui X., Breitenkamp K., Finn M.G., Lotz M., and D'Lima D.D.Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A 18,1304, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedorovich N.E., Schuurman W., Wijnberg H.M., Prins H.J., van Weeren P.R., Malda J., Alblas J., and Dhert W.J.Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng Part C Methods 18,33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooke M.N., Fisher J.P., Dean D., Rimnac C., and Mikos A.G.Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J Biomed Mater Res B Appl Biomater 64,65, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Fedorovich N.E., Alblas J., Hennink W.E., Oner F.C., and Dhert W.J.Organ printing: the future of bone regeneration? Trends Biotechnol 29,601, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Igawa K., Chung U.I., and Tei Y.[Custom-made artificial bones fabricated by an inkjet printing technology]. Clin Calcium 18,1737, 2008 [PubMed] [Google Scholar]
- 25.Lee J.Y., Choi B., Wu B., and Lee M.Customized biomimetic scaffolds created by indirect three-dimensional printing for tissue engineering. Biofabrication 5,045003, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigues M.T., Lee S.J., Gomes M.E., Reis R.L., Atala A., and Yoo J.J.Bilayered constructs aimed at osteochondral strategies: the influence of medium supplements in the osteogenic and chondrogenic differentiation of amniotic fluid-derived stem cells. Acta Biomater 8,2795, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Miot S., Brehm W., Dickinson S., Sims T., Wixmerten A., Longinotti C., Hollander A.P., Mainil-Varlet P., and Martin I.Influence of in vitro maturation of engineered cartilage on the outcome of osteochondral repair in a goat model. Eur Cells Mater 23,222, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Shipley R.J., Jones G.W., Dyson R.J., Sengers B.G., Bailey C.L., Catt C.J., Please C.P., and Malda J.Design criteria for a printed tissue engineering construct: a mathematical homogenization approach. J Theor Biol 259,489, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Aishwarya S., Mahalakshmi S., and Sehgal P.K.Collagen-coated polycaprolactone microparticles as a controlled drug delivery system. J Microencapsul 25,298, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Colter D.C., Class R., DiGirolamo C.M., and Prockop D.J.Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A 97,3213, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira J.M., Rodrigues M.T., Silva S.S., Malafaya P.B., Gomes M.E., Viegas C.A., Dias I.R., Azevedo J.T., Mano J.F., and Reis R.L.Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials 27,6123, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Slivka M.A., Chu C.C., and Adisaputro I.A.Fiber-matrix interface studies on bioabsorbable composite materials for internal fixation of bone fractures. I. Raw material evaluation and measurement of fiber-matrix interfacial adhesion. J Biomed Mater Res 36,469, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Schaefer D., Martin I., Jundt G., Seidel J., Heberer M., Grodzinsky A., Bergin I., Vunjak-Novakovic G., and Freed L.E.Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum 46,2524, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Newitt D.C., Majumdar S., van Rietbergen B., von Ingersleben G., Harris S.T., Genant H.K., Chesnut C., Garnero P., and MacDonald B.In vivo assessment of architecture and micro-finite element analysis derived indices of mechanical properties of trabecular bone in the radius. Osteoporos Int 13,6, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Lima E.G., Bian L., Mauck R.L., Byers B.A., Tuan R.S., Ateshian G.A., and Hung C.T.The effect of applied compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Conf Proc IEEE Eng Med Biol Soc 1,779, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Kelly D.J., and Prendergast P.J.Prediction of the optimal mechanical properties for a scaffold used in osteochondral defect repair. Tissue Eng 12,2509, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Giovannone D., Reyes M., Reyes R., Correa L., Martinez D., Ra H., Gomez G., Kaiser J., Ma L., Stein M.P., and de Bellard M.E.Slits affect the timely migration of neural crest cells via Robo receptor. Dev Dyn 241,1274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsiko A., Levingstone T.J., O'Brien F.J., and Gleeson J.P.Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav Biomed Mater 11,41, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Guarino V., Causa F., Taddei P., di Foggia M., Ciapetti G., Martini D., Fagnano C., Baldini N., and Ambrosio L.Polylactic acid fibre-reinforced polycaprolactone scaffolds for bone tissue engineering. Biomaterials 29,3662, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Cui X., Boland T., D'Lima D.D., and Lotz M.K.Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul 6,149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castilho M., Dias M., Gbureck U., Groll J., Fernandes P., Pires I., Gouveia B., Rodrigues J., and Vorndran E.Fabrication of computationally designed scaffolds by low temperature 3D printing. Biofabrication 5,035012, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Fu M., Lin L., Kong X., Zhao W., Tang L., Li J., and Ouyang J.Construction and accuracy assessment of patient-specific biocompatible drill template for cervical anterior transpedicular screw (ATPS) insertion: an in vitro study. PLoS One 8,e53580, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakansson A., Rantatalo M., Hansen T., and Wanhainen A.Patient specific biomodel of the whole aorta: the importance of calcified plaque removal. Vasa 40,453, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Klammert U., Gbureck U., Vorndran E., Rodiger J., Meyer-Marcotty P., and Kubler A.C.3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Craniomaxillofac Surg 38,565, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q., Mochalin V.N., Neitzel I., Knoke I.Y., Han J., Klug C.A., Zhou J.G., Lelkes P.I., and Gogotsi Y.Fluorescent PLLA-nanodiamond composites for bone tissue engineering. Biomaterials 32,87, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Yeo M., Lee H., and Kim G.Three-dimensional hierarchical composite scaffolds consisting of polycaprolactone, β-tricalcium phosphate, and collagen nanofibers: fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromolecules 12,502, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Lee M., and Wu B.M.Recent advances in 3D printing of tissue engineering scaffolds. Methods Mol Biol 868,257, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Nair L.S., Bhattacharyya S., and Laurencin C.T.Development of novel tissue engineering scaffolds via electrospinning. Expert Opin Biol Ther 4,659, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Ma Z., Kotaki M., Inai R., and Ramakrishna S.Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng 11,101, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Broom N.D., Oloyede A., Flachsmann R., and Hows M.Dynamic fracture characteristics of the osteochondral junction undergoing shear deformation. Med Eng Phys 18,396, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Flachsmann E.R., Broom N.D., and Oloyede A.A biomechanical investigation of unconstrained shear failure of the osteochondral region under impact loading. Clin Biomech (Bristol, Avon) 10,156, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Silyn-Roberts H., and Broom N.D.Fracture behaviour of cartilage-on-bone in response to repeated impact loading. Connect Tissue Res 24,143, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Kumar P., Oka M., Nakamura T., Yamamuro T., and Delecrin J.Mechanical strength of osteochondral junction. Nihon Seikeigeka Gakkai Zasshi 65,1070, 1991 [PubMed] [Google Scholar]
- 54.Hayes D.W., Jr., Brower R.L., and John K.J.Articular cartilage. Anatomy, injury, and repair. Clin Podiatr Med Surg 18,35, 2001 [PubMed] [Google Scholar]
- 55.Kennedy J.C., Grainger R.W., and McGraw R.W.Osteochondral fractures of the femoral condyles. J Bone Joint Surg Br 48,436, 1966 [PubMed] [Google Scholar]
- 56.Imai N., and Tomatsu T.Cartilage lesions in the knee of adolescents and young adults: arthroscopic analysis. Arthroscopy 7,198, 1991 [DOI] [PubMed] [Google Scholar]
- 57.Pope M.H., and Murphy M.C.Fracture energy of bone in a shear mode. Med Biol Eng 12,763, 1974 [DOI] [PubMed] [Google Scholar]
- 58.Hunger P.M., Donius A.E., and Wegst U.G.Structure-property-processing correlations in freeze-cast composite scaffolds. Acta Biomater 9,6338, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Nakai M., Niinomi M., and Ishii D.Mechanical and biodegradable properties of porous titanium filled with poly-L-lactic acid by modified in situ polymerization technique. J Mech Behav Biomed Mater 4,1206, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Rossi R.C.Prediction of the elastic moduli of composites. J Am Cer Soc 51,433, 1968 [Google Scholar]
- 61.Rice R.W.Comparison of stress concentration versus minimum solid area based mechanical property-porosity relations. J Mater Sci 28,2187, 1993 [Google Scholar]
- 62.Liu D.-M.Control of pore geometry on influencing the mechanical property of porous hydroxyapatite bioceramic. J Mater Sci Lett 15,419, 1996 [Google Scholar]
- 63.Caterson E.J., Li W.J., Nesti L.J., Albert T., Danielson K., and Tuan R.S.Polymer/alginate amalgam for cartilage-tissue engineering. Ann N Y Acad Sci 961,134, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Hum M., Wang M., Li Y., Chen H., Chu C., and Jiang H.Evaluation of modifying collagen matrix with RGD peptide through periodate oxidation. J Biomed Mater Res A 73,468, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y.Z., and Da W.M.[In vitro biological characteristics of mesenchymal stem cells from patients with myelodysplastic syndrome and their support to hematopoiesis]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 13,839, 2005 [PubMed] [Google Scholar]
- 66.Poldervaart M.T., Wang H., van der Stok J., Weinans H., Leeuwenburgh S.C., Oner F.C., Dhert W.J., and Alblas J.Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS One 8,e72610, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jager M., Feser T., Denck H., and Krauspe R.Proliferation and osteogenic differentiation of mesenchymal stem cells cultured onto three different polymers in vitro. Ann Biomed Eng 33,1319, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Curran J.M., Chen R., and Hunt J.A.Controlling the phenotype and function of mesenchymal stem cells in vitro by adhesion to silane-modified clean glass surfaces. Biomaterials 26,7057, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Li W.J., Tuli R., Huang X., Laquerriere P., and Tuan R.S.Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 26,5158, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Ishii M., Koike C., Igarashi A., Yamanaka K., Pan H., Higashi Y., Kawaguchi H., Sugiyama M., Kamata N., Iwata T., Matsubara T., Nakamura K., Kurihara H., Tsuji K., and Kato Y.Molecular markers distinguish bone marrow mesenchymal stem cells from fibroblasts. Biochem Biophys Res Commun 332,297, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Farrell E., Byrne E.M., Fischer J., O'Brien F.J., O'Connell B.C., Prendergast P.J., and Campbell V.A.A comparison of the osteogenic potential of adult rat mesenchymal stem cells cultured in 2-D and on 3-D collagen glycosaminoglycan scaffolds. Technol Health Care 15,19, 2007 [PubMed] [Google Scholar]
- 72.Matsubara T., Tsutsumi S., Pan H., Hiraoka H., Oda R., Nishimura M., Kawaguchi H., Nakamura K., and Kato Y.A new technique to expand human mesenchymal stem cells using basement membrane extracellular matrix. Biochem Biophys Res Commun 313,503, 2004 [DOI] [PubMed] [Google Scholar]