Abstract
Background:
Although the potential advantages of the Endocut mode (E-mode) of endoscopic sphincterotomy (EST) over the conventional blended cut mode (C-mode) have been reported, the problems, including the small sample size and retrospective analysis, that occurred in previous studies make it difficult to conclude the advantage of the E-mode regarding the safety and efficacy. We performed a prospective randomized controlled study to compare these modes.
Methods:
A total of 360 patients with choledocholithiasis or stenosis of the bile duct were randomly assigned to one of the modes. To avoid the technical bias due to multiple operators or institutions, the main operator and the institution were restricted to only one experienced doctor and 3 institutions at his place of employment, respectively. We defined pancreatitis, bleeding, and perforation as complications of EST. Besides, bleeding includes endoscopically evident bleeding that was defined as visible during the procedure of sphincterotomy and temporary slight oozing.
Results:
The complications occurred in 20 (11.2%) patients from the E-mode group: pancreatitis in 6 (3.4%) and endoscopically evident bleeding in 14 (7.8%). In contrast, the complications occurred in 25 (13.8%) patients from the C-mode group: pancreatitis in 7 (3.9%) and endoscopically evident bleeding in 18 (9.9%), although these findings were not statistically significant. Overall, there were no severe complications. There were no significant differences in completion ratio of EST and the time taken for the sphincterotomy between both groups.
Conclusions:
The E-mode could not surpass the C-mode in safety and efficacy under the operation by a single endoscopist.
Key Words: endoscopic sphincterotomy, Endocut mode, conventional blended cut mode, complication, efficacy
Endoscopic sphincterotomy (EST) dissects the sphincter of Oddi by cutting and/or causing the coagulation of the tissue using an electrosurgical current. Complications of EST include acute pancreatitis, hemorrhage, duodenal perforation, and sepsis.1–5 These complications occur in 5% to 10% of the population.1–5 Factors involved in the occurrence of complications of EST have been considered to be the Oddi dysfunction,6 types of electrosurgical current,7,8 specialist training, disinfection, drainage, and collaboration with surgical colleagues.4
The characteristics of the electrosurgical current may affect the frequency and extent of the complications of EST because the nature of the thermal tissue injury depends on the characteristics of the electrosurgical current used to perform EST.9,10 A pure cutting current achieves better cutting ability, whereas a low-voltage coagulating current that is not used solely gains better hemostasis in EST.1 A mixed current comprising mixed patterns of both pure cutting and coagulating currents is currently available for EST in 2 modes, which are the blended cut and the Endocut (E)-mode.1,11,12 The blended cut (C)-mode is comprised of cutting and coagulating currents delivered together in one waveform.13 The E-mode (ERBE, Marietta, GA) gains both the effects of cutting and coagulating because the cutting and coagulating currents are mixed together and applied in turn in short bursts with an intermittent pause.13 When compared with the conventional blended C-mode, the E-mode can give a well-defined and constant degree of coagulation of the incision margins for hemostasis by its automatic voltage regulation and controlled cutting speed, which prevents perforation of the superior part of the papilla of Vater based on an uncontrolled cutting speed, by its automatically fractionated cut.14 Thus, the E-mode may reduce complications of EST theoretically compared with the conventional blended C-mode.
An EST with blended currents increases the rate of pancreatitis after EST compared with EST with a pure cutting current.7,8 There is, however, a contradictory report that EST with a pure cutting current has a similar pancreatitis rate, a higher rate of immediate hemorrhage, and poorer control of the incision than that with a blended current.15 A recent meta-analysis shows that the pure cutting current has a similar pancreatitis rate and more episodes of bleeding, primarily mild bleeding, than the mixed current.16 As for the comparison of mixed current modes, the results were controversial in the development of acute pancreatitis and clinically significant bleeding in multiple clinical trials comparing the E-mode with the blended C-mode.14,17–19 However, the problems in the previous studies, including small sample size and retrospective analysis, make it difficult to conclude the advantage of efficacy and safety of the E-mode. Our study aim was to evaluate whether the E-mode surpasses the conventional blended C-mode in safety and efficacy.
METHODS
Study Design
A prospective randomized trial was performed. One and the same endoscopist operated EST in 3 different institutions at his place of employment. Patients at each site were randomly allocated to either EST with the E-mode or conventional blended C-mode according to sequential sealed envelopes in blocks of 20 to ensure equal randomization for the duration of the study. The consecutive patients at 3 institutions were registered. Randomization took place after biliary access and just before an EST. An independent coresearcher in Gunma University, who was not involved in recruitment and treatment allocation, generated the random allocation sequence and the allocation was kept in sealed, opaque envelopes. The allocation was concealed until after the feasibility of EST after biliary access had been confirmed. Another independent coresearcher in Gunma University assigned patients to either mode. The primary endpoint was the safety of these modes and the secondary endpoint was efficacy of these modes.
Our study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of each 3 institution. Written informed consent was obtained from all participating patients.
Study Population
Because an EST needs to be scheduled at the time of therapeutic endoscopy in advance, the baseline diseases eligible for inclusion were restricted to choledocholithiasis or stenosis of the bile duct, which are representative indications for EST, to decrease the bias based on the baseline diseases. However, pancreatitis due to choledocholithiasis was excluded. In addition, acute recurrent pancreatitis, previous pancreatitis, previous postendoscopic retrograde cholangiopancreatography (ERCP) pancreatitis, and suspected sphincter of Oddi dysfunction were excluded. Subjects registered in the study agreed with informed consent before the EST procedure. Exclusion criteria were the incapability to give informed consent, the inability to stop using antiplatelet agents or anticoagulant agents because of a complication, a previous history of Billroth II anastomosis, and a preexisting sphincterotomy or other biliary/pancreatic endotherapy. When antiplatelet agents or anticoagulant agents could be stopped, these drugs were stopped several days before the EST procedure with temporary heparinization if necessary.
Study Intervention
ERCP was performed by using standard duodenoscopes. Biliary EST was performed by using a standard triple-lumen sphincterotome (CleverCut, Olympus, Japan). The length of cutting wires used was 25 mm. A guidewire was used during the sphincterotomy in all subjects. An Olympus UES-20 electrosurgical generator (Olympus, Japan) was used for the conventional blended cut with the blended current set at an output limit of 30 W and the coagulation current set at an output limit of 5 W. An Erbe ICC200 (Erbe, Germany) was used for the E-mode with the effect 3 current set at an output limit of 120 W and the forced coagulation current set at an output limit of 30 W. The Erbe ICC200 electrosurgical generator (Erbe) has a function of feedback monitoring of tissue resistance, and it automatically regulates output correspondingly. In contrast, the Olympus UES-20 electrosurgical generator (Olympus) cannot automatically regulate output and, thus, it is considered working by conventional blended C-mode. The operator of the EST was not blinded to the mode because of the clear differences of the cutting characteristics between both modes. We did not use a prophylactic pancreatic stent or nonsteroidal anti-inflammatory drugs (NSAIDs) in this study because neither prophylactic pancreatic stents nor NSAIDs against post-ERCP pancreatitis are currently approved in Japan.
Data Collection
Patient characteristics and study intervention were recorded by the operator and coresearchers at the end of the EST procedure. All subjects were hospitalized for 1 week after the EST and interviewed 7 days after the procedure or at any time if deemed necessary by the operator and coresearchers. The complications were assessed by research assistants who were blinded to the treatment allocation. The serum total amylase levels were recorded before the ERCP and at 24 hours after the EST. The normal range of serum total amylase levels is the same among 3 institutions and 49 to 136 IU/L. Pancreatitis was defined as upper abdominal pain, new or increased, together with an elevation of the serum total amylase level to at least 3 times at 24 hours after the ERCP. A bleeding complication was classified as endoscopically or clinically evident. The endoscopically evident bleeding was defined as visible during the procedure of sphincterotomy. This bleeding was temporary slight oozing and natural hemostasis was observed without requiring an endoscopic hemostatic therapy. The endoscopically evident bleeding was assessed by blinded research assistants. The clinically evident bleeding was defined by the consensus criteria proposed by Cotton et al.4 The severity of the complications, such as pancreatitis and bleeding, was graded according to the same consensus criteria.4 Patients were followed for evidence of bleeding, such as melena, hematochezia, or black stool, by an interview and laboratory assessment at 24 hours and 7 days after the EST or at any time during hospitalization if necessary.
The efficacy of the EST was judged by completion of EST and the time taken for the sphincterotomy. Completion of EST was defined by the free passage of a fully bowed sphincterotome with a 25 mm wire and spontaneous bile drainage. The manipulation of the endoscopic incision including a “zipper” incision is based on the subjective evaluation by the operator and thus its evaluation is possible lack of accuracy. Thereafter, we did not use the manipulation of the endoscopic incision as an index of efficacy. Supplementary data were collected and comprised of the indication for the procedure, results of therapy, and other required interventions, if any.
Power Calculations and Statistical Analysis
The sample size calculation was based on the safety data from the previous prospective controlled study14 and the large retrospective study.18 The frequencies of the complications (bleeding, pancreatitis, and perforation) of the E-mode and conventional blended C-mode are 12% and 28% in the prospective study14 and 7% and 12% in the large retrospective study,18 respectively. Because these studies include endoscopically evident bleeding, the rate of complications seems to be higher than 5% to 10% of the significant complications of EST reported in several prospective studies.1–4,7 In addition, because the complications are not uniformly defined, the comparison of these complications is difficult. For example, pancreatitis is defined as every increase of serum total amylase with abdominal pain lasting >12 hours in the prospective study,14 but it is defined as clinically apparent pancreatitis without referring serum total amylase levels in the large retrospective study.18 Besides, the latter study18 may underestimate the complications, such as delayed bleeding after patient discharge. Thereafter, we estimated the expected rate of complications as the median values of those 2 studies in our study. Thus, the power calculations were based on the ability to detect a difference in the overall significant complications of 10.5% (eg, 20% vs. 9.5%), with 80% power and a 2-sided α-level of 0.05. This resulted in a total sample size of 360 patients. We aimed to screen 400 patients to randomize 360 patients accounting for dropout for any reason, such as failure of the EST. Continuous data are presented as the median and range. Differences in the proportions were measured by the Fisher exact test or the χ2 test. Continuous variables were compared by the Mann-Whitney U test. Results were considered statistically significant when the P-value was <0.05. The analysis was based on the intention to treat.
RESULTS
The study began on June 1, 2006, and all patients had completed enrollment on May 31, 2011. A total of 400 patients were considered for enrollment in the study. Fifteen patients were excluded because of the evidence of pancreatitis due to choledocholithiasis. In addition, 25 were excluded for the following reasons: use of precut sphincterotomy to access the biliary tree (n=20), difficulty adjusting to EST (n=4), and failure to cannulate the bile duct (n=1). The cases that had difficulty adjusting to EST had the papilla in a diverticulum and were treated with endoscopic papillary balloon dilation. Thus, 360 patients were randomized to EST with the E-mode (n=179) or the conventional blended C-mode (n=181) (Fig. 1). No patients among them were excluded from analysis.
FIGURE 1.
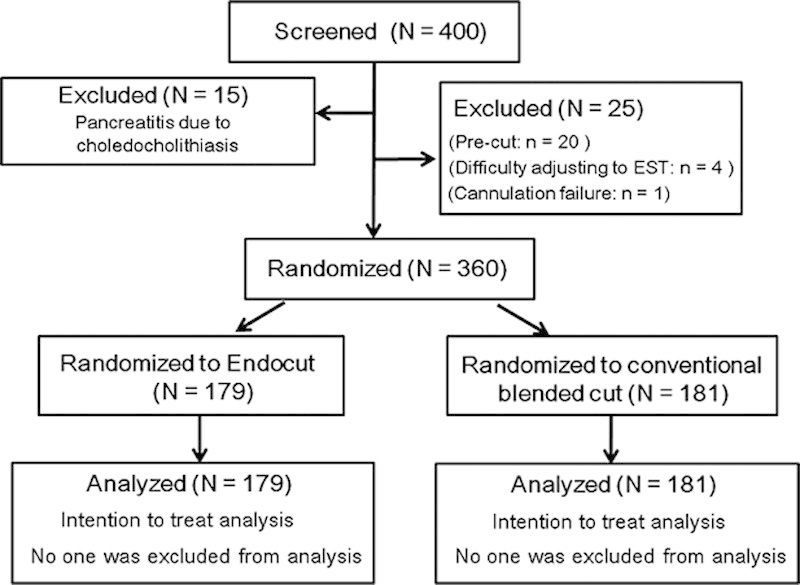
Patient flow during the study.
Baseline characteristics, such as age and gender, were not significantly different between both groups (Table 1). Some patients with stenosis of the bile duct showed high total amylase levels before EST because of pancreatic fluid retention due to pancreatic cancer. The procedure variables, such as difficult cannulation and stone extraction, were not significantly different between both groups (Table 2).
TABLE 1.
Baseline Characteristics of the Study Population
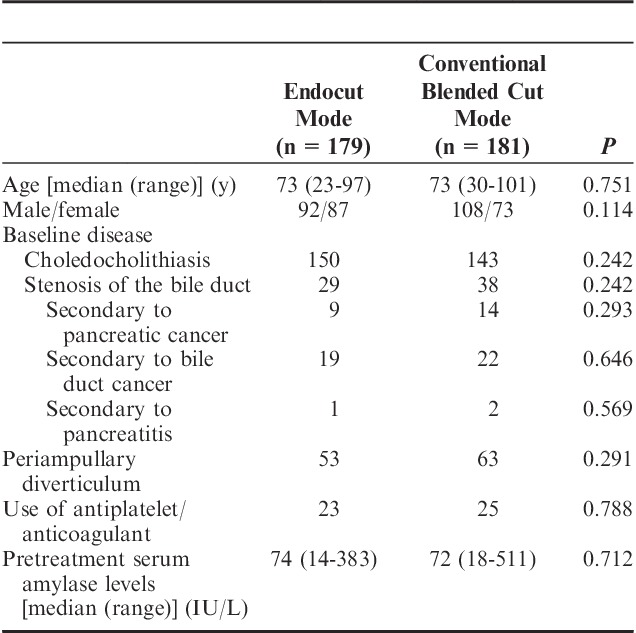
TABLE 2.
Comparison of EST Procedure Variables Between the Modes
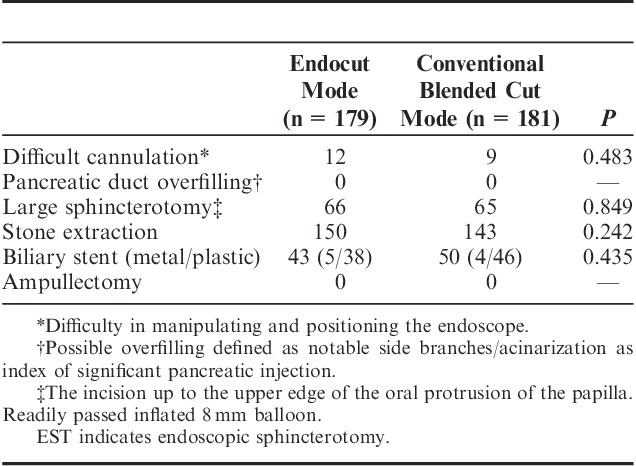
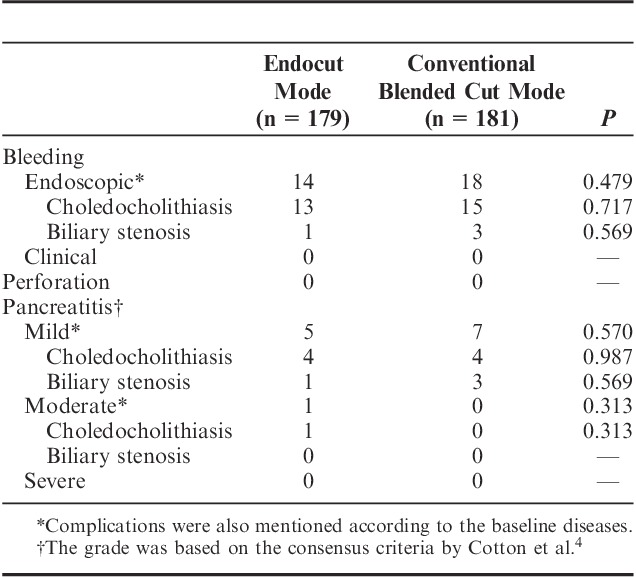
Table 3 shows the comparison of the complications between both groups within 7 days of randomization. The complications occurred in 20 (11.2%) patients of the E-mode group, whereas they occurred in 25 (13.8%) patients of the conventional blended C-mode group. There were 6 (3.4%) patients with pancreatitis in the E-mode group (5 mild and 1 moderate pancreatitis) versus 7 (3.9%) patients with pancreatitis (7 mild pancreatitis) in the conventional blended C-mode group. There was no significant difference of the frequency of pancreatitis between both groups. There were 14 (7.8%) patients with endoscopically evident bleeding in the E-mode group versus 18 (9.9%) patients with endoscopically evident bleeding in the conventional blended C-mode group. The frequency of endoscopically evident bleeding was not significantly different. There was no case of clinically evident hemorrhage in either group at the time of EST, 24 hours after EST, or within 7 days of randomization. Furthermore, endoscopically evident bleeding did not obscure visualization and, thus, did not hamper the procedure. No patients experienced perforation. There were no severe complications, such as bleeding requiring intervention for hemostasis or severe pancreatitis, in either group. Besides, restenosis after EST has not been observed in cases of stenosis of the bile duct in either group at present.
TABLE 3.
Comparison of Complications Between the Modes
Because the definition of pancreatitis varies according to the previous studies, we additionally compared serum total amylase levels after EST between both groups. The median serum total amylase levels after EST was 122 IU/L (range, 29 to 7280 IU/L) and 153 IU/L (range, 18 to 6986 IU/L) in the E-mode group and the conventional blended C-mode group, respectively. Thus, there was no significant clinical difference of the serum total amylase levels after EST between both groups.
In view of efficacy, EST was completed in all patients in either group. The median time taken for the sphincterotomy was 60 seconds (range, 25 to 180 s) and 65 seconds (range, 30 to 340 s) in the E-mode group and the conventional blended C-mode group, respectively. Thus, there were no significant differences in completion ratio of EST and the time taken for the sphincterotomy between both groups.
DISCUSSION
The rates of complications were 11.2% and 13.8% in each group and higher than 5% to 10% of the significant complications in several prospective studies1–4,7 as was expected. The main reason is that endoscopically evident bleeding caused majority of the complications in our study. We estimated 20% as the rate of complications in the conventional blended C-mode group, and the actual rate (13.8%) was lower than the estimated rate. However, the sample calculation can be justified by the estimation based on the results of the previous comparative studies.14,18
Our main result is that the E-mode may have similar safety and efficacy as the conventional blended C-mode. In other words, the differences of these modes may not be an issue in clinical practice under the operation by a single skilled endoscopist of EST. However, the difference of these 2 modes might appear in case of less-experienced ERCP providers and might need to be verified in those operators in the future. Alternatively, albeit speculative, the more “advanced” cutting mode “may” have benefits for those operators. Although the difference between the E-mode and the conventional blended C-mode in view of safety and efficacy had no clinical significance, our study is the first prospective randomized study with an appropriate sample size and thus deserves special consideration.
On the basis of our results, one of the main reasons for employing either mode may be cost rather than safety or efficacy. As the costs of medical devices such as contrast tubes for endoscope and electricity charges were considered roughly equal between 2 modes, the differences of 2 modes mainly depend on those of the cost of main units of electrosurgical generator. These prices at the time of delivery were 1,710,000 yen for the Erbe ICC200 (Erbe) and 1,800,000 yen for the Olympus UES-20 electrosurgical generator (Olympus). Thus, the costs of these modes were also almost equal.
Our study has a number of strengths. It is the largest prospective study to date that addresses whether the E-mode can surpass the conventional blended C-mode in the EST procedure. The earlier studies registered a total of 100 and 134 patients,14,17 and they did not perform the power calculations. However, a larger but retrospective study (n=2309) exists.18 Another strength is that we restricted our study to one operator, in comparison with the earlier studies,14,17,18 except that there was no description in one study.19 This obviously decreased the bias based on the difference of operator experience. However, this is one of our study limitations from another point of view. Multiple operators may be better in view of generalization of the operation of EST. Another strength of our study is that we restricted our analysis to 2 underlying diseases, in comparison with the earlier study.14 Because miscellaneous underlying diseases may provide potential bias, the selection of focused underlying diseases was a good strategy to evaluate both modes. In contrast, another limitation of our study is evaluation of serum total amylase level after EST. Because we often experience elevation of serum salivary amylase levels after endoscopy, serum total amylase levels may be affected by salivary amylase levels because of operation of EST.
The previous large retrospective study18 showed that the EST with the E-mode was associated with a significantly lower frequency of endoscopically evident bleeding but not clinically evident bleeding. Although the study number is overwhelming (1091 in the E-mode versus 1218 in the conventional blended cut), the interpretation of the study requires attention: (1) this study is neither a randomized nor a prospective study; (2) the intervention periods are separated and consecutive between the 2 modes, that is, the conventional blended C-mode was performed earlier than the E-mode. The confounding bias may happen. However, a multivariate analysis may compensate for the quality of their study. Importantly, participating endoscopists were reported to be highly experienced in the performance of the ERCP and EST in the large retrospective study.18 The frequency of endoscopically evident bleeding but not clinically evident bleeding was significantly different between the 2 modes, implying that the differences between these modes may not be an issue in clinical practice under the manipulation of the skilled operator of EST, supporting our data. In our study, there was a trend toward a reduction of endoscopically evident bleeding in the E-mode but no significant difference between the 2 modes. Given that there was no clinically significant bleeding in either mode in our study, although the frequency of endoscopically evident bleeding was higher than that in the large retrospective study,18 the high endoscopic proficiency of our operator was warranted. Although the controversial results showed that operator experience did not affect the complications of EST,15 there are reports that one of the most important factors influencing the complication rate of EST is the case volume per year20 or case volume per week.3 In fact, our operator has performed EST at least twice or 3 times per week and his high proficiency may mask the difference of efficacy and endoscopically evident bleeding between the 2 modes in our study. This may also explain why the overall complication rates between these modes were similar, and the complication rate in conventional blended C-mode was lower than we estimated to decide on the sample size in our study. Although the exact reason why the frequency of endoscopically evident bleeding in our study was higher than that in the large retrospective study18 is unknown, stringent observation by the operator and coresearchers in our prospective study may be more likely to find subtle endoscopically evident bleeding compared with the endoscopy reporting database-based study.18
In our study, all subjects were hospitalized for 1 week. On an outpatient basis, endoscopic therapy of choledocholithiasis may be performed safely21 and therapeutic ERCP may be applicable in a significant proportion of very elderly patients.22 In Japan, however, hospitalization therapy for EST is principally required because most of hospitalization costs are covered by health insurance and there is an inadequate infrastructure construction such as facilities and human resources regarding overnight care or night medical examinations for post-EST outpatients. In addition, hospitalization depends on the facilities because of possible variance including the divergence of underlying diseases, treatments following EST, or emergent treatment for EST-induced complications.
In summary, the E-mode could not surpass the conventional blended C-mode in safety and efficacy under the operation by a single endoscopist. In different clinical settings such as the situation under less-experienced ERCP providers, the comparison between the 2 modes might need to be verified in the future. Otherwise, the more “advanced” cutting mode “may” have benefits for less-experienced ERCP providers, although this is just a conjecture.
Footnotes
Supported by a grant from the Japanese foundation for research and promotion of endoscopy.
The authors declare that they have nothing to disclose.
REFERENCES
- 1.Freeman ML. Complications of endoscopic biliary sphincterotomy: a review. Endoscopy. 1997;29:288–297. [DOI] [PubMed] [Google Scholar]
- 2.Aliperti G. Complications related to diagnostic and therapeutic endoscopic retrograde cholangiopancreatography. Gastrointest Endosc Clin N Am. 1996;6:379–407. [PubMed] [Google Scholar]
- 3.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909–918. [DOI] [PubMed] [Google Scholar]
- 4.Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37:383–393. [DOI] [PubMed] [Google Scholar]
- 5.Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004;59:845–864. [DOI] [PubMed] [Google Scholar]
- 6.Sherman S, Ruffolo TA, Hawes RH, et al. Complications of endoscopic sphincterotomy. A prospective series with emphasis on the increased risk associated with sphincter of Oddi dysfunction and nondilated bile ducts. Gastroenterology. 1991;101:1068–1075. [PubMed] [Google Scholar]
- 7.Stefanidis G, Karamanolis G, Viazis N, et al. A comparative study of postendoscopic sphincterotomy complications with various types of electrosurgical current in patients with choledocholithiasis. Gastrointest Endosc. 2003;57:192–197. [DOI] [PubMed] [Google Scholar]
- 8.Elta GH, Barnett JL, Wille RT, et al. Pure cut electrocautery current for sphincterotomy causes less post-procedure pancreatitis than blended current. Gastrointest Endosc. 1998;47:149–153. [DOI] [PubMed] [Google Scholar]
- 9.Siegel JH, Veerappan A, Tucker R. Bipolar versus monopolar sphincterotomy: a prospective trial. Am J Gastroenterol. 1994;89:1827–1830. [PubMed] [Google Scholar]
- 10.Sherman S, Lehman GA. ERCP- and endoscopic sphincterotomy-induced pancreatitis. Pancreas. 1991;6:350–367. [DOI] [PubMed] [Google Scholar]
- 11.Maydeo A, Borkar D. Techniques of selective cannulation and sphincterotomy. Endoscopy. 2003;35:S19–S23. [DOI] [PubMed] [Google Scholar]
- 12.Taunton JC. Surgical diathermy—a review. J Med Eng Technol. 1981;5:175–183. [DOI] [PubMed] [Google Scholar]
- 13.Slivka A, Bosco JJ, Barkun AN, et al. Electrosurgical generators: May 2003. Gastrointest Endosc. 2003;58:656–660. [DOI] [PubMed] [Google Scholar]
- 14.Kohler A, Maier M, Benz C, et al. A new HF current generator with automatically controlled system (Endocut mode) for endoscopic sphincterotomy—preliminary experience. Endoscopy. 1998;30:351–355. [DOI] [PubMed] [Google Scholar]
- 15.Norton ID, Petersen BT, Bosco J, et al. A randomized trial of endoscopic biliary sphincterotomy using pure-cut versus combined cut and coagulation waveforms. Clin Gastroenterol Hepatol. 2005;3:1029–1033. [DOI] [PubMed] [Google Scholar]
- 16.Verma D, Kapadia A, Adler DG. Pure versus mixed electrosurgical current for endoscopic biliary sphincterotomy: a meta-analysis of adverse outcomes. Gastrointest Endosc. 2007;66:283–290. [DOI] [PubMed] [Google Scholar]
- 17.Akiho H, Sumida Y, Akahoshi K, et al. Safety advantage of endocut mode over endoscopic sphincterotomy for choledocholithiasis. World J Gastroenterol. 2006;12:2086–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perini RF, Sadurski R, Cotton PB, et al. Post-sphincterotomy bleeding after the introduction of microprocessor-controlled electrosurgery: does the new technology make the difference? Gastrointest Endosc. 2005;61:53–57. [DOI] [PubMed] [Google Scholar]
- 19.Shinozuka N, Koyama I, Minoshima T, et al. Effect of automatically controlled system (Endocut mode) in endoscopic sphincterotomy [in Japanese]. Nihongekakeirengougakkaisi. 2001;26:41–44. [Google Scholar]
- 20.Rabenstein T, Schneider HT, Nicklas M, et al. Impact of skill and experience of the endoscopist on the outcome of endoscopic sphincterotomy techniques. Gastrointest Endosc. 1999;50:628–636. [DOI] [PubMed] [Google Scholar]
- 21.Elfant AB, Bourke MJ, Alhalel R, et al. A prospective study of the safety of endoscopic therapy for choledocholithiasis in an outpatient population. Am J Gastroenterol. 1996;91:1499–1502. [PubMed] [Google Scholar]
- 22.Katsinelos P, Kountouras J, Chatzimavroudis G, et al. Outpatient therapeutic endoscopic retrograde cholangiopancreatography is safe in patients aged 80 years and older. Endoscopy. 2011;43:128–133. [DOI] [PubMed] [Google Scholar]


