Photodynamic therapy (PDT) holds promise for treating skin, lung, bladder, and breast cancer.[1] It combines nontoxic photo-sensitizer (PS), harmless visible light, and cell- and tissue-associated oxygen to generate cytotoxic reactive oxygen species (ROS) such as singlet oxygen (1O2). The resultant ROS kills malignant cancer cells by apoptosis and/or necrosis, shuts down the vasculature in tumors, and stimulates the host immune system, and as a result, to inhibit tumor growth and destruct tumors.[2] There has been progress in the development of novel techniques for PDT with advancing efficiency partially because of a better understanding of therapeutic light and the development of fiber optic lasers.[3] Some PSs have been approved by the US Federal Drug Administration (FDA) while many remain in clinical trials.[4] They are efficiently being used to treat several types of cancers and a variety of other diseases.[5] Currently, cancer treatment by PDT is limited by the difficulty in the accumulation of PS in the tumors. Thus, the greatest challenge in PDT of cancer is to find a new strategy for delivering PS to the tumors to achieve efficient tumor destruction.
To overcome the challenge in delivering PS to tumors in breast cancer PDT, here we propose to use mesenchymal stem cells (MSCs) as a drug carrier to deliver PS to breast tumors (Figure 1) for two reasons. First, MSCs can be easily isolated from bone marrow of the patients,[6] then modified (chemically as shown in this work, or genetically as reflected in gene therapy[7]), and finally implanted into patients again for disease treatment to avoid immune rejection.[8] Second, it is now well-accepted that MSCs exhibit a natural high tumor affinity, which allows them to home to tumors and then retain in tumors in vivo[9] although the detailed mechanism remains unclear.[10,11] The tumor affinity of MSCs arises from a mechanism possibly mediated by chemokines such as stromal-derived factor-1, epidermal growth factor, and plate-derived growth factor.[12] It has been verified that the tumor affinity of MSCs can even drive them to home to and retain in breast tumors when injected from the tail vein of mice.[9] However, it is not clear whether the tumor affinity of MSCs will allow the drug-loaded MSCs to retain in tumor sites and make the drug available for destructing the tumor. Hence, this work aims to answer one important question: it is already known that MSCs can home to breast tumors,[9] however, can PS-loaded MSCs with high tumor affinity be exploited to destruct breast tumors by PDT once they are at the tumor sites?
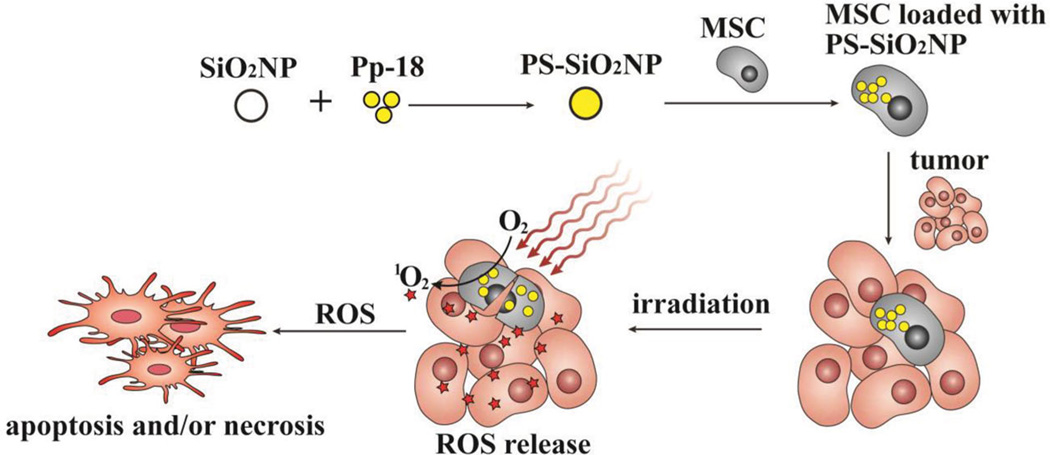
Figure 1.
Schematic illustration of loading PS-loaded SiO2NPs into MSCs and using the resultant PS-loaded MSCs to kill cancer cells and inhibit tumor growth by photodynamic therapy (PDT). The natural high tumor affinity of MSCs is exploited to allow the retention of PS-loaded MSCs in tumors and the consequent accumulation of PS in tumors for effective destruction of tumors by PDT. SiO2 NP: silica nanoparticle; Pp-18: purpurin-18; PS: photosensitizer; ROS: reactive oxygen species.
We first followed a reported similar procedure[13] to synthesize porous hollow silica nanoparticles (SiO2NPs) (Figure S1, Supporting Information). Silica was chosen because it is a biocompatible material.[14] The porous nature of the SiO2NPs allowed us to use a reported protocol[15] to load a hydrophobic PS called purpurin-18 (Pp-18) into the pores. We chose Pp-18 as a PS in this work because it was proved to show low cytotoxicity in the absence of light and could be activated by a red light, which has a better tissue penetration depth than other visible lights.[16] To remove weakly bound PS, PS-loaded SiO2NPs (PS-SiO2NPs) were first sonicated in ethanol and then isolated by high speed centrifugation, and such sonication-centrifugation procedure was repeated three times. When the PS-SiO2NPs were heated, a weight loss, corresponding to the removal of organic PS, was found at around 150–300 °C (Figure S2, Supporting Information), which further confirmed the successful loading of PS into SiO2NPs.
MTT assay suggested that in the absence of light irradiation, PS-SiO2NPs did not show significant toxicity to MSCs derived from the bone marrow of rats when their concentration was lower than 80 µg/mL (Figure S3, Supporting Information). To load the PS-SiO2NPs into the MSCs, which were isolated from rats with a procedure approved by the Institutional Animal Care and Use Committee of the University, we treated the MSCs with PS-SiO2NPs in Dulbecco’s Modified Eagle Medium (DMEM) without fetal bovine serum (FBS) at 37 °C for 4 h to achieve cellular uptake. It was found that nanoparticles could be uptaken by cells through mechanisms such as endocytosis during their incubation with cells.[17] To verify that the loading of PS-SiO2NPs into MSCs was due to cellular uptake, we replaced PS in SiO2NPs with a hydrophobic peptide (which mimics the hydrophobic PS used) labelled with an FITC green dye (WKYMVM-FITC) and then incubated the peptide-loaded SiO2NPs (80 µg/mL) with MSCs. Fluorescence microscopy imaging (Figure 2) showed the green fluorescence around cell nuclei (stained to be blue by 4′,6-diamidino-2-phenylindole (DAPI)), confirming the internalization of the green-dye-loaded SiO2NPs inside the cells. The fluorescence-activated cell sorting (FACS) analysis further confirmed that more than 90% of MSCs were fluorescent due to the uptake of the green-dye-loaded SiO2NPs (Figure 2). Although the peptide is a hydrophobic molecule with a molecular weight slightly higher than the hydrophobic PS, it was still loaded into the pores of the SiO2NPs, which were then uptaken by MSCs. Thus the successful loading of the peptide-loaded SiO2NPs into MSCs could justify the success in loading the PS-loaded SiO2NPs into MSCs using the same protocol.
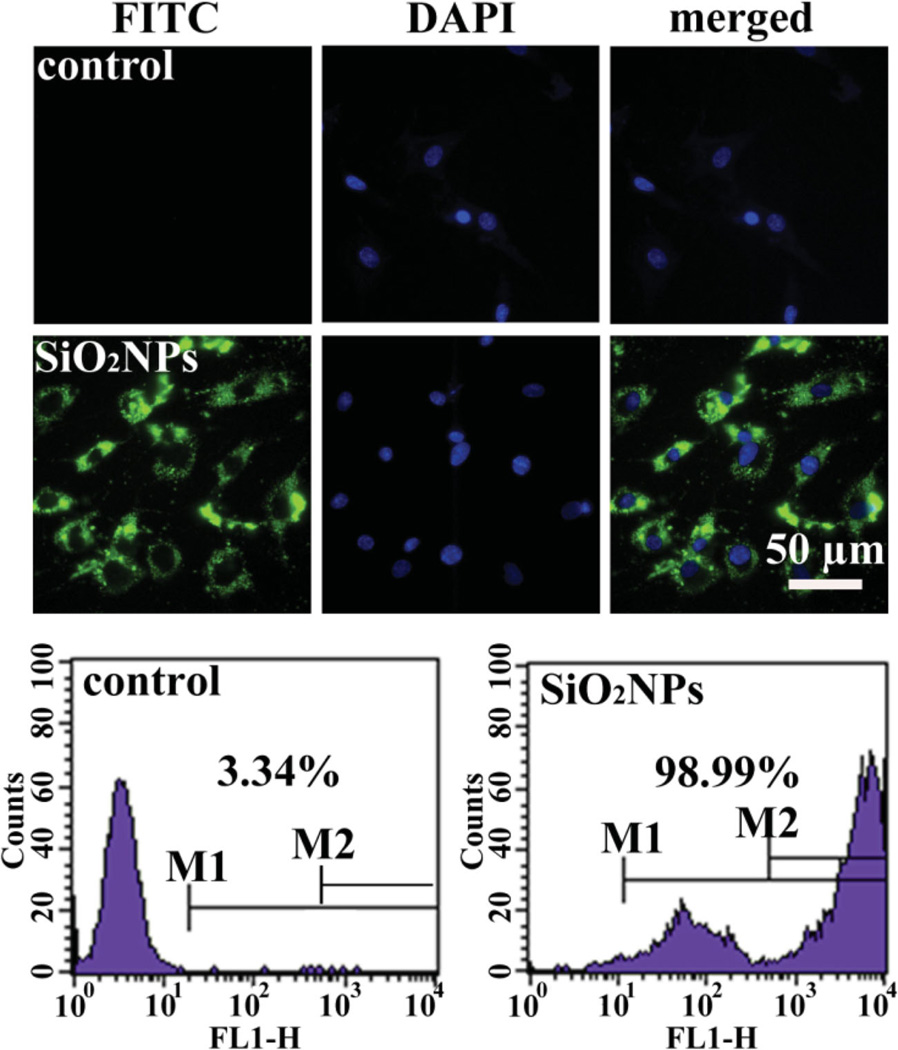
Figure 2.
In vitro cellular uptake of SiO2NPs loaded with FITC-labeled peptide by MSCs. MSCs without interacted with SiO2NPs were used as a control. Top: Fluorescence images showing DAPI-stained nuclei and FITC-stained SiO2NPs internalized in MSCs. Bottom: FACS analysis of MSCs incubated with FITC-stained SiO2NPs for 6 h, showing more than 90% of the cells were loaded with FITC-stained SiO2NPs.
Next, we followed an in vitro migration assay[18] to check whether the MSCs loaded with PS-SiO2NPs (PS-SiO2NPs-MSCs) would inhibit the tumor-homing affinity of MSCs, namely, the migration of MSCs to MCF-7 breast cancer cells. Briefly, the bottom and top wells of a transwell plate were cultured with MCF-7 cells and PS-SiO2NPs-MSCs, respectively. After incubation at 37 °C for 12 h, the cells attached to the top wells were removed whereas those attached to the bottom wells were fixed and counted. We found that the loading of PS-SiO2NPs in MSCs did not significantly reduce the number of MSCs that migrated to MCF-7 cells (Figure S3b, Supporting Information), suggesting that loading PS-SiO2NPs into MSCs did not inhibit the tumor affinity of the MSCs. Such little inhibition of the tumor affinity of MSCs will guarantee that PS-SiO2NPs-MSCs retain in the tumors and allow the PS to be accumulated in the tumor for the destruction of tumors by PDT.
To verify the generation of ROS inside MSCs upon the irradiation of a red light (at a power density of 0.04 W/cm2) on PS-SiO2NPs-MSCs, which is the key to the success of PDT, we used a 2′,7′-dichlorfluorescein-diacetate (DCFH-DA) staining kit (Invitrogen) following the manufacturer’s protocol. In this protocol, MSCs were incubated with a green dye (DCFH-DA) for 30 min, which stained the ROS to show green fluorescence. Thus, the intensity of the green fluorescence reflected the level of ROS generated due to light irradiation. As expected, upon light irradiation on MSCs loaded with PS-SiO2NPs, the intracellular ROS level increased with the increasing concentration of PS-SiO2NPs used to interact with MSCs (Figure S4, Supporting Information). This fact indicates that the internalization of PS-SiO2NPs in MSCs resulted in the presence of PS in MSCs, which was activated by light to trigger the excitation of oxygen into 1O2, the key ROS in PDT for inhibiting tumor growth. Interestingly, the increase of ROS level caused the cell surface to become ruptured, leading to the exposure of internalized PS-SiO2NPs (Figure S5, Supporting Information), which further confirmed the internalization of PS-SiO2NPs in MSCs.
We proceeded to demonstrate that MSCs could serve as a PS carrier for in vivo breast cancer PDT because MSCs were expected to carry PS-SiO2NPs and retain in the tumors due to their high affinity with the tumors. Co-injection of MSCs and cancer cells to generate tumors is a widely-accepted strategy in the demonstration and application of tumor affinity of MSCs.[11,19] Thus, we first generated a breast tumor model by co-injection of MSCs and MCF-7 breast cancer cells subcutaneously on the backs of nude mice, which was approved by the Institutional Animal Care and Use Committee of the University. The high tumor affinity of MSCs enabled the injected MSCs to stay with the tumors induced by MCF-7 breast cancer cells. The PDT was initiated by irradiating a red light onto the injected area with a power density of 0.3 W/cm2 and a spot size of ca. 1 cm2 for 15 min. We designed two groups of six-week-old nude mice (n = 4 for each group). Group 1 received a co-injection of 1 × 106 MCF-7 cells and 1.5 × 106 PS-SiO2NPs-MSCs. The injected area was then irradiated to initiate PDT one day after injection. Group 2 as a control received a co-injection of 1 × 106 MCF-7 cells and 1.5 × 106 MSCs without loading PS-SiO2NPs, followed by light irradiation on the injected area one day after injection. For each group, the inhibition of tumor growth was evaluated by measuring the size and weight of tumors on day 15.
In PDT, the preferential accumulation of a PS in a malignant tumor followed by irradiation with an appropriate wavelength of light can generate cytotoxic ROS, resulting in the death of cancer cells via apoptosis and/or necrosis and shutdown of blood vessels in the tumors, which eventually inhibit tumor growth.[5] Consistent with this already proved theory of PDT, we found that the tumor size and weight were significantly reduced in Group 1 when compared to Group 2 where no PS was loaded in MSCs (Figure 3). This indicates that PDT on day 1 (Group 1) after injection of PS-SiO2NPs-MSCs generated ROS to inhibit tumor growth because MSCs carried PS and retained in the tumors. Hematoxylin and eosin (H&E) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of tumor tissues of Groups 1 and 2 verified that the cancer cells were indeed killed by the mechanisms of necrosis and apoptosis to inhibit tumor growth in Group 1 in comparison to Group 2 (Figure 3 d and e). In a separate study, we applied the light to the tumors after tumors have grown for five days (Figure S6, Supporting Information). We found that the PDT significantly inhibited the tumor growth on the animal group receiving a co-injection of PS-SiO2NPs-MSCs and MCF-7 cells when compared to the animal group receiving a co-injection of unmodified MSCs (i.e., without loading PS-SiO2NPs) and MCF-7 cells (Figure S6, Supporting Information). This study confirms the retention of PS-SiO2NPs-MSCs even after tumors have grown for 5 days. In addition, we also evaluated the effects of the ratios of MSCs to MCF-7 cancer cells on inhibiting the in vivo tumor growth. The results (Figure S7 and S8, Supporting Information) suggested that when fewer PS-SiO2NPs-MSCs were co-injected with cancer cells, weaker tumor inhibition was observed because fewer PS molecules were accumulated within the tumor upon irradiation. Moreover, we found that when other normal cells, such as human embryonic kidney 293 (HEK-293) cells (a gift from Professor Raju V. S. Rajala at the University of Oklahoma Health Science Center), were loaded with PS-SiO2NPs to form PS-SiO2NPs-HEK-293 cells and used to replace PS-SiO2NPs-MSCs while keeping other conditions same as the group of PS-SiO2NPs-MSCs (Group 1) in Figure 3, the tumor growth was not effectively inhibited after light irradiation, just like the case of MSCs group (control group) as well as the case of simply injecting PS-SiO2NPs into the tumors (Figure S9, Supporting Information). Because the silicon content in the PS-SiO2NPs-MSCs present in the tumors reflected the number of PS-SiO2NPs-MSCs that retained in the tumor, we also analyzed the amount of silicon within 1 × 106 of different cells (PS-SiO2NPs-MSCs, PS-SiO2NPs-HEK-293 cells, and MSCs) right before injections (i.e., on day 0 of tumor growth) as well as within the tumors grown from the co-injection of MCF-7 cells and 1 × 106 each of these cells (on day 15) by inductively coupled plasma mass spectroscopy (ICP-MS). The results showed that: i) more PS-SiO2NPs were uptaken by HEK-293 cells (121 ± 11 ng silicon in 1 × 106 cells) than MSCs (82 ± 15 ng silicon in 1 × 106 cells); and ii) after 15 days of tumor growth, the silicon content within the tumors grown from the co-injection of MCF-7 cells and PS-SiO2NPs-MSCs (57 ± 12 ng/tumor) was not significantly decreased when compared with the injected silicon content (82 ± 15 ng silicon in 1 × 106 PS-SiO2NPs-MSCs), while no element silicon could be detected from the tumors of the other two groups (co-injection of MCF-7 cells and PS-SiO2NPs-HEK-293 cells as well as co-injection of MCF-7 cells and MSCs). These results clearly showed the high tumor affinity of MSCs enabled the retention of drug-loaded MSCs at the tumor sites. Taken together, our work indicated that PDT was effective for inhibiting tumor growth only when PS-SiO2NPs-MSCs were co-injected with cancer cells to generate tumors because PS-SiO2NPs-MSCs had a high tumor affinity. When the MSCs were replaced with HEK-293 cells, the PDT was not effective becuase HEK-293 cells did not have a natural tumor affinity and thus the drug carried by HEK-293 could not retain at the tumor sites.
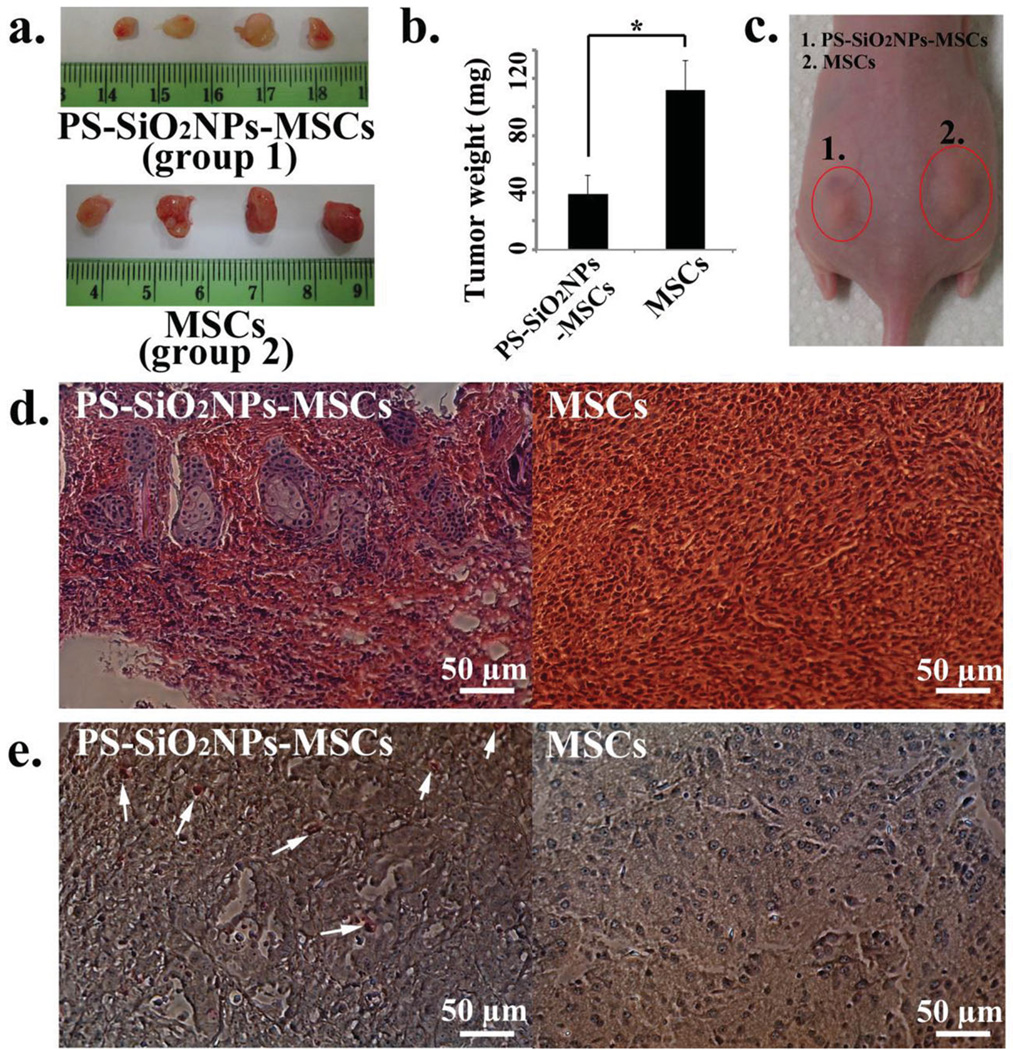
Figure 3.
In vivo PDT treatment on tumors one day after co-injection of MCF-7 cancer cells and MSCs with (group 1: PS-SiO2NPs-MSCs group) or without (group 2: control MSCs group) PS-SiO2NPs loaded. a) Pictures showing the size of tumors isolated from mice (PS-SiO2NPs-MSCs group: MSCs were loaded with PS-SiO2NPs and laser light was applied to trigger PDT; MSCs group: control where MSCs were not loaded with PS-SiO2NPs but laser light was still applied). b) The weight of tumors of PS-SiO2NPs-MSCs group and control MSCs group. c) A picture of a mouse showing the size of tumors one day after light treatment (the tumor on the left and right side was treated with PS-SiO2NPs-MSCs and MSCs, respectively. Both tumors were highlighted by an oval). d) H&E stained tissue sections of tumors (left: PS-SiO2NPs-MSCs group; right: MSCs group). e) TUNEL stained tissue sections of tumors (left: PS-SiO2NPs-MSCs group; right: MSCs group). The white arrows indicate apoptotic cells stained with TUNEL. The asterisk in (b) indicates significant difference between PS-SiO2NPs-MSCs group and MSCs group at p < 0.05.
In summary, we have demonstrated the use of a type of biological particles, MSCs, to deliver PS encapsulated by biocompatible SiO2NPs to tumors. We successfully found that internalization of PS-loaded SiO2NPs did not induce significant toxicity against MSCs, nor did they significantly inhibit the high tumor affinity of MSCs. When the cancer cells were co-injected with PS-loaded MSCs to form tumors, the tumor growth was significantly inhibited by PDT treatment after injection due to the retention of the PS-loaded MSCs (and the consequent accumulation of PS) in the tumors arising from the natural high tumor affinity of MSCs. Since many drugs can be loaded into SiO2NPs, the use of MSCs to deliver drug to tumors is a promising approach to targeted cancer therapy.
Supplementary Material
Acknowledgements
The authors thank the National Institutes of Health (EB009909, EB015190, and HL092526) for support of this work. This work was also in part supported by the National Science Foundation (CBET-0854465, CMMI-1234957, CBET-0854414, and DMR-0847758), the Department of Defense Peer Reviewed Medical Research Program (W81XWH-12–1–0384), the Oklahoma Center for Adult Stem Cell Research (434003) and the Oklahoma Center for the Advancement of Science and Technology (HR11–006). M.Y.Y. also thanks the generous support from the National High Technology Research and Development Program 863 (2013AA102507), the Zhejiang Provincial Natural Science Foundation of China (LZ12C17001), the Projects of Zhejiang Provincial Science and Technology Plans (2012C12910), the National Natural Science Foundation of China (20804037 and 21172194) and the Silkworm Industry Science and Technology Innovation Team (2011R50028). The authors would also like to thank Drs. D. Brackett and H. Zhu for kind help during experiments.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Binrui Cao, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, 101 Stephenson Parkway, Norman, OK 73019–5251, USA.
Mingying Yang, Email: yangm@zju.edu.cn, Institute of Applied Bioresource Research, College of Animal Science, Zhejiang University, Yuhangtang Road 866, Hangzhou, Zhejiang 310058, China.
Ye Zhu, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, 101 Stephenson Parkway, Norman, OK 73019–5251, USA.
Xuewei Qu, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, 101 Stephenson Parkway, Norman, OK 73019–5251, USA.
Chuanbin Mao, Email: cbmao@ou.edu, Department of Chemistry & Biochemistry, Stephenson Life Sciences Research Center, University of Oklahoma, 101 Stephenson Parkway, Norman, OK 73019–5251, USA.
References
- 1.a) Ahn TG, Lee BR, Choi EY, Kim DW, Han SJ. J. Gynecol. Oncol. 2012;23:115. doi: 10.3802/jgo.2012.23.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) De Rosa FS, Bentley MVLB. Pharmaceut. Res. 2000;17:1447. doi: 10.1023/a:1007612905378. [DOI] [PubMed] [Google Scholar]; c) Usuda J, Kato H, Okunaka T, Furukawa K, Tsutsui H, Yamada K, Suga Y, Honda H, Nagatsuka Y, Ohira T, Tsuboi M, Hirano T. J. Thorac. Oncol. 2006;1:489. [PubMed] [Google Scholar]; d) Yano S, Hirohara S, Obata M, Hagiya Y, Ogura S, Ikeda A, Kataoka H, Tanaka M, Joh T. J. Photochem. Photobiol. C. 2011;12:46. [Google Scholar]; e) Gandra N, Abbineni G, Qu X, Huai Y, Wang L, Mao CB. Small. 2013;9:215. doi: 10.1002/smll.201202090. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kalarical JS, Narayan S, Abbineni G, Hayhurst A, Mao CB. Mol. Cancer Ther. 2010;9:2524. doi: 10.1158/1535-7163.MCT-10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Ngweniform P, Li D, Mao CB. Soft Matter. 2009;5:954. [Google Scholar]; h) Ngweniform P, Abbinieni G, Cao B, Mao CB. Small. 2009;5:1963. doi: 10.1002/smll.200801902. [DOI] [PubMed] [Google Scholar]
- 2.Castano AP, Mroz P, Hamblin MR. Nat. Rev. Cancer. 2006;6:535. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J. Natl. Cancer Inst. 1998;90:889. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison RR, Sibata CH. Photodiagn. Photodyn. Ther. 2010;7:61. doi: 10.1016/j.pdpdt.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Dolmans DEJGJ, Fukumura D, Jain RK. Nat. Rev. Cancer. 2003;3:380. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 6.a) Zhu HB, Cao BR, Zhen ZP, Laxmi AA, Li D, Liu SR, Mao CB. Biomaterials. 2011;32:4744. doi: 10.1016/j.biomaterials.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang J, Wang L, Li X, Mao CB. Sci. Rep. 2013;3:1242. doi: 10.1038/srep01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Ma K, Wang DD, Lin YY, Wang JL, Mao CB. Adv. Funct. Mater. 2012 doi: 10.1002/adfm.201102963. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gandra N, Wang DD, Zhu Y, Mao CB. Angew. Chem. Int. Ed. 2013;52:11278. doi: 10.1002/anie.201301113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Stem Cells. 2008;26:831. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 9.a) Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Stem Cells. 2009;27:2614. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ren YJ, Zhang H, Huang H, Wang XM, Zhou ZY, Cui FZ, An YH. Biomaterials. 2009;30:1036. doi: 10.1016/j.biomaterials.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 10.a) Bexell D, Scheding S, Bengzon J. Mol. Ther. 2010;18:1067. doi: 10.1038/mt.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Loebinger MR, Eddaoudi A, Davies D, Janes SM. Cancer Res. 2009;69:4134. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Roger M, Clavreul A, Venier-Julienne MC, Passirani C, Sindji L, Schiller P, Montero-Menei C, Menei P. Biomaterials. 2010;31:8393. doi: 10.1016/j.biomaterials.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JAJM, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Proc. Natl. Acad. Sci. USA. 2009;106:4822. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.a) Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O’Brien T, Kerin MJ. Clin. Cancer Res. 2007;13:5020. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]; b) Mishra PJ, Mishra PJ, Glod JW, Banerjee D. Cancer Res. 2009;69:3240. doi: 10.1158/0008-5472.CAN-08-3562. [DOI] [PubMed] [Google Scholar]; c) Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. Stem Cells. 2007;25:1737. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]; d) Zhang XY, La Russa VF, Bao L, Kolls J, Schwarzenberger P, Reiser J. Mol. Ther. 2002;5:555. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 13.Li D, Zhu Y, Mao CB. J. Mater. Chem. B. 2013;1:5515. doi: 10.1039/C3TB20733A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a) Qiu P, Qu X, Brackett DJ, Lerner MR, Li D, Mao CB. Adv. Mater. 2013;25:2492. doi: 10.1002/adma.201204472. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Mao CB, Wang F, Cao B. Angew. Chem. Int. Ed. 2012;51:6411. doi: 10.1002/anie.201107824. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang F, Nimmo S, Cao B, Mao CB. Chem. Sci. 2012;3:2639. doi: 10.1039/C2SC00583B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo B, Xu SA, Ma WF, Wang WR, Wang SL, Guo J, Yang WL, Hu JH, Wang CC. J. Mater. Chem. 2010;20:7107. [Google Scholar]
- 16.a) Di Stefano A, Ettorre A, Sbrana S, Giovani C, Neri P. Photochem. Photobiol. 2001;73:290. doi: 10.1562/0031-8655(2001)073<0290:picwll>2.0.co;2. [DOI] [PubMed] [Google Scholar]; b) Sharma S, Dube A, Bose B, Gupta PK. Cancer Chemother. Pharmacol. 2006;57:500. doi: 10.1007/s00280-005-0072-x. [DOI] [PubMed] [Google Scholar]
- 17.a) Canton I, Battaglia G. Chem. Soc. Rev. 2012;41:2718. doi: 10.1039/c2cs15309b. [DOI] [PubMed] [Google Scholar]; b) Sahay G, Alakhova DY, Kabanov AV. J. Controlled Release. 2010;145:182. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park SH, Sung YC, Jeun SS. Cancer Res. 2008;68:9614. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 19.a) Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Cancer Res. 2010;70:10044. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Nature. 2007;449:557. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.