Abstract
MJC13 is a novel molecule that has potential use for the treatment of hormone refractory prostate cancer (HRPC). The purpose of this work was to develop a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for quantification of MJC13. Itraconazole was used as the internal standard (IS). Acetonitrile was used to extract MJC13 from rat plasma and urine samples. A LC system equipped with a Waters XTerra MS C18 column (125Å, 3.5 µm, 4.6×150 mm) was used for chromatographic separation with acetonitrile-water as mobile phase. The API 3200 QTRAP triple quadrupole mass spectrometer was used for chromatographic analysis by multiple reaction monitoring (MRM) at positive mode with the transitions m/z 272→m/z 162 for MJC13, and m/z 705→m/z 392 for IS. The retention times for MJC13 and IS were 4.98 min and 4.42 min, respectively. The standard curves of MJC13 in solution, rat plasma, and rat urine were linear in the concentration range of 1 – 1000, 1 – 1000 and 1 – 200 ng/mL, respectively. The intra- and inter-day accuracy (relative error) ranged from 1.99 – 4.20% and 1.83 – 4.39%, respectively. The intra- and inter-day precision (coefficient of variation) ranged from 2.27 – 3.88% and 2.80 – 4.79%, respectively. The extraction recovery rates of rat plasma and urine samples were 95.3% and 96.2%, respectively. No measurable matrix effect interfered with MJC13 identification and quantification in rat plasma and urine. In summary, a rapid, specific, sensitive, and reproducible LC-MS/MS method was developed and validated to quantify MJC13 in solution, rat plasma, and rat urine.
Keywords: LC-MS/MS, MJC13, Quantification, Solution, Rat, Plasma, Urine
1. Introduction
Prostate cancer is the second leading cause of cancer deaths and the most commonly diagnosed cancer in American males (Siegel et al., 2013). All current therapies act as classic antagonists by competing with androgens, the major stimulator of prostate tumor growth, for binding the androgen receptor (AR) hormone binding pocket. However, those treatments become ineffective in late stage prostate cancer. Thus, recent strategies have focused on targeting alternative sites on AR, such as binding function 3 (BF3) region (Estebanez-Perpina et al., 2007). The 52kDa FK506-binding protein (FKBP52) has been shown to be a specific, positive regulator of AR, glucocorticoid receptor (GR) and progesterone receptor (PR) in both cellular and whole animal models (Sivils et al., 2011, Storer et al., 2011). Given that FKBP52 is functionally specific for a small subset of Hsp90 client proteins (Riggs et al., 2004), targeting FKBP52 regulation of AR activity represents a promising therapeutic approach with the possibility of fewer side effects.
A series of small molecules that can specifically inhibit FKBP52-mediated potentiation of AR signaling through the putative targeting of the AR BF3 surface were recently identified and characterized (De Leon et al., 2011). The lead molecule, MJC13 (N-(2,3-dichlorophenyl)-cyclohexanecarboxamide, see Fig 1 for structure), is an attractive therapeutic options for the treatment of prostate cancer.
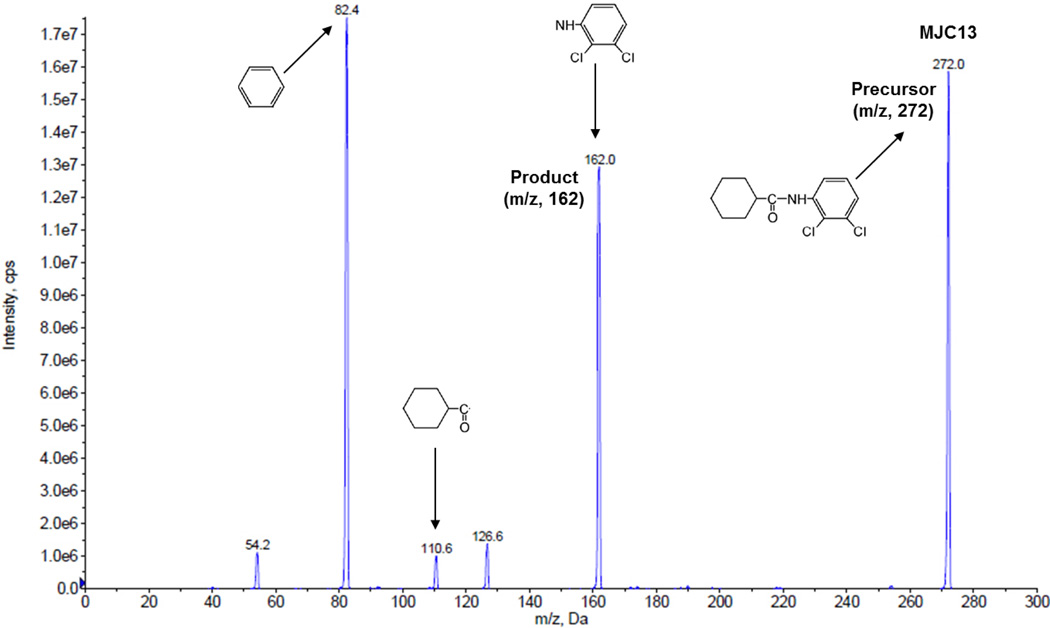
Fig 1.
The product ion scan spectra for MJC13.
Systematic evaluations on MJC13 are needed to facilitate preclinical application. Further progress in the pharmacological and pharmaceutical development of this compound strongly depends on the availability of modern analytical methods to evaluate MJC13 in biological material. A quantitative method must be developed to show adequate selectivity and sensitivity to be acceptable for regulatory authorities. Thus, we developed and validated a rapid, sensitive and robust liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay for quantification of MJC13 in solution, rat plasma, and rat urine.
2. Materials and methods
2.1. Materials
MJC13 was custom synthesized (purity ≥ 99%) by Chembridge (San Diego, CA). Itraconazole (purity ≥ 98%), formic acid, HPLC grade acetonitrile and water were purchased from Sigma-Aldrich (St. Louis, MO). All chemicals and reagents were used as received. Freshly obtained blank rat plasma and urine were collected from male Sprague–Dawley rats (Harlan Laboratories, Houston, TX) and frozen at −80 °C till use.
2.2. Preparation of standard and quality control samples
Stock solutions of MJC13 and itraconazole (IS) were prepared separately by dissolving solid compound in acetonitrile at the concentration of 1 mg/mL, and stored at −80 °C till use. Standard samples of MJC13 in rat plasma were prepared by diluting the stock solution with acetonitrile and then spiking in blank rat plasma at seven different concentrations ranging from 1 to 1000 ng/mL. Quality control (QC) samples of MJC13 in rat plasma were 5 ng/mL (low), 400 ng/mL (medium), and 800 ng/mL (high). Standard samples of MJC13 in rat urine were prepared by diluting the stock solution with acetonitrile and then spiking in blank rat urine at six different concentrations ranging from 1 to 200 ng/mL. QC samples of MJC13 in rat urine were 5 ng/mL (low), 90 ng/mL (medium), and 180 ng/mL (high). Standard and QC samples of MJC13 in net solution were prepared in parallel using acetonitrile instead of blank rat plasma or urine.
2.3. Plasma and urine sample preparation
The protein precipitation method was used for the preparation of rat plasma samples as described previously (Liang et al., 2013). Briefly, the rat plasma sample (50 µL) was well mixed with 200 µL of IS solution in acetonitrile (200 ng/mL). Following centrifugation, an aliquot of the supernatant was applied to the LC-MS/MS for quantitative analysis.
For rat urine sample, an aliquot (500 µL) was evaporated to dryness under a stream of nitrogen. The residue was reconstituted with 200 µL of IS solution in acetonitrile (200 ng/mL). Then the same procedure as above was followed.
2.4. Instruments and conditions
A LC system, Agilent 1200 series HPLC (Foster City, CA) equipped with a Waters XTerra MS C18 column (125Å, 3.5 µm, 4.6×150 mm, Milford, MA), was used for chromatographic separation. The two-phase gradient elution was summarized in Table 1. The sample injection volume was 10 µL.
Table 1.
Summary of gradient elution applied in separation of MJC13 from matrix and IS.
| Time (min) | Flow Rate (mL/min) | Mobile Phase A* (%) | Mobile Phase B* (%) |
|---|---|---|---|
| 0 | 1 | 95 | 5 |
| 1 | 1 | 95 | 5 |
| 2 | 1 | 5 | 95 |
| 5 | 1 | 5 | 95 |
| 6 | 1 | 95 | 5 |
| 7 | 1 | 95 | 5 |
Mobile phase A = HPLC grade water; Mobile phase B = 0.2% formic acid in acetonitrile.
A MS/MS system, API 3200 QTRAP triple quadrupole mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, CA) equipped with a TurboIonSpray source, was used for chromatographic analysis. Itraconazole was applied as an internal standard (IS) to analyze MJC13 in solution, rat plasma and rat urine. The quantification was performed by multiple reaction monitoring (MRM) at positive mode with the transitions m/z 272 → m/z 162 for MJC13, and m/z 705 → m/z 392 for IS. Fig 1 shows the product ion scan spectra of MJC13. The compound-dependent parameters for MJC13 were optimized and set as follows: declustering potential (DP), 56 V; collision cell entrance potential (CEP), 15 V; collision energy (CE), 23 V; and collision cell exit potential (CXP), 4 V.
2.5. Method validation
Calibration curves in solution, rat plasma and urine were created by plotting the peak area ratio of MJC13 to IS against the known concentrations of the MJC13. The least-squares linear regression method with 1/x2 weighting was applied to generate the slope, intercept, and correlation coefficient of each linear regression equation. The lower limit of quantification (LLOQ) was evaluated based on the signal-to-noise ratio of at least 5:1.
To examine the intra-day or inter-day assay accuracy and precision, we analyzed QC samples on the same day or three subsequent days. In order to analyze rat plasma samples with even higher concentrations, samples with 2500 or 5000 ng/mL of MJC13 after 5 or 10 times dilution were evaluated. Experiments were conducted in sextuplicate. The assay accuracy and precision were expressed in relative error from the theoretical drug concentration and coefficient of variation, respectively.
Extraction recovery and matrix factor was estimated by analyzing QC samples at three concentration levels. The extraction recovery of MJC13 was calculated according to Eq. 1:
| (1) |
where Responsepre-extraction spike is the average area count for MJC13 that has been through the extraction process, and Responsepost-extraction spike is for MJC13 spiked into extracted matrix after the extraction procedure. The matrix factor of MJC13 was calculated according to Eq. 2:
| (2) |
where Responsepost-extraction spike is the average area count for the MJC13 spiked into extracted matrix after the extraction procedure, and Responsematrix-free spike is for the same concentration of MJC13 in neat solution (acetonitrile). Experiments were conducted in triplicate.
2.6. Plasma and urine sample stability
Short-term (bench-top) stability: three sets of MJC13 QC plasma samples were freshly prepared and remained on the bench-top for 2, 4, or 6 h, respectively. Another set of plasma samples were freshly prepared and stored at −80 °C for 7 days. All the samples were compared with freshly prepared samples at the same concentrations. Experiments were conducted in triplicate.
Freeze-thaw stability: MJC13 plasma QC samples which were exposed for three cycles of freeze (at −20 °C) and thaw (room temperature) were compared with freshly prepared samples at the same concentrations. Experiments were conducted in triplicate.
Processed sample (on-instrument or autosampler) stability: one set of plasma QC samples were extracted with IS solution in acetonitrile, and another set were extracted with pure acetonitrile without IS. Before injecting into the LC-MS/MS for quantitative analysis, the plasma extracts were kept in the autosampler for 2, 4, or 6 h. All the samples were compared with freshly prepared samples at the same concentrations. Experiments were conducted in triplicate.
The stability of MJC13 in rat urine was conducted in the same fashion as in rat plasma.
3. Results
3.1. Method development and validation
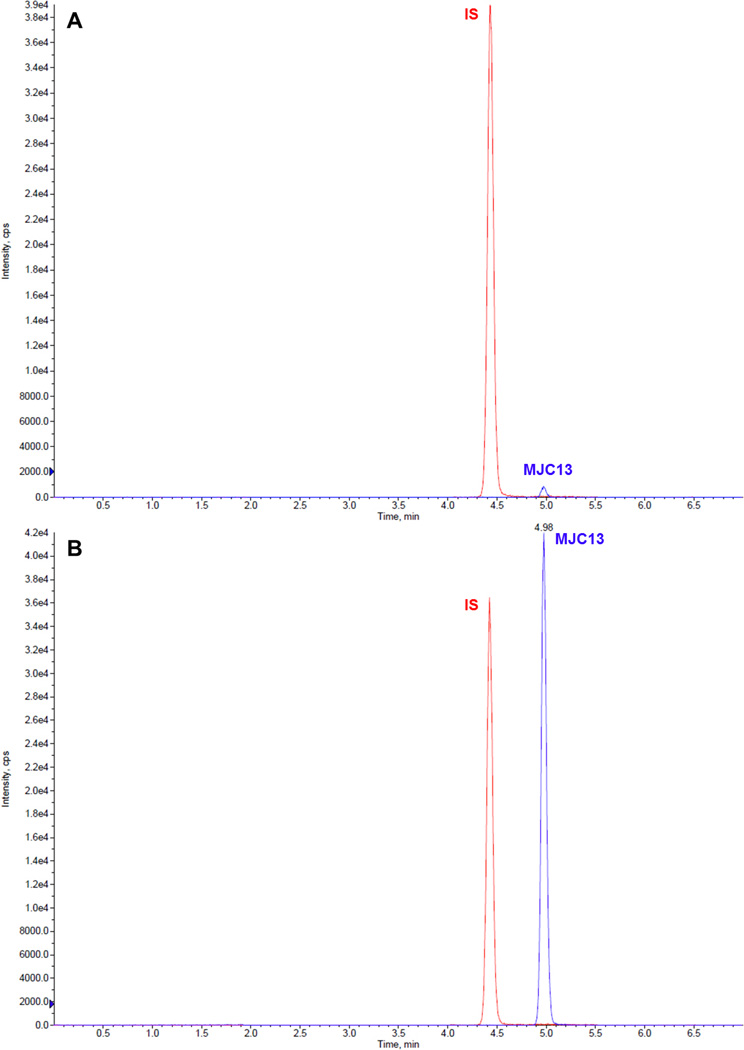
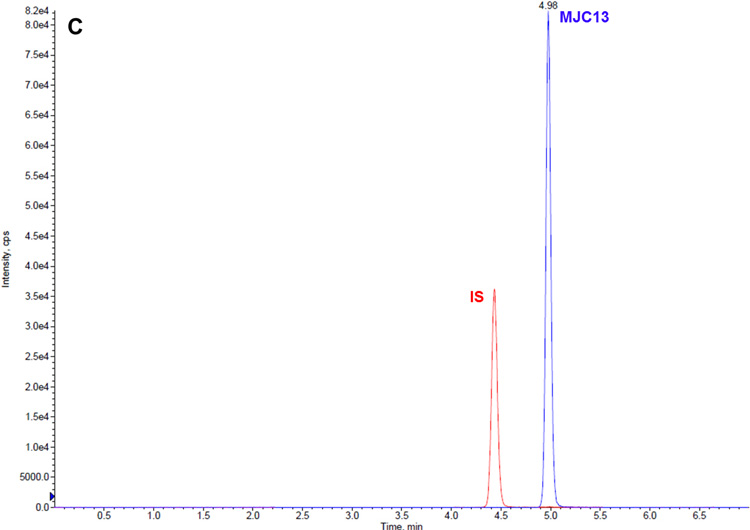
The retention times for MJC13 and IS were 4.98 and 4.42 min, respectively. Fig 2 shows typical representative chromatograms from blank rat plasma spiked with MJC13 at concentration of 1 ng/mL (A), 500 ng/mL (B), and 1000 ng/mL (C).
Fig 2.
Representative LC-MS/MS chromatograms for blank rat plasma spiked with MJC13 at concentrations of (A) 1 ng/mL, (B) 500 ng/mL, and (C) 1000 ng/mL.
The standard curves of MJC13 in solution, rat plasma and rat urine were linear in the concentration ranges of 1 – 1000, 1 – 1000, and 1 – 200 ng/mL, respectively, with correlation coefficient values > 0.998. The intra- and inter-day accuracy and precision of the LC-MS/MS method for analysis of MJC13 in solution, rat plasma, and rat urine are summarized in Table 2. These data indicated that the accuracy and precision were well within the 15% acceptance range, therefore, this LC-MS/MS assay was validated to be accurate and precise in solution and rat plasma over a MJC13 concentration range of 1 – 1000 ng/mL, and 1 – 200 ng/mL in rat urine. The accuracy and precision in analysis of diluted rat plasma samples were 8.93% (relative error) and 8.55% (coefficient of variation), respectively, for 5 times dilution; and 9.24% (relative error) and 8.94% (coefficient of variation), respectively, for 10 times dilution. The data suggest that the LC-MS/MS method can be used to analyze MJC13 concentrations from a 5 or 10 times diluted plasma sample.
Table 2.
Intra- and inter-day accuracy and precision of MJC13 LC-MS/MS analysis.
| Solution | Concentration (ng/mL) |
Intra-day (n=6) | Inter-day (n = 6) | ||
| Accuracy (RE*, %) |
Precision (CV*, %) |
Accuracy (RE, %) |
Precision (CV, %) |
||
| 5 | 3.25 | 3.76 | 3.25 | 4.23 | |
| 400 | 2.12 | 2.85 | 2.14 | 3.19 | |
| 800 | 1.99 | 2.27 | 1.83 | 2.80 | |
| Plasma | Concentration (ng/mL) |
Intra-day (n=6) | Inter-day (n = 6) | ||
| Accuracy (RE*, %) |
Precision (CV*, %) |
Accuracy (RE, %) |
Precision (CV, %) |
||
| 5 | 4.07 | 3.76 | 4.39 | 4.07 | |
| 400 | 2.38 | 2.83 | 2.63 | 3.54 | |
| 800 | 2.65 | 3.47 | 3.92 | 4.79 | |
| Urine | Concentration (ng/mL) |
Intra-day (n=6) | Inter-day (n = 6) | ||
| Accuracy (RE*, %) |
Precision (CV*, %) |
Accuracy (RE, %) |
Precision (CV, %) |
||
| 5 | 4.20 | 3.88 | 3.33 | 4.76 | |
| 90 | 3.61 | 3.12 | 4.27 | 3.48 | |
| 180 | 3.95 | 3.54 | 3.83 | 4.35 | |
RE = relative error; CV = coefficient of variation.
The extraction recovery rate and matrix factor of the LC-MS/MS method for analysis of MJC13 in rat plasma and urine are summarized in Table 3. The high and stable extraction recovery rate indicated that MJC13 can be efficiently extracted from biological fluid by the one-step protein precipitation method. Meanwhile, there was no measurable matrix effect that interfered with MJC13 identification and quantification in rat plasma and urine.
Table 3.
Extraction recovery rate and matrix factor of the LC-MS/MS method for analysis of MJC13 in rat plasma and urine.
| Plasma | Concentration (ng/mL) |
Extraction recovery rate (mean ± SD, %) |
Matrix factor (mean ± SD, %) |
| 5 | 94.3 ± 3.9 | −7.3 ± 4.2 | |
| 400 | 95.7 ± 4.8 | −9.4 ± 5.8 | |
| 800 | 95.9 ± 3.1 | −6.3 ± 3.1 | |
| Urine | Concentration (ng/mL) |
Extraction recovery rate (mean ± SD, %) |
Matrix factor (mean ± SD, %) |
| 5 | 96.6 ± 3.5 | −9.5 ± 5.4 | |
| 90 | 96.7 ± 3.7 | −9.7 ± 6.1 | |
| 180 | 95.4 ± 5.0 | −8.8 ± 6.5 |
3.2. Plasma and urine sample stability
Short-term stability: the rat plasma samples displayed 98.6 ± 1.7%, 97.6 ± 2.0%, and 97.4 ± 1.2% average MJC13 amount remaining after 2, 4, and 6 h; and rat urine samples displayed 97.9 ± 2.5%, 99.1 ± 1.9%, and 96.7 ± 2.8%, respectively. These data indicated that MJC13 in rat plasma and rat urine were stable at least for 6 h on the bench-top. The other set of rat plasma and rat urine samples that were stored at −80 °C for 7 days displayed 96.4 ± 1.4% and 95.9 ± 2.7% average MJC13 amount remaining, which suggested that the MJC13 in rat plasma and rat urine were stable in the freezer for at least 7 days.
Freeze-thaw stability: the rat plasma and rat urine samples displayed 96.7 ± 2.0% and 97.5 ± 2.4% average MJC13 amount remaining, which indicated that MJC13 in rat plasma and urine were stable after three freeze-thaw cycles.
Processed sample stability: the plasma samples that were extracted by acetonitrile with IS displayed 97.6 ± 1.3%, 98.3 ± 1.5%, and 96.7 ± 0.7% average recoveries at 2, 4, and 6 h; and the rat urine samples displayed 95.8 ± 3.2%, 98.6 ± 2.5%, and 97.9 ± 1.7% respectively. The rat plasma samples that were extracted by acetonitrile without IS displayed 96.1 ± 1.3%, 97.6 ± 1.1%, and 97.7 ± 2.4% recoveries at 2, 4, and 6 h; and rat urine samples displayed 97.4 ± 2.2%, 97.1 ± 1.9%, and 96.4 ± 3.3% recoveries. These data indicated that the processed MJC13 rat plasma extracts and rat urine extracts were stable for at least 6 h, and not affected by the presence of the IS.
4. Discussion
Since MJC13 is a recently identified lead molecule, no chromatographic method has been reported for its analysis in net solution or biological fluids. Therefore, a rapid, sensitive, and robust LC-MS/MS method is needed to quantify MJC13 in solution, rat plasma, and rat urine for preformulation, formulation, and preclinical studies. The selection of IS is critical for development of a robust and reproducible LC-MS/MS method. Generally, the IS is a compound that is as close as possible, but not identical to the analyte, to correct for the loss of analyte during sample preparation or sample inlet. For the LC-MS/MS method, a stable isoform of the analyte is the first choice, and an analog is second. However, given that MJC13 is a newly identified lead molecule, the isoforms and analogs are not commercially available. Thus, in current work, itraconazole was selected as the IS mainly for the following reasons: it is easy to obtain (commercially available); it is stable (stable in autosampler for 24 h) (Yao et al., 2001); it has extraction recovery very close to MJC13 (extraction recovery rate of itraconazole via protein precipitation with acetonitrile was 96%) (Yao et al., 2001); its retention time is close to MJC13 due to similar lipophilicity (logP of itraconazole is 5.66) (Fromtling, 1987); and it can be detected by the MS/MS system using MRM method in the positive mode like MJC13. In order to better separate the drug and the IS from endogenous impurities in the rat plasma and rat urine, the gradient elution method was developed, starting with high aqueous percentage, which can elute out the hydrophilic blood matrix such as phospholipid early in the process. The chromatograms (Fig 2) showed very clear baseline separated peaks for MJC13 and the IS with no interference from the matrix. The one-step protein precipitation method used in rat plasma and rat urine sample preparation was simple and quick, and offered good extraction recovery and matrix effect.
5. Conclusions
The recently identified anti-cancer molecule, MJC13, has great potential to be developed as a novel treatment of HRPC. In this work, we report a chromatographic method for determination of MJC13 in net solution or biological fluid for the first time. A rapid, specific, sensitive, and reproducible LC-MS/MS method was developed to quantify MJC13. The assay was properly validated over the concentration ranges of 1 – 1000, 1 – 1000, and 1 – 200 ng/mL in solution, rat plasma, and rat urine, respectively. This method can be applied to the ongoing preformulation, formulation, and preclinical studies.
Acknowledgements
HX has research grant support from an NIH/RTRN grant (5U54RR022762-05), NIH/NIGMS grant (1SC3GM102018-01) and from NIH/NIMHD/RCMI grant (5G12RR003045-21). MBC has research grant support from NIH/NCRR/RCMI grant (5G12RR008124), NIH/NIMHD/RCMI grant (G12MD007592), NIH/NIGMS grant (SC1GM084863) and State of Texas CPRIT grant (RP110444-P2). We also thank Dr. Dong Liang for his technical support and proofreading.
References
- De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, Chen Y, Ning YM, Riggs DL, Fletterick RJ, Guy RK, Trepel JB, Neckers LM, Cox MB. Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci U S A. 2011;108(29):11878–11883. doi: 10.1073/pnas.1105160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estebanez-Perpina E, Arnold LA, Nguyen P, Rodrigues ED, Mar E, Bateman R, Pallai P, Shokat KM, Baxter JD, Guy RK, Webb P, Fletterick RJ. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc Natl Acad Sci U S A. 2007;104(41):16074–16079. doi: 10.1073/pnas.0708036104. Epub 2007 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling RA. Recent trends in the discovery, development, and evaluation of antifungal agents: proceedings of an international telesymposium. J.R. Prous; 1987. May, 1987. [Google Scholar]
- Liang S, Bian X, Ma J, Arogunjo M, Deorukhkar AA, Krishnan S, Xie H. Development and validation of a sensitive LC/MS/MS method for the determination of gamma-tocotrienol in rat plasma: application to pharmacokinetic studies. Biomed Chromatogr. 2013;27(1):58–66. doi: 10.1002/bmc.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. doi: 10.1080/10409230490892513. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- Sivils JC, Storer CL, Galigniana MD, Cox MB. Regulation of steroid hormone receptor function by the 52-kDa FK506-binding protein (FKBP52) Curr Opin Pharmacol. 2011;11(4):314–319. doi: 10.1016/j.coph.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab. 2011;22(12):481–490. doi: 10.1016/j.tem.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M, Chen L, Srinivas NR. Quantitation of itraconazole in rat heparinized plasma by liquid chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;752(1):9–16. doi: 10.1016/s0378-4347(00)00505-3. [DOI] [PubMed] [Google Scholar]