Abstract
Objectives
This study aimed to determine whether regional cerebral blood flow (rCBF) is abnormal in patients who have Transient Global Amnesia (TGA).
Methods
We obtained noninvasive rCBF measurements using Tc-99m-ethyl cysteinate diamer Single Photon Emission Computed Tomography (SPECT) in 7 patients diagnosed with TGA within 4 days of onset of the amnestic episode while the patients were still symptomatic and in 17 age-matched healthy control subjects. We assessed memory functioning using the Hopkins's Verbal Learning Test (HVLT) and Statistical Parametric Mapping to compare rCBF across diagnostic groups.
Results
The patients with TGA were significantly impaired in their performance on the 20-minute delayed recall of the HVLT. They also exhibited significantly decreased rCBF on their SPECT scans in the inferior and middle frontal gyrus bilaterally, with more prominent left-sided reductions in the superior temporal, precentral, and postcentral gyri, as well as increased rCBF primarily in the right hemisphere within the middle temporal, superior temporal, and inferior frontal gyri, cerebellum, and thalamus, compared with the normal control group.
Conclusion
These findings suggest that lateralized abnormalities in brain functioning are an important component of the pathophysiology of TGA. Lateralized abnormalities may disrupt functions that are relatively specific to the left hemisphere, including receptive language, symbolic representation, and the processing of local features in the environment, while preserving anterograde memory processes. Increased flow to the right hemisphere centered on regions that subserve the functions of expressive language and visuospatial processing, and may represent processes that compensate for flow reductions to the left hemisphere.
Keywords: Transient global amnesia, Brain perfusion SPECT, Statistical parametric mapping
Introduction
Transient Global Amnesia (TGA) is defined by a sudden onset of transient impairment of memory for recent events, a transient inability to retain new information, and retrograde amnesia in the absence of other neurological signs and symptoms. The annual incidence of TGA in the general population is approximately 3 per 100,000 people, with a peak incidence between the ages of 50 and 60 years (Schmidtke et al., 2002). Although its etiology is unknown, transient ischemic attacks, epilepsy, and migraines have been proposed as possible causes or precipitating events (Hodges and Oxbury, 1990a; Hodges and Ward, 1989).
Studies of cerebral perfusion during episodes of TGA may improve our understanding of the pathogenesis of TGA. Because TGA is both rare and short-lived, however, neuroimaging studies performed during or immediately following the attack of amnesia in close temporal proximity to the inciting cerebral event are also rare, the numbers of subjects in those studies have been small, and the findings thus far have been inconclusive (Asada et al., 2000; Evans et al., 1993; Jung et al.,1996; Schmidtke et al.,1998; Stillhard et al.,1990; Tanabe et al., 1991; Warren et al., 2000). Indeed, most studies have been performed remotely from the time of onset, long after the resolution of amnesia.
Previous imaging studies of cerebral perfusion in TGA have employed Region-of-Interest (ROI) analyses to detect abnormalities in cerebral blood flow (CBF). ROI analyses can suffer from inefficiency and inaccuracy in detecting abnormalities that are small relative to the size of the ROI or that extend across the boundaries of two or more ROIs. Voxel-based approaches largely avoid these difficulties when testing statistically for group differences, and they may in addition afford an advantage in diminishing operator bias (Furutani et al., 2005). Thus, voxel-based approaches such as Statistical Parametric Mapping (SPM) are now being applied increasingly in rCBF studies using Single Photon Emission Computed Tomography (SPECT) (Acton and Friston, 1998; Friston et al., 1991; Lingford-Hughes et al., 1998).
The aim of the present study was to identify disturbances of rCBF in TGA patients using SPM-based analyses. Seven patients who were recently diagnosed with TGA underwent Tc-99m-ethyl cysteinate diamer SPECT scanning 1–4 days from the onset of the acute amnestic episode and while they were still exhibiting memory disturbances. These rCBF maps were compared with those of 17 age-matched, healthy control subjects.
Methods
Subjects and characterization
Seven patients with TGA (M:F = 4:3, ages: 52–69 years, mean 63.4 ± 4.84 years, all right-handed) were recruited upon being diagnosed with TGA. The diagnosis was established using standard clinical diagnostic criteria3 applied to a semi-structured interview and neurological exam developed in-house and administered by a senior neurologist. During the TGA episode, neurological examination disclosed no abnormalities or cognitive deficits other than amnesia. The Mini-Mental State Examination (MMSE) (Folstein et al.,1975) was performed after the initial diagnosis of TGA, and then the subtest assessing memory for three objects was repeated every 2 h during the patients' waking hours, using different objects upon each administration, to determine the time course of memory disturbance and recovery. Seventeen age-matched healthy controls (M:F = 7:10, ages: 56–69 years, mean 62.2 ± 6.84 years, all right-handed) were recruited through local advertisements They reported no psychiatric or neurological problems themselves or in their families, and no history of memory disturbance. The health of the normal control group was confirmed using a structured questionnaire for dementia (Christensen et al., 1991), the MMSE, and a clinical diagnostic interview. All participants denied any history of epilepsy, migraine, cerebral ischemic events, or drug use, and none were taking psychoactive medications.
The Hopkins's Verbal Learning Test (HVLT) (Brandt, 1991), shown previously to be of use in verifying the verbal memory impairment associated with TGA, was administered to the patient group immediately after the SPECT scan. The HVLT assesses verbal memory performance by measuring the recognition, immediate recall, and 20-minute delayed recall of 12 words read at random by the examiner from a larger list of 24 words belonging to 3 semantic categories, at a rate of 1 word every 2 s (Table 1).
Table 1.
Clinical and laboratory characteristics of TGA patients.
| TGA patient | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Sex | M | F | M | M | M | F | F |
| Age | 70 | 63 | 67 | 67 | 68 | 63 | 68 |
| Time to recovery (hours) | 7 | 17 | 14 | 12 | 10 | 12 | 16 |
| Precipitating event | Physical abuse | Swimming | Emotional distress | Tennis playing | Emotional distress | Emotional distress | Cold stress |
| EEG | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| MRI | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| SPECT timing (days after TGA onset) | 3 | 1 | 3 | 2 | 4 | 2 | 2 |
| HVLT free recall | 1+2+6 = 9 (15) | 4+8+6 = 18(40) | NA | 6+7+7 = 20 (40) | 0+5+5 = 10 (25) | 1+4+5 = 10 (30) | 0+4+5 = 9 (35) |
| HVLT recognition index | 9−4 = 5(60) | 7−2 = 5(60) | NA | 9−2 = 7(70) | 7−4 = 3(30) | 6−3 = 3(30) | 7−4 = 3(30) |
M=Male, F=Female.
HVLT, Hopkins Verbal Learning Test reported as percentile of clinical norms in parentheses; NA, not available.
HVLT Recognition (Discriminability) Index: true positive-false positive responses. Parentheses indicate percentile relative to clinical norms.
HVLT Free Recall: 1st+2nd+3rd recall = total.
All subjects gave informed written consent to participate, and the Institutional Review Board of the Catholic Medical Center in Seoul, South Korea, approved the study.
SPECT scanning
All subjects were scanned within 4 days from the onset of TGA (Table 1), as soon as serial examinations revealed resolution of their acute confusional state, at a time when they were capable of providing informed consent and cooperating with the scanning protocol. SPECT imaging was initiated 20 min after intravenous injection of approximately 740–925 MBq of Tc-99m-ECD (ethyl cysteinate diamer) using a multi-detector scanner (ECAM plus; Siemens, Erlangen, Germany) equipped with a low-energy, fan-beam collimator. The head unit consisted of two rotating rings of 59 probe-type detectors. Data were reconstructed in a 128×128 matrix with a pixel size of 3.9×3.9×3.9 mm (FOV = 240 mm, slices thickness = 7 mm) and a 20% symmetric window at 140 keV. Continuous transaxial tomograms of the brain were reconstructed after back-projection using a Butterworth filter (cutoff frequency 0.4 cycles/pixel, order 5) to reduce statistical noise. Tc-99 m ECD images were corrected for tissue attenuation using a standard commercial correction routine (Siemens Inc., Erlangen, Germany), assuming uniform attenuation and a circular head shape.
Image preprocessing
All subsequent image manipulation and data analyses were performed on an IBM personal computer running a Windows XP operating system. The software for image manipulation included Matlab version 5.3 (Mathworks, Inc., Natick, MA) and Statistical Parametric Mapping 99 (SPM99: Institute of Neurology, University College of London, UK) (Friston et al., 1995b). The SPECT data with attenuation and scatter correction were converted into ANALYZE format (Mayo Foundation, Baltimore, MD, USA) having voxel dimensions of 3.9 mm in each spatial dimension. The mean pixel intensity across all slices of the imaging volume was calculated. Each pixel was then thresholded at 80% of this value to eliminate background noise and partial volume effects at the edge of the brain. Each SPECT scan was then spatially normalized using a 12-parameter affine warp and sinc-linear interpolation to the SPECT template brain from the Montreal Neurological Institute (Friston et al., 1995a), reformatted to a 16-bit image having 79×95×68 voxels, each 2×2×2 mm in size. These images were spatially smoothed using a Gaussian filter of 16 mm Full-Width at Half Maximum. Normalized rCBF values were calculated by dividing CBF at each voxel by global CBF in each individual.
Statistical analysis
Intensity-normalized SPECT data from the TGA group were compared with similarly normalized data from the 17 healthy subjects. Group contrasts in rCBF were estimated at each voxel using the General Linear Model of SPM99. A two-sample t-test model was fitted, and a t-statistic image was constructed and then thresholded at t>3.30, corresponding to an uncorrected p-value <0.01, in conjunction with a cluster filter of 200 voxels in the reformatted, template imaging space. This combined application of a statistical threshold and cluster filter has previously been shown to reduce substantially the false positive identification of activated pixels at any given threshold (Forman et al., 1995). For purposes of visualization and anatomic localization, the t-score clusters were projected onto the standard high-resolution T1-weighted MRI.
Results
The clinical characteristics of the participants and measures of their memory performance are summarized in Table 1. Diagnostic groups did not differ on age (Student's t = 0.964, df = 6, p = 0.13), gender (t = 0.854, df = 6, p = 0.50), or educational level (t = 0.768, df = 6, p = 0.66). Even after recovery from their acute confusional state, the TGA patients demonstrated grossly impaired 20-minute delayed recall relative to published norms, despite good preservation of verbal recognition memory, on the HVLT administered immediately after the SPECT scan. We therefore regard our SPECT findings as representing ongoing, intra-episode disturbances in neural functioning during the amnestic episode.
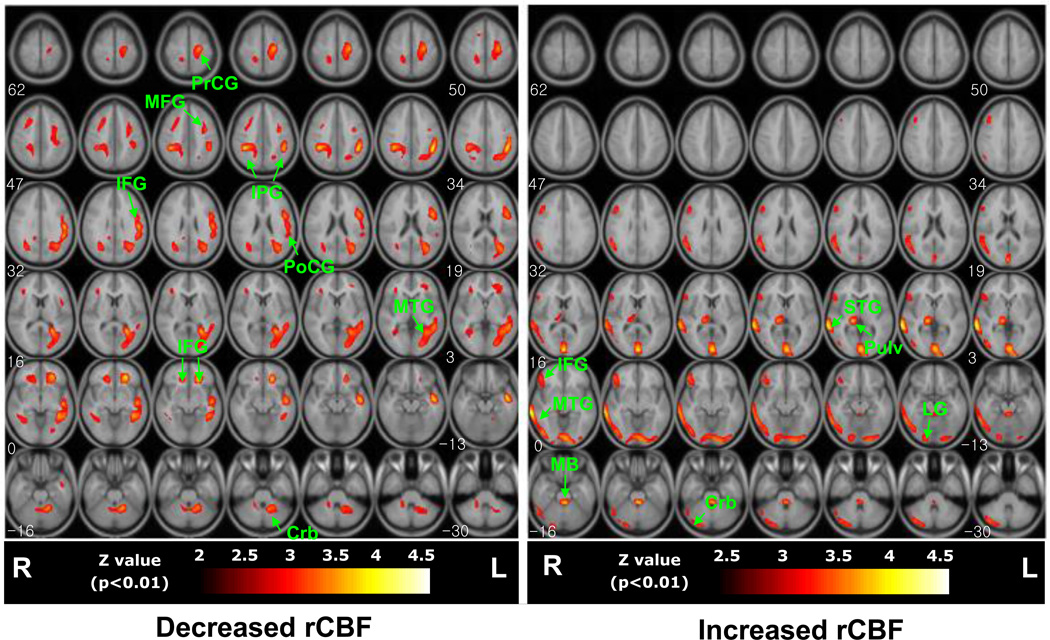
The mean global CBF for the TGA group (155.93 ± 10.81 counts/min) did not differ significantly from that of the controls (160.14 ± 15.87 counts/min) (Student's t = −.475, df = 6, p = 0.65). Voxel-wise comparisons of globally normalized rCBF across diagnostic groups revealed reduced flow in the TGA patients in the inferior and middle frontal gyrus bilaterally, with more prominent left-sided reductions in the superior temporal, precentral, and postcentral gyri (Table 2). Increases in rCBF were detected in the TGA patients primarily in the right hemisphere, within the right middle temporal, superior temporal, and inferior frontal gyri, and in the right cerebellum and right pulvinar nucleus of the thalamus (Table 3). These results therefore indicate in general that blood flow in the TGA patients was reduced in the left and increased in the right cerebral hemisphere relative to controls (Fig. 1). These lateralized findings were also evident in the individual rCBF maps of each of the 7 TGA patients compared with the controls (data not shown).
Table 2.
Anatomical sites of decreased rCBF in TGA patients compared with controls.
| Regions | Abbreviation | Number of voxels in cluster | t-statistic (z-level) | Location (x, y, z mm) | Brodmann's area |
|---|---|---|---|---|---|
| Lt frontal lobe, Inferior frontal gyrus | IFG | 5792 | 5.57 (4.04) | −52, 8, 30 | 9 |
| Lt parietal lobe, Postcentral gyrus | PoCG | 5792 | 5.30 (3.92) | −52, −20, 26 | 2 |
| Lt frontal lobe, Middle frontal gyrus | MFG | 5792 | 5.09 (3.82) | −34, 24, 40 | 8 |
| Rt parietal lobe, Inferior parietal gyrus | IPG | 202 | 4.71 (3.63) | 40, −40, 42 | 40 |
| Lt parietal lobe, Inferior parietal gyrus | IPG | 398 | 4.22 (3.37) | −52, −42, 42 | 40 |
| Lt frontal lobe, Precentral gyrus | PrCG | 768 | 4.20 (3.36) | −24, −18, 60 | 6 |
| Lt frontal lobe, Inferior frontal gyrus | IFG | 248 | 3.94 (3.21) | −23, 31, −10 | 47 |
| Rt frontal lobe, Inferior frontal gyrus | IFG | 233 | 3.37 (2.86) | 24, 31, −10 | 47 |
| Lt cerebellum | Crb | 234 | 3.32 (2.83) | −18, −50, −24 | NA |
| Lt temporal lobe, Middle temporal gyrus | MTG | 233 | 3.31 (2.83) | −58, −36, 6 | 37 (Wernicke's) |
Lt = left; NA = not applicable.
Table 3.
Anatomical sites of increased rCBF in TGA patients compared with controls.
| Regions | Abbreviation | Number of voxels in cluster | t-statistic (z-statistic) | Location (x, y, z mm) | Brodmann's area |
|---|---|---|---|---|---|
| Rt temporal lobe, Superior temporal gyrus | STG | 1493 | 5.91 (4.18) | 66, −42, 8 | 22 |
| Brain stem, midbrain | MB | 240 | 5.82 (4.15) | 2, −30, −16 | NA |
| Rt occipital lobe, Lingual gyrus | LG | 1842 | 5.39 (3.96) | 4, −90, −12 | NA |
| Rt thalamus, pulvinar | Pulv | 243 | 4.58 (3.57) | 18, −24, 6 | NA |
| Rt temporal lobe, Middle temporal gyrus | MTG | 1493 | 4.36 (3.44) | 66, −32, 0 | 21 |
| Rt frontal lobe, Inferior frontal gyrus | IFG | 1493 | 4.28 (3.41) | 60, 26, 0 | 45 (Broca's) |
| Rt cerebellum | Crb | 520 | 4.03 (3.25) | 40, −84, −20 | NA |
Rt = right; NA = not applicable.
Fig. 1.
Group comparisons of regional CBF significant differences in voxel-wise comparisons of rCBF across groups (thresholded at p<0.01) are superimposed on T1-weighted the high-resolution anatomical MRI template. Areas of significant decreases (left panel) and increases (right panel) in rCBF are mapped separately. Regional abbreviations are defined in Tables 2 and 3.
Discussion
This study demonstrated that within 4 days of the onset of amnesia—when serious impairment of delayed recall was still clearly present on detailed neuropsychological testing but after the resolution of the acute confusional state—rCBF in the patients with TGA was significantly increased in the broad expanses of the right hemisphere (in the right hemisphere homologue of Broca's region, in the temporal cortices, and thalamus), but significantly reduced in frontal cortices bilaterally and in the left hemisphere elsewhere (in pericentral, parietal, and temporal cortices, including Wernicke's region). To our knowledge, this is the first study to demonstrate prominent hemispheric specificity in abnormalities of cerebral blood flow during the period of continuing memory impairment within an amnestic episode of TGA.
Findings from previous functional imaging studies of TGA patients have been inconsistent and include abnormalities in perfusion of the temporal lobes (Asada et al., 2000; Evans et al., 1993; Jung et al., 1996; Matsuda et al., 1993; Schmidtke et al., 2002; Stillhard et al., 1990; Strupp et al., 1998; Tanabe et al., 1991; Warren et al., 2000), thalamus (Goldenberg et al.,1991; Sakashita et al.,1997; Schmidtke et al., 2002), and striatum (Baron et al., 1994; Schmidtke et al., 2002). Although most studies of TGA have reported decreased perfusion, hyperperfusion in persons with TGA has also been reported in a handful of studies, particularly in the hippocampus, amygdala, and thalamus of the left hemisphere (Asada et al., 2000; Jung et al., 1996; Matsuda et al., 1993). The inconsistency in findings across studies are probably attributable to differences in variability in patient characteristics, including the timing of scan acquisition relative to clinical recovery from the amnestic episode, the degree of ischemia present during the scan, and the presence of varying cognitive deficits during and after the episode (Baron et al., 1994; Eustache et al., 1997; Hodges and Oxbury,1990a; Hodges andWarlow,1990; Jung et al.,1996; Stillhard et al., 1990). Although the perfusion abnormalities detected after clinical recovery are generally thought to reflect those detected during the acute episode (Pai and Yang, 1999; Takeuchi et al., 2004), this assumption has yet to be supported conclusively by experimental data. Differences in methods of image analysis, which typically have assessed rCBF in TGA patients within variably defined ROIs, have also likely contributed to the inconsistency in findings across studies. Our use of a voxel-based analysis may have facilitated detection of hemisphere-specific abnormalities in rCBF in the TGA patients, as several of these abnormalities, such as those in Broca's region, may have been too small to detect in ROI-based analyses.
Most blood flow studies performed during or soon after the amnestic episode have demonstrated abnormalities in perfusion of brain regions that subserve memory processes. Whether these perfusion deficits cause TGA (as they might in the presence of ischemia, for example) or whether they simply reflect reductions in the underlying neural activity of those brain structures, with causes for the reduced neural activity residing elsewhere, is unknown (Jovin et al., 2000; Pai and Yang, 1999; Schmidtke et al., 1998; Takeuchi et al., 1998). Our findings do not include disturbances in blood flow to mesial temporal lobe structures that have been reported in prior studies, suggesting that abnormal blood flow to these regions may not be a necessary feature of the pathophysiology of TGA. It is possible, however, that abnormal flow to these structures resolved immediately after the acute onset of amnesia in our sample, prior to scanning. Thus, mesial temporal regions could be involved during the initial phase of the attack, with areas of abnormal perfusion moving from the mesial temporal to temporal-parietal cortices during the early stages of resolution of the most severe anterograde amnesia and confusion. A more likely explanation for our failure to detect disturbances in mesial temporal lobe structures, however, is the imprecision of spatial normalization procedures across individuals and the need to implement large spatial filters that may obscure group differences in perfusion in small brain regions, such as the hippocampus (Peterson, 2003), if perfusion is indeed altered in those regions. Another possible explanation for our failure to detect disturbances in flow in the mesial temporal lobe is the relative insensitivity of blood flow measurements in detecting early ischemia. Consistent with this possibility is a study using diffusion weighted imaging, a more sensitive measure of early ischemia than are blood flow measures, to demonstrate the evolution of ischemic lesions in the hippocampus in a large sample of persons with TGA within the first 48 h of illness (Sedlaczek et al., 2004).
Instead of implicating the hippocampus, our findings suggest that hemisphere-specific abnormalities in rCBF may contribute to the pathophysiology of TGA. In particular, our findings suggest that hemisphere-specific disturbances in blood flow to receptive and expressive language regions may contribute either to the cause of TGA or to compensatory responses in the service of its resolution. Flow to Wernicke's receptive language region was significantly reduced in the left hemisphere, whereas flow to the homologue of Broca's expressive language regions was increased in the right hemisphere in the patient group. That the hemisphere-specific disturbances in flow centered primarily on language regions is perhaps not surprising, given that the strongest evidence for functional lateralization of the CNS is in the language system (Hugdahl, 2000).
Why the hemisphere-specific abnormalities involved decreased flow to receptive language regions in the left hemisphere and increased flow to anterior language regions in the right hemisphere, however, is unclear. Perhaps under-activity of receptive language regions, together with reduced activity of other putative left hemisphere functions—functions such as the processing of local features (Christman, 1993; Hellige, 1993), formation of categorical judgments (Kosslyn, 1987), processing of temporal information (Hutsler and Galuske, 2003), and the symbolic representation of local features of the environment that are communicated to conspecifics (Hugdahl, 2000)—fundamentally disrupted in persons with TGA the language-based processes that support the formation and consolidation of conscious memories. Under-activity of these left hemisphere functions could induce attempts at compensation by complementary neural systems in the right hemisphere, which include expressive language functions in the frontal lobe and visuospatial systems in the right midtemporal cortex and pulvinar nucleus of the thalamus (Vogel et al., 2003). Similar right hemisphere processes that compensate for deficiencies in functioning of receptive language regions in the left hemisphere have been documented extensively in persons with dyslexia (Pugh et al., 2001). This interpretation of hemisphere-specific abnormalities in blood flow in persons with TGA is of course speculative, although it does lead to the testable hypothesis that detailed neuropsychological testing of components of the language systems in persons with TGA will reveal significant deficits compared with healthy controls, either because receptive language functions within the left hemisphere are disturbed, or because the tests exceed the limited capacity of already-taxed compensatory systems in the right hemisphere. Preliminary experimental support for this hypothesis includes evidence of deficits in verbal fluency (Kessler et al., 2001) and selective deficits in tests of verbal but not spatial memory after resolution of the acute, global amnestic episode in large, independent cohorts of patients with TGA (Hodges and Oxbury, 1990b; Mazzucchi and Parma, 1990).
Alternatively, hemisphere-specific deficits in rCBF could contribute to the pathophysiology of TGA by disrupting hemisphere-specific systems that support the perception of emotional valence, thus reducing the emotional salience of environmental stimuli that facilitate mnemonic encoding and retrieval. Anterior portions of the left cerebral hemisphere are thought to contribute to the perception of positive emotions, and anterior portions of the right hemisphere support the experience of negative emotions (Davidson, 1995; Shenal et al., 2003). Left hemisphere hypofunctionality, reflected in reduced rCBF in our sample, could interfere with positively valenced experiences and thereby increase stress responsivity, perhaps accounting in part for the widely recognized fact that stressful life experiences frequently seem to accompany the onset of the acute amnestic episode in persons with TGA (Fisher, 1982; Inzitari et al., 1997; Pantoni et al., 2000).
Limitations of this study include the absence of detailed neuropsychological assessments, particularly measures of language functioning. Also, the number of TGA patients, though larger than in most prior studies, is still small and may have contributed to false negative findings, particularly in the hippocampus. The accuracy of spatial normalization would have been improved through the simultaneous acquisition of high-resolution MRI scans and the use of extracerebral fiducial markers for coregistration of the SPECT images. Finally, detailed angiography was not performed in any of the patients, and therefore we were unable to exclude underlying vascular causes for the observed hemispheric abnormalities.
Given our findings of hemisphere-specific abnormalities in rCBF and the unique cognitive and emotional functions that each of the cerebral hemispheres is thought to subserve, future studies of TGA should include detailed neuropsychological assessments of receptive and expressive language functions, hemispheric biases for language functioning, local and global processing, symbolic representation, categorical judgments, time perception, and the perception of emotional valence—all of which are thought to represent the functioning of neural systems that are relatively hemisphere-specific. Those neuropsychological assessments should be coupled with quantitative brain imaging studies of cerebral perfusion and more direct measures of neuronal activity, such as electroencephalography and magnetoencephalography, which would be expected to show reduced activity in temporal-parietal portions of the left cerebral hemisphere and markedly altered measures of electrical coherence across hemispheres.
Supplementary Material
Acknowledgments
This work was supported by the Korean government (MOST) (No. R01-2007-000-21094-0 and No. M10644000028-06N4400-02810), NIMH grants MH59139, MH068318, K02-74677, the Suzanne Crosby Murphy Endowment at Columbia University College of Physicians and Surgeons, and the Thomas D. Klingenstein & Nancy D. Perlman Family Fund.
References
- Acton PD, Friston KJ. Statistical parametric mapping in functional neuroimaging: beyond PET and fMRI activation studies. Eur. J. Nucl. Med. 1998;25:663–667. [PubMed] [Google Scholar]
- Asada T, Matsuda H, Morooka T, Nakano S, Kimura M, Uno M. Quantitative single photon emission tomography analysis for the diagnosis of transient global amnesia: adaptation of statistical parametric mapping. Psychiatry Clin. Neurosci. 2000;54:691–694. doi: 10.1046/j.1440-1819.2000.00771.x. [DOI] [PubMed] [Google Scholar]
- Baron JC, Petit-Taboue MC, Le Doze F, Desgranges B, Ravenel N, Marchal G. Right frontal cortex hypometabolism in transient global amnesia. A PET study. Brain. 1994;117(Pt 3):545–552. doi: 10.1093/brain/117.3.545. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin. Neuropsychol. 1991;5:125–142. [Google Scholar]
- Christensen KJ, Multhaup KS, Nordstrom S, Voss K. A cognitive battery for dementia: development and measurement characteristics. Psychol. Assess. 1991;3:168–174. [Google Scholar]
- Christman SD. On the complex relation between perceptual characteristics and hemispheric asymmetry. Brain Cogn. 1993;21:123–129. doi: 10.1006/brcg.1993.1009. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Cerebral asymmetry, emotion, and affective style. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. Cambridge, MA: MIT Press; 1995. pp. 361–387. [Google Scholar]
- Eustache F, Desgranges B, Petit-Taboue MC, de la Sayette V, Piot V, Sable C, Marchal G, Baron JC. Transient global amnesia: implicit/explicit memory dissociation and PET assessment of brain perfusion and oxygen metabolism in the acute stage. J. Neurol. Neurosurg. Psychiatry. 1997;63:357–367. doi: 10.1136/jnnp.63.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Wilson B, Wraight EP, Hodges JR. Neuropsychological and SPECT scan findings during and after transient global amnesia: evidence for the differential impairment of remote episodic memory. J. Neurol. Neurosurg. Psychiatry. 1993;56:1227–1230. doi: 10.1136/jnnp.56.11.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM. Transient global amnesia. Precipitating activities and other observations. Arch. Neurol. 1982;39:605–608. doi: 10.1001/archneur.1982.00510220003001. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Comparing functional (PET) images: the assessment of significant change. J. Cereb. Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowia RSJ. Spatial registration and normalization of images. Hum. Brain Mapp. 1995a;3:165–189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1995b;2:189–210. [Google Scholar]
- Furutani K, Harada M, Minato M, Morita N, Nishitani H. Regional changes of fractional anisotropy with normal aging using statistical parametric mapping (SPM) J. Med. Investig. 2005;52:186–190. doi: 10.2152/jmi.52.186. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Podreka I, Pfaffelmeyer N, Wessely P, Deecke L. Thalamic ischemia in transient global amnesia: a SPECT study. Neurology. 1991;41:1748–1752. doi: 10.1212/wnl.41.11.1748. [DOI] [PubMed] [Google Scholar]
- Hellige JB. Hemispheric Asymmetry: What's Right and What's Left. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- Hodges JR, Ward CD. Observations during transient global amnesia. A behavioural and neuropsychological study of five cases. Brain. 1989;112(Pt 3):595–620. doi: 10.1093/brain/112.3.595. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Warlow CP. Syndromes of transient amnesia: towards a classification. A study of 153 cases. J. Neurol. Neurosurg. Psychiatry. 1990;53:834–843. doi: 10.1136/jnnp.53.10.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Oxbury SM. Persistent memory impairment following transient global amnesia. J. Clin. Exp. Neuropsychol. 1990a;12:904–920. doi: 10.1080/01688639008401030. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Oxbury SM. Persistent memory impairment following transient global amnesia. J. Clin. Exp. Neuropsychol. 1990b;12:904–920. doi: 10.1080/01688639008401030. [DOI] [PubMed] [Google Scholar]
- Hugdahl K. Lateralization of cognitive processes in the brain. Acta. Psychol. (Amst.) 2000;105:211–235. doi: 10.1016/s0001-6918(00)00062-7. [DOI] [PubMed] [Google Scholar]
- Hutsler J, Galuske RA. Hemispheric asymmetries in cerebral cortical networks. Trends Neurosci. 2003;26:429–435. doi: 10.1016/S0166-2236(03)00198-X. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Pantoni L, Lamassa M, Pallanti S, Pracucci G, Marini P. Emotional arousal and phobia in transient global amnesia. Arch. Neurol. 1997;54:866–873. doi: 10.1001/archneur.1997.00550190056015. [DOI] [PubMed] [Google Scholar]
- Jovin TG, Vitti RA, McCluskey LF. Evolution of temporal lobe hypoperfusion in transient global amnesia: a serial single photon emission computed tomography study. J. Neuroimaging. 2000;10:238–241. doi: 10.1111/jon2000104238. [DOI] [PubMed] [Google Scholar]
- Jung HH, Baumgartner RW, Burgunder JM, Wielepp JP, Lourens S, Wielepp JP. Reversible hyperperfusion of the right medial temporal lobe in transient global amnesia. J. Neurol. Neurosurg. Psychiatry. 1996;61:654–655. doi: 10.1136/jnnp.61.6.654-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J, Markowitsch HJ, Rudolf J, Heiss WD. Continuing cognitive impairment after isolated transient global amnesia. Int. J. Neurosci. 2001;106:159–168. doi: 10.3109/00207450109149746. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM. Seeing and imagining in the cerebral hemispheres: a computational approach. [erratum appears in Psychol Rev 1988 Apr;95(2):255] Psychol. Rev. 1987;94:148–175. [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJ, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. Br. J. Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Higashi S, Tsuji S, Sumiya H, Miyauchi T, Hisada K, Yamashita J. High resolution Tc-99m HMPAO SPECT in a patient with transient global amnesia. Clin. Nucl. Med. 1993;18:46–49. doi: 10.1097/00003072-199301000-00011. [DOI] [PubMed] [Google Scholar]
- Mazzucchi A, Parma M. Transient global amnesia and related disorders. In: Markowitsch HJ, editor. Neuropsychological Testing of Transient Global Amnesia During Attack and During Follow-Up. Toronto: Hogrefe & Huber Publs; 1990. pp. 152–167. [Google Scholar]
- Pai MC, Yang SS. Transient global amnesia: a retrospective study of 25 patients. Zhonghua Yixue Zazhi (Taipei) 1999;62:140–145. [PubMed] [Google Scholar]
- Pantoni L, Lamassa M, Inzitari D. Transient global amnesia: a review emphasizing pathogenic aspects. Acta Neurol. Scand. 2000;102:275–283. doi: 10.1034/j.1600-0404.2000.102005275.x. [DOI] [PubMed] [Google Scholar]
- Peterson BS. Conceptual, methodological, and statistical challenges in brain imaging studies of developmentally based psychopathologies. Dev. Psychopathol. 2003;15:811–832. doi: 10.1017/s0954579403000385. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Neurobiological studies of reading and reading disability. J. Commun. Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- Sakashita Y, Kanai M, Sugimoto T, Taki S, Takamori M. Changes in cerebral blood flow and vasoreactivity in response to acetazolamide in patients with transient global amnesia. J. Neurol. Neurosurg. Psychiatry. 1997;63:605–610. doi: 10.1136/jnnp.63.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidtke K, Reinhardt M, Krause T. Cerebral perfusion during transient global amnesia: findings with HMPAO SPECT. J. Nucl. Med. 1998;39:155–159. [PubMed] [Google Scholar]
- Schmidtke K, Manner H, Kaufmann R, Schmolck H. Cognitive procedural learning in patients with fronto-striatal lesions. Learn. Mem. 2002;9:419–429. doi: 10.1101/lm.47202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlaczek O, Hirsch JG, Grips E, Peters CN, Gass A, Wöhrle J, Hennerici M. Detection of delayed focal MR changes in the lateral hippocampus in transient. global amnesia. Neurology. 2004 Jun 22;62(12):2165–2170. doi: 10.1212/01.wnl.0000130504.88404.c9. [DOI] [PubMed] [Google Scholar]
- Shenal BV, Harrison DW, Demaree HA. The neuropsychology of depression: a literature review and preliminary model. Neuropsychol. Rev. 2003;13:33–42. doi: 10.1023/a:1022300622902. [DOI] [PubMed] [Google Scholar]
- Stillhard G, Landis T, Schiess R, Regard M, Sialer G. Bitemporal hypoperfusion in transient global amnesia: 99m-Tc-HM-PAO SPECT and neuropsychological findings during and after an attack. J. Neurol. Neurosurg. Psychiatry. 1990;53:339–342. doi: 10.1136/jnnp.53.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp M, Bruning R, Wu RH, Deimling M, Reiser M, Brandt T. Diffusion-weighted MRI in transient global amnesia: elevated signal intensity in the left mesial temporal lobe in 7 of 10 patients. Ann. Neurol. 1998;43:164–170. doi: 10.1002/ana.410430206. [DOI] [PubMed] [Google Scholar]
- Takeuchi R, Matsuda H, Yoshioka K, Yonekura Y. Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur. J. Nucl. Med. Mol. Imaging. 2004;31:578–589. doi: 10.1007/s00259-003-1406-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi R, Yonekura Y, Matsuda H, Nishimura Y, Tanaka H, Ohta H, Sakahara H, Konishi J. Resting and acetazolamide-challenged technetium-99m-ECD SPECT in transient global amnesia. J. Nucl. Med. 1998;39:1360–1362. [PubMed] [Google Scholar]
- Tanabe H, Hashikawa K, Nakagawa Y, Ikeda M, Yamamoto H, Harada K, Tsumoto T, Nishimura T, Shiraishi J, Kimura K. Memory loss due to transient hypoperfusion in the medial temporal lobes including hippocampus. Acta Neurol. Scand. 1991;84:22–27. doi: 10.1111/j.1600-0404.1991.tb04897.x. [DOI] [PubMed] [Google Scholar]
- Vogel JJ, Bowers CA, Vogel DS. Cerebral lateralization of spatial abilities: a meta-analysis. Brain Cogn. 2003;52:197–204. doi: 10.1016/s0278-2626(03)00056-3. [DOI] [PubMed] [Google Scholar]
- Warren JD, Chatterton B, Thompson PD. A SPECT study of the anatomy of transient global amnesia. J. Clin. Neurosci. 2000;7:57–59. doi: 10.1054/jocn.1998.0129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.