Abstract
Context
The basal ganglia and thalamus together connect in parallel closed-loop circuits with the cortex. Previous imaging studies have shown modifications of the basal ganglia and cortical targets in individuals with Tourette syndrome (TS), but less is known regarding the role of the thalamus in TS pathogenesis.
Objective
To study the morphological features of the thalamus in children and adults with TS.
Design
A cross-sectional, case-control study using anatomical magnetic resonance imaging.
Setting
University research center.
Participants
The 283 participants included 149 with TS and 134 normal control individuals aged 6 to 63 years.
Main Outcome Measures
Conventional volumes and measures of surface morphology of the thalamus.
Results
Analyses of conventional volumes and surface morphology were consistent in demonstrating an enlargement in TS-affected thalami. Overall volumes were 5% larger in the group composed of children and adults with TS. Statistical maps of surface contour demonstrated enlargement over the lateral thalamus. Post hoc testing indicated that differences in IQ, comorbid illnesses, and medication use did not account for these findings.
Conclusions
Morphological abnormalities in the thalamus, together with the disturbances reported in the sensorimotor cortex, striatum, and globus pallidus, support the hypothesis of a circuit wide disorder within motor pathways in TS. The connectivity and function of the numerous and diverse thalamic nuclei within cortical-subcortical circuits constitute an anatomical crossroad wherein enlargement of motor nuclei may represent activity-dependent hypertrophy within this component of cortical-subcortical motor circuits, or an adaptive response within a larger putative compensatory system that could thereby directly modulate activity in motor circuits to attenuate the severity of tics.
Anatomical and functional disturbances in corticostriatothalamocortical (CSTC) circuits are thought to contribute to the pathogenesis of Tourette syndrome (TS). Previous imaging studies have suggested the presence of hypoplasia in motor portions of these loops, particularly in the caudate nucleus1,2 and in inferior portions of sensory, motor, and premotor cortices.3 Enlargement in other portions of CSTC circuits, including the frontal and parietal cortices, the hippocampus, and their associated commissural pathways,4–6 are thought to compensate for disturbances in motor pathways and thereby reduce the severity of tic symptoms in persons with TS.
The thalamus is an integral component of the CSTC circuits that are involved in the genesis of tic symptoms and their associated compensatory responses. A previous functional magnetic resonance imaging study demonstrated activation of the thalamus, together with the basal ganglia and frontal and parietal cortices, during the willful suppression of tic symptoms.7 The change in activity of the thalamus correlated with the change inactivity of basal ganglia nuclei, suggesting that a coordinated effort between the thalamus and basal ganglia (defined as the striatum, globus pallidus, substantia nigra, and subthalamic nucleus)8 is required to suppress tics. In addition, inducing lesions in or stimulating thalamic subregions may attenuate tics,9,10 whereas space-occupying lesions of the thalamus seem to exacerbate them.11 These and other findings have prompted some to hypothesize that dysregulation of thalamocortical activity may generate tic behaviors.12
Several preliminary anatomical studies have yielded contradictory findings for overall thalamic volume. One reported an increased size of the left hemithalamus in 18 treatment-naive boys with TS.13 Another in which treatment history was unspecified found decreased thalamic volumes in 23 children with TS.14 A third study detected no morphological differences in the thalamus of 15 neuroleptic-naive adults.15 The small numbers of participants, their differing histories of medication use, the differing age and sex compositions of the samples, and the markedly differing image-processing techniques across these studies renders a coherent interpretation of their findings impossible.
A large-scale study is needed to clarify the effects of TS on thalamic morphology. We assessed overall thalamic volumes and localized morphological features over individual thalamic subregions in a large sample of children and adults to improve our understanding of the role of the thalamus in the pathophysiological processes of TS. We hypothesized that the volumes of the thalamus and its subregions would differ between diagnostic groups.
METHODS
PARTICIPANTS
Participants with TS (n=149) were recruited from the Tic Disorders Specialty Clinic at the Yale Child Study Center, New Haven, Connecticut. Normal control subjects (NC [n=134]) were recruited from a list of 10 000 names purchased from a telemarketing company and group-matched with the participants with TS by zip code and age range. Written informed consent was obtained from all participants, and protocols were approved by the human investigation committee at Yale School of Medicine and New York State Psychiatric Institute.
Participants were aged 6 to 63 years and were predominantly right-handed.16 Exclusion criteria for the TS group included another movement disorder or a major psychiatric disorder other than obsessive-compulsive disorder (OCD) or attention-deficit/hyperactivity disorder (ADHD). Exclusion criteria for controls included history of tic disorder, OCD, or ADHD and any current Axis I diagnosis. Additional exclusion criteria for both groups included seizure activity, head trauma with loss of consciousness, any history of substance abuse, and IQ below 80.
Diagnoses were established by using a portion of the Schedule for Tourette Syndrome and Other Behavioral Disorders,17 which includes the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version,18 and a best-estimate consensus procedure that considered all available study materials, including medical records. Clinical ratings of current and worst-ever severity of tic symptoms were rated with the Yale Global Tic Severity Scale,19 OCD symptoms with the Yale-Brown Obsessive Compulsive Scale,20,21 and ADHD symptoms with the DuPaul-Barkley ADHD Scale.22 Socioeconomic status was estimated using the Hollingshead Four-Factor Index.23 Estimates of full-scale IQ were made using the Wechsler Abbreviated Scale of Intelligence.24
The present sample overlaps closely with samples for which morphological analyses of other brain regions have been reported.2,4,5 The samples differ slightly because of minor differences in image quality or partial volume effects locally where the boundaries of the brain regions of interest are defined. If a blind review of the supervised region definitions suggested that regional boundaries could not be defined with great confidence, a decision was rarely made to exclude a participant’s imaging data from further analysis so as to reduce noise from measurement error that could undermine the ability to detect real group differences. Fewer than 2% of participants have been excluded from our various studies on this basis.
MAGNETIC RESONANCE IMAGING
High-resolution T1-weighted magnetic resonance images were obtained on a single 1.5-T scanner (GE Signa, Milwaukee, Wisconsin) during a 7-year period. Assessments were performed at least monthly to ensure the stability of image quality over time and included analyses of signal-to-noise and contrast-to-noise ratios, geometric distortion, and intensity uniformity in phantoms and living participants. Head positioning was standardized with the use of canthomeatal landmarks. Brain scans were acquired using a 3-dimensional volume spoiled gradientecho sequence (repetition time, 24 milliseconds; echo time, 5 milliseconds; flip angle, 45°; frequency encoding, superior/inferior; no wrap; 256 × 192 matrix; field of view, 30 cm; 2 excitations; section thickness, 1.2 mm; and 124 contiguous sections encoded for sagittal section reconstructions).
MORPHOMETRIC PROCEDURES
Morphometric analyses were performed on workstations (Sun Ultra 10; Sun Microsystems, Inc, Santa Clara, California) using a commercially available software system (ANALYZE 7.5; Biomedical Imaging Resource, Mayo Foundation, Rochester, Minnesota) by technicians unaware of the clinical status of the subject or left-right orientation of the image. A second operator (K.D.) confirmed the accuracy of all procedures.
IMAGE QUALITY
Images were rated blindly on a 6-point scale describing motion artifact. Images scoring 4 or higher on this scale were excluded from analysis and are not included in the reported sample size. Rates of inclusion differed only slightly between diagnostic groups (TS group, 78%; NC group, 68%; [P=.03]).
THALAMUS DEFINITION
After removal of nonbrain tissue and cortical gray matter, an anisotropic diffusion filter was applied to the remaining brain image (k = 2, iterations = 20; eFigure 1 [http://www.archgenpsychiatry.com]).25–27 The thalamus was segmented by sampling gray scale values throughout the brain image and averaging the peaks for white and gray matter. An isointensity contour function grown from a seed within the thalamus provided an initial definition of the structure, which was then manually edited. The thalamus was distinguished from the hypothalamus by a line defining the hypothalamic sulcus on sagittal views, which excluded a portion of the geniculate nuclei and pulvinar (Pu) from the analysis. Interrater intraclass reliability coefficients28 assessed on 10 scans obtained at times spaced equally throughout the study were greater than 0.90. An expert in these procedures (B.S.P.) reviewed all the tracings used for this study for spatial accuracy.
WHOLE BRAIN VOLUME
We measured whole brain volume (WBV) for use as a covariate to control for global scaling effects in statistical analyses of conventional volumes. This measure included not only gray and white matter but also cerebrospinal fluid within the ventricles and cortical sulci to ensure the exclusion of any possible confound of age-related effects of tissue atrophy with this general measure of body scaling.29
SURFACE ANALYSIS
We assessed group differences in surface contour while controlling for age and sex. We calculated the distance from a voxel-sized point on the surface of each participant’s thalamus to the corresponding point on the surface of the thalamus in a template brain. Procedures for selecting the template brain are described elsewhere.30 This previously validated method of surface analysis31 was customized to accommodate independent analysis of the right and left hemithalami. Briefly, a rigid-body similarity transformation with global scaling was used to register the entire brain of each participant with the template brain, thereby eliminating the need to further adjust for differences in WBV. The thalamus was then rigidly coregistered to the template thalamus. This second transformation created a refined registration in which to compare surfaces of isolated hemithalami. Each hemithalamus was warped to the corresponding anatomy of the template by using a high-dimensional, nonrigid algorithm based on techniques used in fluid-flow dynamics. Warping permitted point-to-point matching of homologous tissue between the test thalamus and the template thalamus. Subsequently, the high-dimensionally warped images were unwarped to the refined registration while maintaining the point-to-point correspondences. This procedure permitted calculation of the signed euclidean distance of each surface point from the corresponding point on the surface of the template thalamus.
ATLAS-BASED LABELING OF THALAMIC NUCLEI
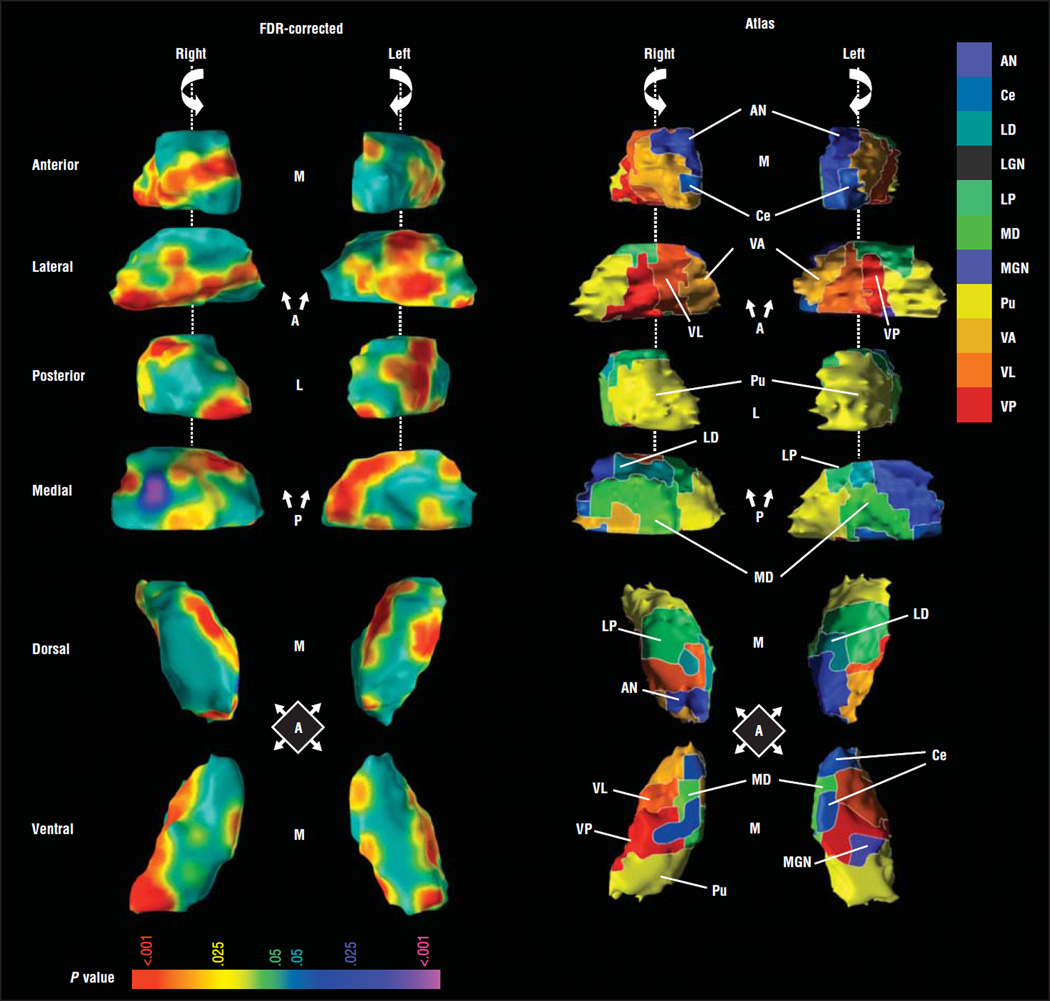
Boundaries of nuclei within the thalamus of the template brain were estimated using a modified version of a digital brain atlas32 with a widely used parcellation scheme and nomenclature.33 The atlas was registered to the template thalamus using a 3-dimensional nonlinear transformation based on voxel intensity. After smoothing this image manually, a simplified outline of 11 identified nuclei (listed in the legend for Figure 1) was overlaid on the template thalamus to aid localization of findings to individual thalamic nuclei.
Figure 1.
Main effects of diagnosis in thalamic surface morphology. Right and left hemithalami are shown in rotating views of the anterior (A), lateral (L), posterior (P), and medial (M) aspects as guided by the arrow showing direction of rotation. Dorsal and ventral views are also shown. The images to the right of the vertical line display the atlas warped to the template brain. Cytoarchitectonic boundaries are depicted, and each of the 11 defined nuclei is uniquely colored. Images to the left of the vertical line show color-coded maps of statistical significance illustrated on the template brain. The color bar provides the color coding for P values associated with the main effect of diagnosis. The statistical model includes age and sex as covariates. Yellows and reds indicate protruding surfaces, presumably from larger underlying volumes, and blues and purples indicate indented surfaces, presumably from smaller volumes underlying those regions. Maps are shown for images that have been corrected for multiple comparisons using false discovery rate (FDR). Widespread areas of the thalamic surface are affected by the presence of Tourette syndrome. Most affected surfaces are red, indicating bulging surfaces. Specifically affected were large portions of the lateral surface and the posterior half of the left medial surface. As depicted by the cytoarchitectonic boundaries, these areas overlie the ventroanterior nucleus (VA), ventrolateral nucleus (VL), ventroposterior nucleus (VP), lateral posterior nucleus (LP), and the pulvinar (Pu). The 11 nuclei defined in the atlas include the anterior nucleus (AN); central nuclei including the central medial, central lateral, center median, and parafascicular nuclei (Ce); lateral dorsal nucleus (LD); lateral geniculate nucleus (LGN); LP; medial dorsal nucleus (MD); medial geniculate nucleus (MGN); Pu; VA; VL; and VP. All central nuclei are delineated in grayish blue. The central medial nucleus is visible in the anterior view, and the parafascicular nucleus is visible in the medial view. The LGN was defined in the atlas but is not shown.
STATISTICAL ANALYSES
Conventional Volumes
Statistical analyses of thalamic volumes were performed using commercially available software (SAS, version 9.0; SAS Institute Inc, Cary, North Carolina). Our a priori hypothesis was tested using a mixed-models analysis with repeated measures (PROC MIXED; SAS Institute Inc) that accounted for the intercorrelation of hemithalamic volumes across the cerebral hemispheres. The model included a 2-level within-subjects factor of hemisphere (left or right) and a between-subjects factor of diagnosis (TS or NC). Covariates included age, sex, lifetime diagnosis of ADHD or OCD, and WBV to control for scaling effects. In addition to the independent variables and covariates, we considered all 2- and 3-way interactions of diagnosis, sex, hemisphere, and age and the 2-way interactions of WBV and hemisphere. Non-significant terms were eliminated via backward stepwise regression, with the constraint that the model at each step was hierarchically well formulated. Our a priori hypothesis was tested by assessing the statistical significance of the main effect of diagnosis. Statistical significance was set at P <.05, with all P values being 2-sided.
Surface Morphometry
We used linear regression at each voxel on the surface of the thalamic template to compare differences in the average distance of the surfaces between the TS and NC groups. Our a priori hypothesis of group differences in thalamic morphology was tested by assessing the significance of the main effect of diagnosis across the entire sample. Post hoc analyses included assessment of the effects of age and sex on our findings of group differences, which we tested in the interactions of diagnosis × age and diagnosis × sex. To ensure the stability of our findings across children and adults, we also assessed maps of effect size and variance for the whole sample, a subsample of adults, and a subsample of children. Models covaried for age and sex and were hierarchically well formulated.34 We used the theory of false discovery rate to correct P values for multiple comparisons in the presence of intercorrelated measures of distance.31,35 Voxels with P values of less than .05 were color coded and displayed on the template brain.
Correlation With Tic Severity
We explored the associations of thalamic morphology with the severity of tic symptoms at the time of the scan or at the period of greatest reported lifetime severity. Two analyses correlated tic severity (current and lifetime) with conventional volumes using age, WBV, and sex as covariates. Two additional analyses correlated tic severity (current and lifetime) with measures of distance from the thalamic template at each point on the thalamic surface while including age and sex as covariates. A third analysis assessed the sex differences in the correlation of tic severity with surface morphology by assessing the statistical significance of the interaction of tic severity with sex while including within the model the main effects of age, sex, and tic severity.
Assessment of Possible Confounds
We explored the association of thalamic surface morphology and use of α-agonists, antipsychotics, or antidepressants in 3 separate linear regressions. Models exploring medication use (coded dichotomously) included the main effect of use of the particular medication in question with age and sex as covariates. The effects of medication on our findings were also assessed by repeating a priori hypothesis testing using a subgroup of individuals with TS who were medication free. The effect of IQ was assessed by repeating a priori hypothesis testing using IQ as a covariate. Finally, we assessed the effects of comorbid ADHD or OCD on our findings in several ways. First, we assessed the stability of our findings in the subgroup of participants with TS who had no lifetime history of those disorders. Second, we included ADHD and OCD as covariates in our statistical models assessing the effects of a diagnosis of TS on thalamic morphology. Third, regression models explored the effects of comorbid ADHD or OCD on surface measures by evaluating the main effects of scores from the DuPaul-Barkley ADHD Scale or Yale-Brown Obsessive Compulsive Scale while including age and sex as covariates.
RESULTS
PARTICIPANT DEMOGRAPHICS
Consistent with the male predominance in this disorder,36,37 the TS group had a higher proportion of male participants (Table 1). In addition, the difference in mean age between diagnostic groups was small but statistically significant. The mean IQ score in the TS group was lower than that in the NC group, although the IQ scores were above the national average in both groups. Other demographic characteristics were similar between groups.
Table 1.
Demographic Characteristics
| Research Participantsa | ||||||
|---|---|---|---|---|---|---|
| Characteristic | TSb (n=149) |
NC (n=134) |
Test Statistic | P Value | ||
| Mean age (SD), y | 18.7 (13.0) | 22.9 (13.7) | t281 =2.69 | .008 | ||
| <18 y | 105 | 58 | <.001 | |||
| ≥18 y | 44 | 76 | ||||
| Sex | ||||||
| Male | 112 | 69 | <.001 | |||
| Female | 37 | 65 | ||||
| SES at birth, mean (SD)c | 46.5 (11.4) | 46.0 (11.2) | t273 =0.41 | .68 | ||
| FSIQ, mean (SD) | 113.8 (16.8) | 120.0 (17.1) | t218 =2.68 | .008 | ||
| Nonwhite ethnicity, % | 5 | 10 | .17 | |||
| Nondextral handedness, % | 15 | 8 | .06 | |||
Abbreviations: FSIQ, full-scale IQ; NC, normal control; SES, socioeconomic status; TS, Tourette syndrome.
Unless otherwise indicated, data are expressed as number of participants.
In the TS group, 55 (36.9%) had a comorbid lifetime diagnosis of obsessive-compulsive disorder, 42 (28.2%) had attention-deficit/hyperactivity disorder, and 10 (6.7%) had both. At the time of scanning, 87 participants with TS (58.4%) were taking psychotropic medication (some more than 1 type), including typical neuroleptics (n=15), atypical neuroleptics (n=7), stimulants (n=5), α-agonists (n=28), selective serotonin reuptake inhibitors (n=20), and tricyclic antidepressants (n=12). Eighty-one participants with TS were medication free.
Computed SES scores ranged from a high of 66 to a low of 8. Score was estimated at the time of the participant’s birth to avoid bias attributable to downward drift in adults with TS, whose educational and occupational opportunities can be compromised by persistent illness.
HYPOTHESIS TESTING
The test for fixed effects in our mixed model revealed significantly larger conventional thalamic volumes in the TS group compared with the NC group (diagnosis main effect, F=4.96 [P=.03]; Table 2). An analysis of least squares means (SEs) indicated that conventional thalamic volumes were on average 5% larger in the TS group (TS group, 7000 [98]mm3; NC group, 6689 [148]mm3; SE, 139.66; t275=−2.23 [P=.03]).
Table 2.
Final Model for Conventional Volumesa
| Variable | df | F Value | P Value |
|---|---|---|---|
| TS | 1,275 | 4.96 | .03 |
| Age | 1,275 | 2.45 | .12 |
| Sex | 1,275 | 0.03 | .86 |
| Hemisphere | 1,281 | 0.76 | .38 |
| OCD | 1,275 | 0.09 | .76 |
| ADHD | 1,275 | 0.20 | .65 |
| WBV | 1,275 | 42.11 | <.001 |
| Age×hemisphere | 1,282 | 5.55 | .02 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; df, degrees of freedom; OCD, obsessive-compulsive disorder; TS, Tourette syndrome; WBV, whole-brain volume.
The model was determined through a backward stepwise procedure for variable selection in which all main effects were forced into the model and the least significant higher-order terms were successively removed, with the constraint that the model was hierarchically well formulated at each step. The significant main effect of TS reflected larger overall thalamic volumes in the TS group. Additional significant terms included WBV, indicating the presence of scaling effects in the data (the larger the brain, the larger the thalamus), and the age×hemisphere interaction, indicating that the association of age with volume varied by hemisphere independent of tic status. The value for the mean volume of the right thalamus was larger than that for the left thalamus regardless of age; however, least squares means analysis suggested that the volume difference between hemispheres was significantly larger in adults (estimate, 268.92; SE, 48.15; t118=5.59; P <.001) than children (estimate, 92.48; SE, 41.57; t162=2.22; P=.03).
SURFACE ANALYSIS
Maps comparing morphological surface features across diagnostic groups indicated that the overall increase in thalamic volume in the TS group derived from protrusions that were widespread across the thalamic surface (Figure 1). Significant enlargement was localized along the horizontal length of the right lateral thalamic surface and over the posteromedial and lateral surfaces of the left thalamus. Regions with robust bilateral effects included the ventrolateral (VL) and ventroposterior (VP) motor nuclei and the posterior complex formed by the lateral posterior (LP) and Pu nuclei (LP-Pu complex).
SEX × DIAGNOSIS INTERACTION
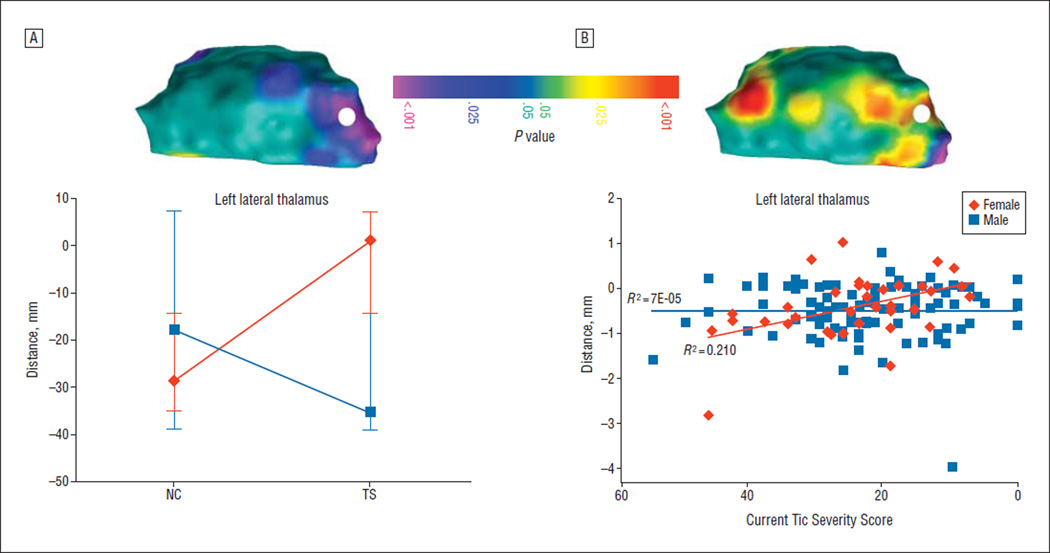
A portion of the left posterior thalamus exhibited a significant diagnosis × sex interaction that survived false discovery rate correction. Local volumes in the region protruded in female participants with TS compared with female NC participants, whereas local volumes were similar between male participants with TS and male NC participants (Figure 2A).
Figure 2.
Post hoc analyses. A, Sex effects. The topographic color map demonstrates the sex × diagnosis interaction present in a statistical model that covaries for age. The color coding represents false discovery rate–corrected P values associated with the interaction term calculated at each point on the surface of the right and left thalamus. Only the left thalamus is shown from the lateral view. The color bar, orientation, and abbreviations are as described in Figure 1. A single point, located under the white circle, was probed to further define the nature of the association. The point was selected by a computerized algorithm to demonstrate a maximal P value for the effect of tic severity. At the point underlying the white circle, female participants with Tourette syndrome (TS) showed a substantive outward deformation compared with female normal control participants (NCs), surpassing that seen in male NCs. Male participants with TS, on the other hand, showed minimal change in surface contour compared with male NCs. B, Correlation with tic severity. The topographic color map demonstrates the interaction of sex and tic severity in a statistical model that covaries for age. The color coding represents false discovery rate–corrected P values associated with the severity term calculated at each point on the surface of the right and left thalamus. Only the left thalamus is shown from the posterior view. The same point as in part A, located under the white circle, was probed to further define the nature of the association. The scatterplot demonstrates a correlation between tic severity and surface deformation in female participants only.
CORRELATION WITH TIC SEVERITY
We did not detect a significant main effect for the correlation of tic severity with conventional thalamic volume (r=0.016 [P=.4]) or with morphological features of the thalamic surface. We did, however, detect a significant interaction of current tic severity with sex over the Pu in the same location where the sex × diagnosis interaction was also detected. A plot of this interaction indicated that a greater outward deformation accompanied less severe tic symptoms in female participants only (Figure 2B).
AGE EFFECTS
In our model for conventional volumes, neither the main effect of age nor the age × diagnosis interaction was significant, indicating that the average size of the thalamus was similar in children and adults regardless of diagnosis. Similarly, an age × diagnosis interaction did not survive correction for multiple comparisons in surface maps (data not shown; eFigure 2 shows additional analyses, including effect size and variance maps in the whole sample and samples of children and adults separately), indicating that the effects of diagnosis on thalamic morphology were similar in children and adults.
COMORBIDITY EFFECTS
We did not detect significant main effects for the lifetime diagnosis of OCD or ADHD in conventional thalamic volumes. Correlation of thalamic surface morphology with comorbid symptom severity resulted in inward and outward deformations across anterior and medial thalamic surfaces (eFigure 3). An analysis of the participants who had a sole diagnosis of TS (pure TS) compared with the NC group yielded findings that demonstrated the outward deformation in the lateral thalamus in the TS group and that were consistent with the findings in analyses of all participants with TS (eFigure 4A).
MEDICATION EFFECTS
Medications had various effects on thalamic morphology (eFigure 5). The participants with TS who were taking selective serotonin receptor inhibitors exhibited local volume reductions across the anterior thalamic surface that included the anterior nucleus and the left Pu. Use of α-agonists was associated with outward deformation across the ventral thalamus. The deformation corresponded to a portion of the left lateral Pu that did not overlap with the main effect of diagnosis. Participants using second-generation antipsychotics demonstrated an outward deformation across the anterior surface of the thalamus and an inward deformation of the left lateral Pu. Analyses of surface morphology comparing medication-free participants in the TS group with the NC group revealed robust findings in the motor nuclei and the LP-Pu complex (eFigure 4B) confirming that medication effects were not producing the differences detected in lateral thalamic contour across diagnostic groups.
IQ SCORES
A surface map demonstrating the main effect of diagnosis while controlling for age, sex, and IQ was indistinguishable from the model in which IQ was not included as a covariate (not shown), indicating that the small but statistically significant difference in IQ scores across diagnostic groups did not account for the large group differences in thalamic morphology.
COMMENT
We detected significant enlargement in conventional thalamic volumes in a group of children and adults with TS. Surface maps localized the source of this enlargement to the lateral thalamus bilaterally, overlying several motor nuclei (VL/VP) and the Pu (eTable). Post hoc findings included a significant sex × diagnosis interaction such that female participants with TS showed a proportionally larger outward deformation. Also at this location, a relatively greater outward deformation accompanied less severe tics in female participants only.
LATERAL THALAMUS IN THE PATHOGENESIS OF TS
Anatomical, pharmacologic, clinical, and functional imaging studies have implicated CSTC circuits in the pathogenesis of TS.38 The CSTC circuits are arranged in parallel, topographical, tripartite loops. The first portion of the loop consists of projections from a specified cortical region that terminate in the striatum. Postsynaptic projections from the striatum traverse the direct and indirect pathways through the basal ganglia output nuclei to terminate in the thalamus. The final projection of the loop exits the thalamus and closes the loop by terminating within the cortical areas of origin.39 This topographic arrangement is loosely classified by region of cortical origin into 4 sets of loops originating and returning to the sensorimotor, orbitofrontal, association, and anterior cingulate cortices. Comparing the locations of prominent group differences in thalamic morphology to CSTC loop topography suggests that the circuits containing thalamic enlargement include those from caudal motor portions of the frontal lobe. Specifically, the CSTC circuits involved likely include the supplementary motor and cingulate motor cortices that project to the VL motor nucleus via the dorsolateral caudate and dorsocentral putamen and those from the primary motor and premotor cortices that project to lateral portions of the VP motor nucleus via the dorsolateral putamen.
The importance of the basal ganglia for motor control and movement disorders historically contributed to the general assumption that dysfunction of the basal ganglia was of primary importance in causing TS. However, reports of thinning of motor and premotor cortices that control movement of the tic-prone face and larynx3 and reports of abnormal electrophysiological properties of primary motor cortex in persons with TS40 suggested that morphological and functional disturbances in structures outside the basal ganglia may contribute to a wider circuit-based dysfunction in TS. Combined with data showing thinning of the somatosensory cortex in TS-affected brains,3 our data suggest that motor and sensorimotor circuits may be altered in persons with TS. The presence of abnormalities in sensorimotor pathways would support hypotheses asserting that TS results from an inability to effectively gate sensory information given that tics are often executed to alleviate premonitory sensory urges.41,42
MODULATION AND INTEGRATION OF INFORMATION IN CSTC PATHWAYS BY THE THALMUS
Modern anatomical and physiological studies have shown that portions of the thalamus actively modulate information coming to thalamic nuclei from the basal ganglia as well as the cortexandother subcortical structures.43–47Monosynaptic projections from intralaminar48 and motor nuclei,49 for example, directly influence basal ganglia functioning. Thalamic innervation to the dorsal-most portions of the caudate may be entirely from the VL motor nucleus,49–51 in effect creating a closed subcortical loop that functions in parallel to CSTC loops.52 This anatomical arrangement places the VL motor nucleus in a position to be a primary modulator of the basal ganglia nuclei that have been hypothesized to be involved in the pathogenesis of TS.
The thalamus also serves as a locus for cross-talk between CSTC loops. Anatomical studies demonstrate that thalamic motor nuclei contain not only the synapses that relay information through parallel CSTC loops but also the terminals of nonreciprocal projections from the cortex.53–55 Multiple nonreciprocal projections are arranged in a rostral-to-caudal topographic orientation within the thalamus such that the terminals of nonreciprocal projections interdigitate with terminals of reciprocal projections. This coalescence of projections permits the integration of information originating from higher-order association cortices with signals emanating from the primary motor and sensory cortices.56 Thalamic nuclei therefore provide an anatomical locus whereby information from higher-order association cortices, which are thought to play a compensatory role in TS, can integrate with and modulate information from dysfunctional motor and sensorimotor circuits.
ROLE OF THE LP-Pu COMPLEX
Unlike classic motor and sensory nuclei that relay ascending information from peripheral and subcortical structures to the cortex, the LP-Pu complex relays higher-order information from one cortical area to another.57 Efferents of the LP-Pu complex terminate in association cortices of the prefrontal, parietal, occipital, and temporal lobes as well as in limbic regions such as the amygdala.58–69 Combined with incoming information from the superior colliculus and reticular brainstem, information relayed through the LP-Pu complex is thought to subserve arousal, selective attention, and orientation to visual and auditory stimuli.70–75 Thus, a disturbance in the Pu such as the enlargement described herein would presumably disrupt attentional processing.76–79 Disturbed attentional processing could in turn exacerbate tic symptoms, as supported by the following considerations: first, persons with TS must allocate enormous attentional reserves to control their tic symptoms80; second, the presence of comorbid ADHD bodes poorly for the long-term prognosis of tic symptoms40,81; and third, the successful treatment of ADHD symptoms has been shown to reduce the severity of tics in children with TS.82
POSSIBLE CAUSES OF THALAMIC ENLARGEMENT IN TS
The causes of thalamic enlargement in our TS sample are unclear. Enlargement could represent an activity-dependent hypertrophy83–86 of relay nuclei within dysfunctional, hyperactive motor circuits in persons with TS.87 This interpretation is consistent with evidence of reduced inhibitory interneurons in the striatum and reduced inhibition of motor cortices in persons with TS.3,88,89 It is also consistent with the long-postulated excess activity within the basal ganglia’s direct output pathway, which would disinhibit thalamocortical projections and thereby produce excess synaptic activity within thalamic motor nuclei.90 Weighing somewhat against this interpretation of the source of thalamic enlargement, however, is the absence of significant correlation of the degree of enlargement of the motor nuclei with the severity of tic symptoms, which would have been predicted if enlargement represented activity-dependent hypertrophy associated with excess tic-related activity in motor circuits.
A competing hypothesis asserts that larger local thalamic volumes serve a compensatory function in persons with TS. This interpretation is supported by previous studies in which enlargement of the prefrontal cortex and hippocampus were associated with less severe symptoms, seeming therefore to represent a compensatory response that helped to attenuate symptoms.4,5 The pattern of enlargement in the thalamus shown herein, along with the enlargement in the hippocampus and frontal cortex demonstrated previously, could represent an extended network of CSTC circuit-based hypertrophy that increases executive control over hypoplastic and dysfunctional motor circuits in persons with TS.4 Indeed, the nuclei of the lateral thalamus would likely be the locus where executive systems interact with and influence activity in dysfunctional motor circuits.56
Finally, previous studies have provided evidence of reduced white matter integrity in the right thalamus14 and findings consistent with decreased fiber branching in the right motor nuclei that correlated with symptom severity91 in individuals with TS. These previous findings suggest that the increase in thalamic volume that we detected could represent physical expansion of gray matter into space made available by abnormalities in adjacent white matter. Alternatively, alterations in nonneuronal cells within the thalamus itself, such as expansion produced by a reactive astrocytosis, could conceivably contribute to the observed increase in thalamic volume.
SEXUAL DIMORPHISMS IN THE PATHOGENESIS OF TS
The significant sex × diagnosis interaction detected in the Pu derived from an enlargement in female participants with TS relative to female NCs that surpassed the size in male participants with TS and male NCs. An increased volume in the posterior thalamus in female participants with TS could be interpreted as a relative masculinization, as has been reported to occur in the amygdalae of female participants with TS.4 The direct projection described between the LP-Pu complex and the amygdala92 and a similar pattern of TS-related enlargement are consistent with the idea that both structures play a similar role in the pathophysiological processes of TS. Moreover, more maletyped cognitive profiles have been reported in both girls and women who have TS.93 Together our findings and those in previous reports are consistent with the long-standing hypothesis that a relative masculinization of the brain in female participants with TS may counter the protective effects that female sex usually confers on the risk of developing TS.93,94
An alternative hypothesis is that relative Pu enlargement in female participants signifies that the Pu is part of the larger distributed compensatory network in TS. Our correlation analyses indicated that in female participants relative outward deformation of the Pu was associated with less severe tics. In other words, relatively greater Pu volumes appeared to be protective, implying that the development of interventions that enhance Pu plasticity and function might provide new ways to attenuate the symptoms of TS.
LIMITATIONS
Findings from previous preliminary studies of thalamic volume have been inconsistent in TS.13–15 The large sample size and advanced imaging and statistical techniques of the present study help to resolve some of these contradictory findings. Nevertheless, limitations of this study must be acknowledged. We examined only morphological features of the thalamic surface and therefore were unable to study the intralaminar nuclei of the thalamus, which project heavily to the basal ganglia nuclei95,96 and which are frequent targets for electrical stimulation in the treatment of intractable TS symptoms.97 We also could not visualize directly the reticular thalamus, which surrounds the body of the thalamus and modulates its activity via inhibitory projections secreting γ-aminobutyric acid.98 Further clarification of the morphological differences associated with TS will require additional studies, ideally longitudinal, that are designed and powered to detect the morphology of individual subcortical nuclei. Finally, although we devised statistical modeling and numerous post hoc analyses to exclude the likelihood that thalamic enlargement was produced by factors other than a diagnosis of TS, we cannot entirely exclude the possibility that developmental stage, sex, comorbid symptoms, or treatment history contributed to our findings.
In conclusion, these findings add to growing evidence of the presence of circuitwide disturbances in motor and sensorimotor pathways in persons with TS. They also suggest that the thalamus is a promising anatomical locus where hypertrophic executive portions of CSTC circuits interact with and modulate activity in hyperexcitable and hypoplastic motor and sensorimotor portions of CSTC circuits. The thalamus therefore may be an anatomical crossroad where pathogenesis and compensation meet and where compensatory systems can directly influence dysfunctional activity in motor circuits to attenuate the severity of tic symptoms.
Supplementary Material
Acknowledgments
Funding/Support: The design and conduct, data collection and analysis, and manuscript preparation were funded in part by grants MH01232, MH59139, MH068318-01, MH-K0274677, MH36197, and T32 MH16434-27 from the National Institutes of Mental Health; by grant EB008235-01A1 from the National Institutes of Health; by a grant from the Tourette Syndrome Association; and by the Opening Project of Shanghai Key Laboratory of Functional Magnetic Resonance Imaging at the East China Normal University.
Additional Contributions: We thank the families who participated in this research and made these findings possible.
Footnotes
Financial Disclosure: None reported.
Previous Presentations: This report was presented in part at the Annual Meetings of the American Association for Child and Adolescent Psychiatry, October 21, 2005, Toronto, and November 1, 2008, Chicago, Illinois; at the Annual Meeting for the Society of Biological Psychiatry, May 2, 2008, Washington, DC; and the Annual Meeting for the American College of Neuropharmacology, December 9, 2008, Scottsdale, Arizona.
Online-Only Material: The eFigures and eTable are available at http://www.archgenpsychiatry.com.
REFERENCES
- 1.Wolf SS, Jones DW, Knable MB, Gorey JG, Lee KS, Hyde TM, Coppola R, Weinberger DR. Tourette syndrome: prediction of phenotypic variation in monozygotic twins by caudate nucleus D2 receptor binding. Science. 1996;273(5279):1225–1227. doi: 10.1126/science.273.5279.1225. [DOI] [PubMed] [Google Scholar]
- 2.Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L. Basal Ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60(4):415–424. doi: 10.1001/archpsyc.60.4.415. [DOI] [PubMed] [Google Scholar]
- 3.Sowell ER, Kan E, Yoshii J, Thompson PM, Bansal R, Xu D, Toga AW, Peterson BS. Thinning of sensorimotor cortices in children with Tourette syndrome. Nat Neurosci. 2008;11(6):637–639. doi: 10.1038/nn.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterson BS, Choi HA, Hao X, Amat JA, Zhu H, Whiteman R, Liu J, Xu D, Bansal R. Morphologic features of the amygdala and hippocampus in children and adults with Tourette syndrome. Arch Gen Psychiatry. 2007;64(11):1281–1291. doi: 10.1001/archpsyc.64.11.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58(5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- 6.Plessen KJ, Wentzel-Larsen T, Hugdahl K, Feineigle P, Klein J, Staib LH, Leckman JF, Bansal R, Peterson BS. Altered interhemispheric connectivity in individuals with Tourette’s disorder. Am J Psychiatry. 2004;161(11):2028–2037. doi: 10.1176/appi.ajp.161.11.2028. [DOI] [PubMed] [Google Scholar]
- 7.Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55(4):326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- 8.Kandel ER, Schwartz JH, Jessel TM, editors. Principles of Neural Science. 4th ed. New York, NY: McGraw-Hill Medical; 2000. [Google Scholar]
- 9.Maciunas RJ, Maddux BN, Riley DE, Whitney CM, Schoenberg MR, Ogrocki PJ, Albert JM, Gould DJ. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107(5):1004–1014. doi: 10.3171/JNS-07/11/1004. [DOI] [PubMed] [Google Scholar]
- 10.Rauch SL, Baer L, Cosgrove GR, Jenike MA. Neurosurgical treatment of Tourette’s syndrome: a critical review. Compr Psychiatry. 1995;36(2):141–156. doi: 10.1016/s0010-440x(95)90109-4. [DOI] [PubMed] [Google Scholar]
- 11.Peterson BS, Bronen RA, Duncan CC. Three cases of symptom change in Tourette’s syndrome and obsessive-compulsive disorder associated with paediatric cerebral malignancies. J Neurol Neurosurg Psychiatry. 1996;61(5):497–505. doi: 10.1136/jnnp.61.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: a relentless drumbeat—driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47(6):537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Yoo SS, Cho SY, Ock SM, Lim MK, Panych LP. Abnormal thalamic volume in treatment-naïve boys with Tourette syndrome. Acta Psychiatr Scand. 2006;113(1):64–67. doi: 10.1111/j.1600-0447.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- 14.Makki MI, Behen M, Bhatt A, Wilson B, Chugani HT. Microstructural abnormalities of striatum and thalamus in children with Tourette syndrome. Mov Disord. 2008;23(16):2349–2356. doi: 10.1002/mds.22264. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Lee DY, Bailey E, Hartlein JM, Gado MH, Miller MI, Black KJ. Validity of large-deformation high dimensional brain mapping of the basal ganglia in adults with Tourette syndrome. Psychiatry Res. 2007;154(2):181–190. doi: 10.1016/j.pscychresns.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.Pauls DL, Hurst CR. Schedule for Tourette and Other Behavioral Syndromes. New Haven, CT: Yale University Child Study Center; 1996. [Google Scholar]
- 18.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- 20.Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 21.Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 22.DuPaul GJ. Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. J Clin Child Psychol. 1991;20(3):245–253. [Google Scholar]
- 23.Hollingshead AB. Four-Factor Index of Social Status. New Haven, CT: Yale University Press; 1975. [Google Scholar]
- 24.Wechsler Abbreviated Scale of Intelligence. San Diego, CA: Psychological Corp; 1999. [Google Scholar]
- 25.Perona P, Malik J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans Pattern Anal Mach Intell. 1990;12(7):629–639. [Google Scholar]
- 26.Gerig G, Kubler O, Kikinis R, Jolesz FA. Nonlinear anisotropic filtering of MRI data. IEEE Trans Med Imaging. 1992;11(2):221–232. doi: 10.1109/42.141646. [DOI] [PubMed] [Google Scholar]
- 27.Nordstrom N. Biased anisotropic diffusion: a unified regularization and diffusion approach to edge detection. Image Vis Comput. 1990;8(4):318–327. [Google Scholar]
- 28.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 29.Skullerud K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, Alzheimer changes, and cerebral atherosclerosis. Acta Neurol Scand Suppl. 1985;102:1–94. [PubMed] [Google Scholar]
- 30.Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63(7):795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bansal R, Staib LH, Whiteman R, Wang YM, Peterson BS. ROC-based assessments of 3D cortical surface-matching algorithms. Neuroimage. 2005;24(1):150–162. doi: 10.1016/j.neuroimage.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage. 2006;30(2):359–376. doi: 10.1016/j.neuroimage.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 33.Hirai T, Jones EG. A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev. 1989;14(1):1–34. doi: 10.1016/0165-0173(89)90007-6. [DOI] [PubMed] [Google Scholar]
- 34.Morrell CH, Pearson JD, Brant LJ. Linear tranformations of linear mixed-effects models. Am Stat. 1997;51(4):338–343. [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 36.Burd L, Kerbeshian J, Wikenheiser M, Fisher W. A prevalence study of Gilles de la Tourette syndrome in North Dakota school-age children. J Am Acad Child Psychiatry. 1986;25(4):552–553. doi: 10.1016/s0002-7138(10)60016-7. [DOI] [PubMed] [Google Scholar]
- 37.Khalifa N, von Knorring AL. Prevalence of tic disorders and Tourette syndrome in a Swedish school population. Dev Med Child Neurol. 2003;45(5):315–319. doi: 10.1017/s0012162203000598. [DOI] [PubMed] [Google Scholar]
- 38.Marsh R, Leckman JF, Bloch MH, Yazgan Y, Peterson BS. Tics and compulsions: disturbances of self-regulatory control in the development of habitual behaviors. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2nd ed. Cambridge, MA: MIT Press; 2008. pp. 717–737. [Google Scholar]
- 39.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 40.Orth M, Rothwell JC. Motor cortex excitability and comorbidity in Gilles de la Tourette syndrome. J Neurol Neurosurg Psychiatry. 2009;80(1):29–34. doi: 10.1136/jnnp.2008.149484. [DOI] [PubMed] [Google Scholar]
- 41.Leckman JF, Bloch MH, Scahill L, King RA. Tourette syndrome: the self under siege. J Child Neurol. 2006;21(8):642–649. doi: 10.1177/08830738060210081001. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow NR, Sutherland AN. Preclinical models relevant to Tourette syndrome. Adv Neurol. 2006;99:69–88. [PubMed] [Google Scholar]
- 43.Guillery RW, Sherman SM. The thalamus as a monitor of motor outputs. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1809–1821. doi: 10.1098/rstb.2002.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones EG. The thalamic matrix and thalamocortical synchrony. Trends Neurosci. 2001;24(10):595–601. doi: 10.1016/s0166-2236(00)01922-6. [DOI] [PubMed] [Google Scholar]
- 45.Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- 46.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456(7220):391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimura M, Minamimoto T, Matsumoto N, Hori Y. Monitoring and switching of cortico-basal ganglia loop functions by the thalamo-striatal system. Neurosci Res. 2004;48(4):355–360. doi: 10.1016/j.neures.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Lacey CJ, Bolam JP, Magill PJ. Novel and distinct operational principles of intralaminar thalamic neurons and their striatal projections. J Neurosci. 2007;27(16):4374–4384. doi: 10.1523/JNEUROSCI.5519-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mengual E, de las Heras S, Erro E, Lanciego JL, Giménez-Amaya JM. Thalamic interaction between the input and the output systems of the basal ganglia. J Chem Neuroanat. 1999;16(3):187–200. doi: 10.1016/s0891-0618(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka D, Jr, Isaacson LG, Trosko BK. Thalamostriatal projections from the ventral anterior nucleus in the dog. J Comp Neurol. 1986;247(1):56–68. doi: 10.1002/cne.902470104. [DOI] [PubMed] [Google Scholar]
- 51.de las Heras S, Mengual E, Giménez-Amaya JM. Double retrograde tracer study of the thalamostriatal projections to the cat caudate nucleus. Synapse. 1999;32(2):80–92. doi: 10.1002/(SICI)1098-2396(199905)32:2<80::AID-SYN2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 52.McHaffie JG, Stanford TR, Stein BE, Coizet V, Redgrave P. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28(8):401–407. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 53.McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22(18):8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catsman-Berrevoets CE, Kuypers HG. Differential laminar distribution of corticothalamic neurons projecting to the VL and the center median. An HRP study in the cynomolgus monkey. Brain Res. 1978;154(2):359–365. doi: 10.1016/0006-8993(78)90706-0. [DOI] [PubMed] [Google Scholar]
- 56.Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Res Bull. 2009;78(2–3):69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33(2):163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 58.Gutierrez C, Cola MG, Seltzer B, Cusick C. Neurochemical and connectional organization of the dorsal pulvinar complex in monkeys. J Comp Neurol. 2000;419(1):61–86. doi: 10.1002/(sici)1096-9861(20000327)419:1<61::aid-cne4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 59.Cola MG, Gray DN, Seltzer B, Cusick CG. Human thalamus: neurochemical mapping of inferior pulvinar complex. Neuroreport. 1999;10(18):3733–3738. doi: 10.1097/00001756-199912160-00002. [DOI] [PubMed] [Google Scholar]
- 60.Cola MG, Seltzer B, Preuss TM, Cusick CG. Neurochemical organization of chimpanzee inferior pulvinar complex. J Comp Neurol. 2005;484(3):299–312. doi: 10.1002/cne.20448. [DOI] [PubMed] [Google Scholar]
- 61.Abramson BP, Chalupa LM. Multiple pathways from the superior colliculus to the extrageniculate visual thalamus of the cat. J Comp Neurol. 1988;271(3):397–418. doi: 10.1002/cne.902710308. [DOI] [PubMed] [Google Scholar]
- 62.Berson DM, Graybiel AM. Parallel thalamic zones in the LP-pulvinar complex of the cat identified by their afferent and efferent connections. Brain Res. 1978;147(1):139–148. doi: 10.1016/0006-8993(78)90778-3. [DOI] [PubMed] [Google Scholar]
- 63.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357(1428):1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Preuss TM, Goldman-Rakic PS. Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. J Comp Neurol. 1987;257(2):269–281. doi: 10.1002/cne.902570211. [DOI] [PubMed] [Google Scholar]
- 65.Shipp S. The functional logic of cortico-pulvinar connections. Philos Trans R Soc Lond B Biol Sci. 2003;358(1438):1605–1624. doi: 10.1098/rstb.2002.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romanski LM, Giguere M, Bates JF, Goldman-Rakic PS. Topographic organization of medial pulvinar connections with the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1997;379(3):313–332. [PubMed] [Google Scholar]
- 67.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8(11):4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sadikot AF, Parent A, François C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315(2):137–159. doi: 10.1002/cne.903150203. [DOI] [PubMed] [Google Scholar]
- 69.Buchsbaum MS, Buchsbaum BR, Chokron S, Tang C, Wei TC, Byne W. Thalamocortical circuits: fMRI assessment of the pulvinar and medial dorsal nucleus in normal volunteers. Neurosci Lett. 2006;404(3):282–287. doi: 10.1016/j.neulet.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 70.Kaas JH, Lyon DC. Pulvinar contributions to the dorsal and ventral streams of visual processing in primates. Brain Res Rev. 2007;55(2):285–296. doi: 10.1016/j.brainresrev.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8(5):223–230. doi: 10.1016/j.tics.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Van Essen DC. Corticocortical and thalamocortical information flow in the primate visual system. Prog Brain Res. 2005;149:173–185. doi: 10.1016/S0079-6123(05)49013-5. [DOI] [PubMed] [Google Scholar]
- 73.Kastner S, Pinsk MA. Visual attention as a multilevel selection process. Cogn Affect Behav Neurosci. 2004;4(4):483–500. doi: 10.3758/cabn.4.4.483. [DOI] [PubMed] [Google Scholar]
- 74.Casanova C, Merabet L, Desautels A, Minville K. Higher-order motion processing in the pulvinar. Prog Brain Res. 2001;134:71–82. doi: 10.1016/s0079-6123(01)34006-2. [DOI] [PubMed] [Google Scholar]
- 75.Grieve KL, Acuña C, Cudeiro J. The primate pulvinar nuclei: vision and action. Trends Neurosci. 2000;23(1):35–39. doi: 10.1016/s0166-2236(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 76.Vecera SP, Rizzo M. Spatial attention: normal processes and their breakdown. Neurol Clin. 2003;21(3):575–607. doi: 10.1016/s0733-8619(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 77.Coull JT, Jones ME, Egan TD, Frith CD, Maze M. Attentional effects of noradrenaline vary with arousal level: selective activation of thalamic pulvinar in humans. Neuroimage. 2004;22(1):315–322. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 78.Annoni JM, Khateb A, Gramigna S, Staub F, Carota A, Maeder P, Bogousslavsky J. Chronic cognitive impairment following laterothalamic infarcts: a study of 9 cases. Arch Neurol. 2003;60(10):1439–1443. doi: 10.1001/archneur.60.10.1439. [DOI] [PubMed] [Google Scholar]
- 79.Wester K, Irvine DR, Hugdahl K. Auditory laterality and attentional deficits after thalamic haemorrhage. J Neurol. 2001;248(8):676–683. doi: 10.1007/s004150170113. [DOI] [PubMed] [Google Scholar]
- 80.Silva RR, Munoz DM, Barickman J, Friedhoff AJ. Environmental factors and related fluctuation of symptoms in children and adolescents with Tourette’s disorder. J Child Psychol Psychiatry. 1995;36(2):305–312. doi: 10.1111/j.1469-7610.1995.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 81.Sukhodolsky DG, Scahill L, Zhang H, Peterson BS, King RA, Lombroso PJ, Katsovich L, Findley D, Leckman JF. Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry. 2003;42(1):98–105. doi: 10.1097/00004583-200301000-00016. [DOI] [PubMed] [Google Scholar]
- 82.Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology. 2002;58(4):527–536. doi: 10.1212/wnl.58.4.527. [DOI] [PubMed] [Google Scholar]
- 83.van Pelt J, van Ooyen A, Corner MA. Growth cone dynamics and activity-dependent processes in neuronal network development. Prog Brain Res. 1996;108:333–346. doi: 10.1016/s0079-6123(08)62550-9. [DOI] [PubMed] [Google Scholar]
- 84.Johnston MV. Brain plasticity in paediatric neurology. Eur J Paediatr Neurol. 2003;7(3):105–113. doi: 10.1016/s1090-3798(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 85.Van der Linden A, Van Meir V, Tindemans I, Verhoye M, Balthazart J. Applications of manganese-enhanced magnetic resonance imaging (MEMRI) to image brain plasticity in song birds. NMR Biomed. 2004;17(8):602–612. doi: 10.1002/nbm.936. [DOI] [PubMed] [Google Scholar]
- 86.Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129(pt 10):2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- 87.Peterson BS, Leckman JF, Arnsten A, Anderson G, Staib LH, Gore JC, Bronen RA, Malison R, Scahill L, Cohen DJ. The neuroanatomical substrate of Tourette’s syndrome-related disorders. In: Leckman JF, editor. Tourette Syndrome and Tic-Related Obsessive Compulsive Disorder: Natural History, Genetics, Neurobiology and Treatment. New York: John Wiley & Sons; 2004. [Google Scholar]
- 88.Kalanithi PS, Zheng W, Kataoka Y, DiFiglia M, Grantz H, Saper CB, Schwartz ML, Leckman JF, Vaccarino FM. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102(37):13307–13312. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154(9):1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- 90.Mink JW. Neurobiology of basal ganglia and Tourette syndrome: basal ganglia circuits and thalamocortical outputs. Adv Neurol. 2006;99:89–98. [PubMed] [Google Scholar]
- 91.Thomalla G, Siebner HR, Jonas M, Bäumer T, Biermann-Ruben K, Hummel F, Gerloff C, Müller-Vahl K, Schnitzler A, Orth M, Münchau A. Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain. 2009;132(pt 3):765–777. doi: 10.1093/brain/awn339. [DOI] [PubMed] [Google Scholar]
- 92.Tye KM, Stuber GD, de Ridder B, Bonci A, Janak PH. Rapid strengthening of thalamoamygdala synapses mediates cue-reward learning. Nature. 2008;453(7199):1253–1257. doi: 10.1038/nature06963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alexander GM, Peterson BS. Testing the prenatal hormone hypothesis of ticrelated disorders: gender identity and gender role behavior. Dev Psychopathol. 2004;16(2):407–420. doi: 10.1017/s095457940404458x. [DOI] [PubMed] [Google Scholar]
- 94.Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ, Cohen DJ. Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette’s syndrome. Psychoneuroendocrinology. 1992;17(6):553–563. doi: 10.1016/0306-4530(92)90015-y. [DOI] [PubMed] [Google Scholar]
- 95.Benarroch EE. The midline and intralaminar thalamic nuclei: anatomic and functional specificity and implications in neurologic disease. Neurology. 2008;71(12):944–949. doi: 10.1212/01.wnl.0000326066.57313.13. [DOI] [PubMed] [Google Scholar]
- 96.Parent M, Parent A. Single-axon tracing and three-dimensional reconstruction of centre median-parafascicular thalamic neurons in primates. J Comp Neurol. 2005;481(1):127–144. doi: 10.1002/cne.20348. [DOI] [PubMed] [Google Scholar]
- 97.Servello D, Porta M, Sassi M, Brambilla A, Robertson MM. Deep brain stimulation in 18 patients with severe Gilles de la Tourette syndrome refractory to treatment: the surgery and stimulation. J Neurol Neurosurg Psychiatry. 2008;79(2):136–142. doi: 10.1136/jnnp.2006.104067. [DOI] [PubMed] [Google Scholar]
- 98.Guillery RW, Feig SL, Lozsádi DA. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21(1):28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.