Abstract
Quality of the medical imaging is a key component for accurate disease diagnosis. How to optimize image quality while maintain scan time efficiency and patient comfort is important for the clinical routine MRI exams. In this chapter, we review both practical and advanced techniques to achieve high image quality, especially focusing on optimizing the trade-offs between the image quality (such as signal-to-noise and spatial resolution) and acquisition time. We provide practical examples for optimizing the image quality and scan time.
Keywords: Motion, gating, acceleration, parallel imaging, compressed sensing
Introduction
Cardiac MRI provides images with a variety of tissue contrasts and this can be exploited in the diagnosis of different cardiac conditions. All of these contrasts require sufficient image quality to provide accurate diagnoses. Image quality in MR is based on the underlying MR physics, and usually requires trade-offs between the signal-to-noise, spatial resolution, acquisition time, and so on. A major limitation in cardiac MRI is the motion caused by the beating heart and by respiration, which further constrains the trade-offs. The utilization of multiple-channel coils generally provides improved image quality, and higher field strength scanners provide higher SNR (signal-to-noise ratio). In this paper, we will introduce current practical approaches to optimize cardiac MRI image quality and scan time. We will review motion compensation methods, acceleration techniques, and practical examples for optimizing the image quality and scan time.
Cardiac and respiratory motion
Central to cardiac MRI is how to address the cardiac and respiratory motion. These two sources of motion are commonly managed independently to reduce image artifacts.
Cardiac triggering
Cardiac MR Images are typically reconstructed from data acquired over several heart beats. To account for cardiac motion the acquisition is synchronized with the echocardiographic (ECG) signal. Morphological single time frame images are commonly acquired by acquiring multiple data samples during mid-diastole when the heart has minimal motion. This allows for a relatively long temporal window with minimal motion artifacts, resulting in a shorter acquisition time. For functional time resolved data, the trade-off between temporal window and the total acquisition time becomes more apparent. A higher temporal resolution requires a narrower temporal window for each time frame which requires a longer total acquisition time. On the other hand, lowering the temporal resolution widens the temporal window used to reconstruct the images, which makes it more prone to temporal blurring.
Prospective and retrospective
Time resolved cardiac image can be acquired in two different modes: prospective or retrospective. In prospective imaging the data acquisition starts at the detection of the ECG-trigger and data is acquired for a predefined number of temporal phases, after which the acquisition is idle until the next trigger occur. The predefined number of temporal phases is prescribed to cover an interval that is slightly shorter than the duration of the cardiac cycle to ensure that the system is ready for the next trigger. If the number of phases is low, the acquisition is limited to the early parts of the cardiac cycle, and if the number is too high, the acquisition may continue into the next cardiac cycle, thereby missing the trigger of that cardiac cycle. Missed triggers result in longer acquisition times. Retrospective acquisition, on the other hand, does not require a predefined number of time frames as the data is sorted after it is acquired. Furthermore, retrospective sequences run continuously, without any breaks in data acquisition between cardiac cycles, and the steady state can therefore be preserved.
Respiratory motion
The heart is located next to the diaphragm, and respiration is therefore a major cause of motion artifacts for cardiac MRI. Analogous to cardiac motion, the remedy for respiratory motion is to acquire data at certain stages in the respiratory cycle. This can be achieved by breathholds, navigator gating, or pneumatic bellows triggering.
Breathhold methods are a simple and reliable means to minimize respiratory motion as long as the acquisition time is sufficiently short. However, patients often find even short breathholds challenging. Furthermore, to cover an image volume multiple breath-holds are usually required, which may result in slice misregistration error due to the difficulty in consistently reproducing the same breath-hold position. To obtain short breathholds, the breathhold duration can be reduced using acceleration techniques described later in this paper.
Navigator gating is a suitable option for sequences that require longer acquisition time. In navigated sequences a separate readout is introduced to detect the movement of the diaphragm. A threshold is set for the maximum allowable excursion of the diaphragm through the acquisition, and data acquired outside that threshold is rejected and reacquired when the diaphragm returns back within the threshold. A narrow acceptance window will therefore reduce the extent to which respiration degrades image quality but will increase the total acquisition time. Navigator gating is a relatively accurate method for tracking respiratory motion, since it measures the actual displacement of the diaphragm. However it continuously interrupts data acquisition, which breaks the steady-state of the readout and may result in artifacts.
Acceleration
Compared to other medical imaging modalities such as Ultrasound and CT, MR imaging is relatively time consuming, resulting in patient discomfort, motion-related artifacts, or limitations of imaging capabilities. The speed of the acquisition is fundamentally limited by physical and physiological constrains. The most common approach used to accelerate the acquisition is therefore to reduce the amount of data acquired to reconstruct an image. However, the missing information that results from data undersampling will cause artifacts in the reconstructed image. The goal of different acceleration methods is therefore to recover images with minimal artifacts even in the presence of undersampled data.
Partial acquisition and non-Cartesian acquisition
Most of the image energy is concentrated around the low frequency k-space center whereas the high frequency outer k-space contains less visual information. This can be exploited to reduce the acquisition time. One commonly used method is partial Fourier acquisition which takes advantage of the symmetry properties of k-space. In this method, only one half of k-space is fully sampled in the phase or the slice encoding direction (1). With this method nearly 50% of k-space need not be explicitly acquired but can be mathematically reconstructed correspondingly reducing the total acquisition time. Instead of sampling the data uniformly, non-Cartesian acquisitions such as spiral and radial sampling can be designed to accelerate scan time by undersampling the outer portions of k-space and sufficiently or overly sampling the central k-space (2, 3).
Sliding window, Keyhole, TRICKS
For dynamic imaging with multiple time frames the data can be undersampled by exploiting redundancies in the temporal domain, generally called as k-t methods. View sharing is a commonly used method to accelerate time-resolved acquisitions (4–6). The k-space is interleaved into multiple segments, and the time frames are reconstructed by sharing data with adjacent time frames. Usually the k-space center is acquired at each time frame while the high frequencies are interleaved. The fully sampled high frequency data is combined from all the time frames and is shared for all the frames.
Parallel Imaging
Each coil element in a multiple receiver coil has its unique spatial location, and when combined that data contains spatial redundancies. In parallel imaging these spatial redundancies are exploited to reconstruct images from undersampled data. In theory the greatest acceleration factor that can be achieved is of the order of the number of the coil elements. In practice the SNR loss related to the parallel acquisition often limits the achievable acceleration factor. Clinically used parallel imaging methods include SENSE and GRAPPA (7, 8), which appear as various names with different vendors. Parallel imaging also allows combination with k-t methods (9, 10).
Compressed sensing
Recently, the compressed sensing technique has been introduced into the field of MRI to achieve scan time acceleration by recovering images with undersampled data (11). Compression techniques are commonly used in applications such as MP3 audios and JPG images. Similarly, under certain constraints, the MR images can be compressed with specific transformations and can be represented by a small portion of the data. This permits the user to acquire less data (shorter scan time) but maintain sufficient image quality. Furthermore, the compressed sensing technique can also be combined with parallel imaging and k-t techniques, achieving high acceleration factors(12, 13).
Practical exams for optimizing image quality and scan time
Practical exam implementations achieve a tradeoff between image quality and scan time. In this section, we describe examples of practical exams to explain the relationships between the scan parameters and introduce how the tradeoff between the image quality and scan time can be optimized.
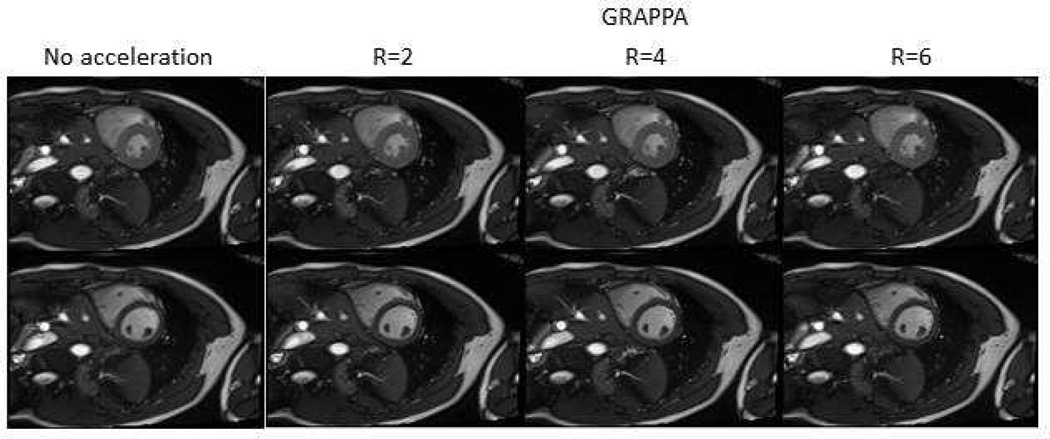
Parallel imaging is an efficient way to reduce scan time, but sacrifices the SNR, and may introduce technique depended artifacts. Figure 1 shows data from accelerated acquisitions using GRAPPA, for which the end-systolic and diastolic phases are shown.
Figure 1.
Parallel imaging acceleration can be used to reduce the acquisition time. Short axis views at endsystolic (top row) and end-diastolic (bottom row) phases can be seen for different acceleration factors using GRAPPA. Notice the decreased SNR with increased acceleration factor.
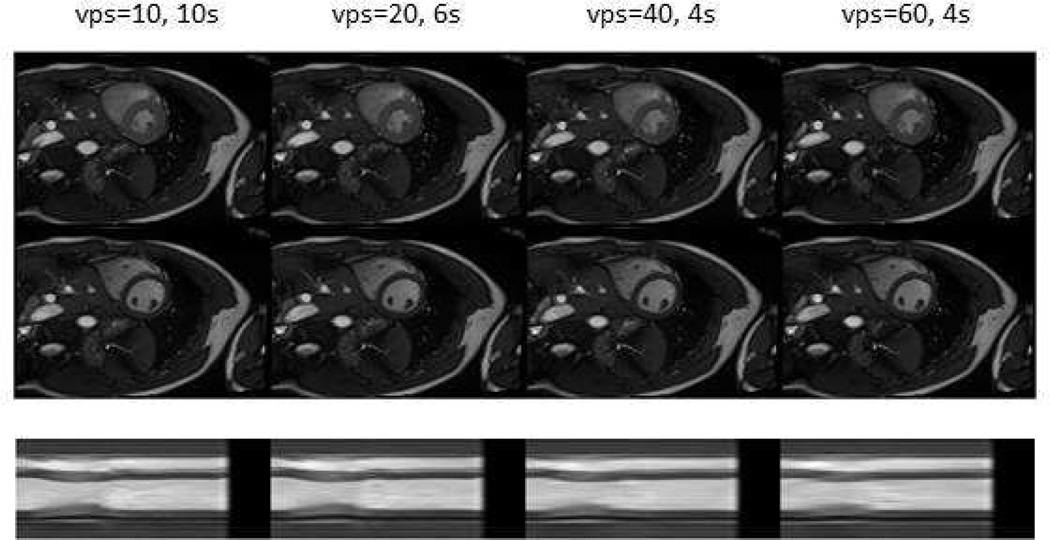
By modifying the number of k-space lines per segments per view the acquisition time can be reduced at the expense of the temporal resolution. In Figure 2, the number of views per segment varies, ranging between 20 and 60. The reconstructed number of cardiac phases was chosen to be the same by applying sliding window to share the data among different cardiac phases.
Figure 2.
Short axis view images at end-systolic (first row) and end-diastolic (second row) phases with different selections of views per segment (10, 20, 40, and 60). The third row show a cross section profile (a vertical line in the short axis view image) plotted along time (horizontal axis). Notice the increased temporal blurring (horizontally in the third row) with increased views per segment.
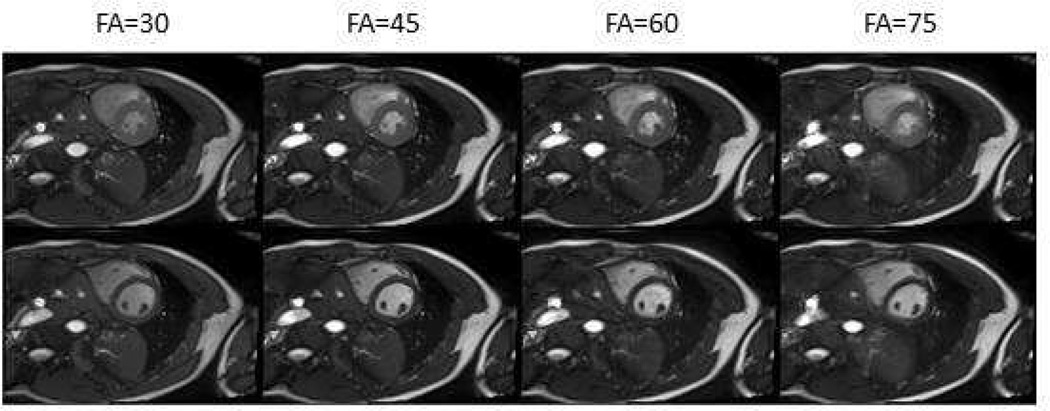
The flip angle influence both SNR and contrast of the blood and myocardium in the cardiac image. In Figure 3 data were acquire with different flip angles. The measured SNRs of the blood in the left ventricles were 3.9, 5.2, 4.8 and 2.8 for flip angles of 30°, 45°, 60° and 75° respectively, and the corresponding CNRs (contrast-to-noise ratio) between blood and myocardium were 3.9, 5.1, 4.8, and 2.7. It suggests a flip angle of 45°~60° could be chosen to achieve the optimal blood SNR and blood-to-myocardium CNR.
Figure 3.
Short axis images acquired with different flip angles. The top row shows the images at end-systolic phase, and the bottom row shows the end-diastolic phase. Different SNR and contrast are seen for the different flip angles.
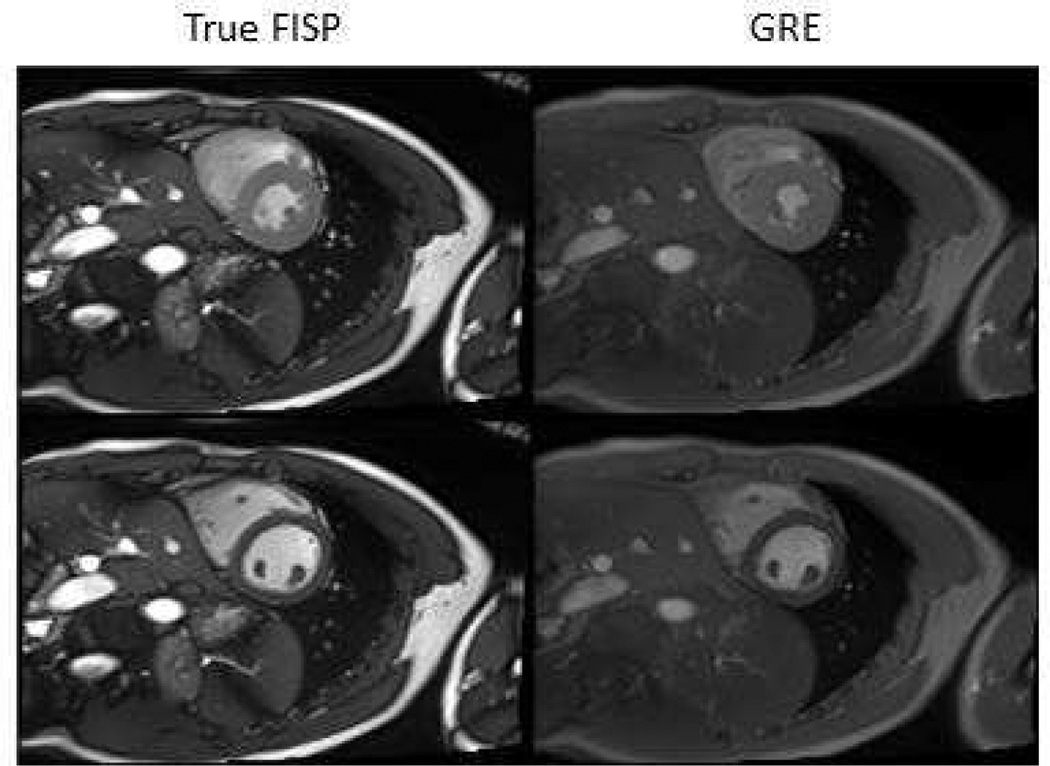
Some applications in cardiac MRI require specific sequences while other applications allow multiple choices. The choice does in these cases affect many aspects of the image quality. Figure 4 show images acquired with TrueFISP and GRE, and illustrates the differences in SNR, contrast, and technique specific artifacts.
Figure 4.
Images acquired with True FISP and GRE. The TrueFISP acquisition provides a greater SNR. Black bands are present in the arm in the lower right corner in the TrueFISP acquisition, but are located outside the region of interest.
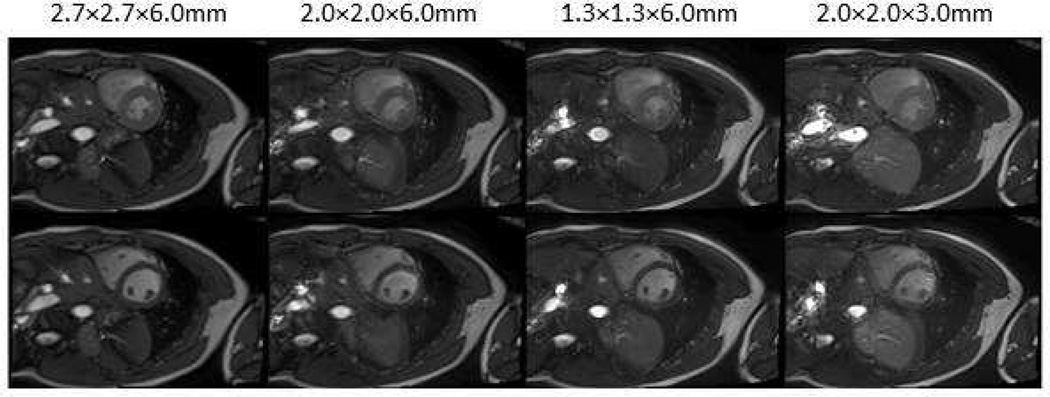
Reduced spatial resolution increases the SNR, which could be traded for a shorter acquisition time. Figure 5 shows the trade-off between images acquired with different image spatial resolution.
Figure 5.
Images acquired with various spatial resolutions. Reduced spatial resolution increases the SNR, which can be traded for a shortened acquisition time.
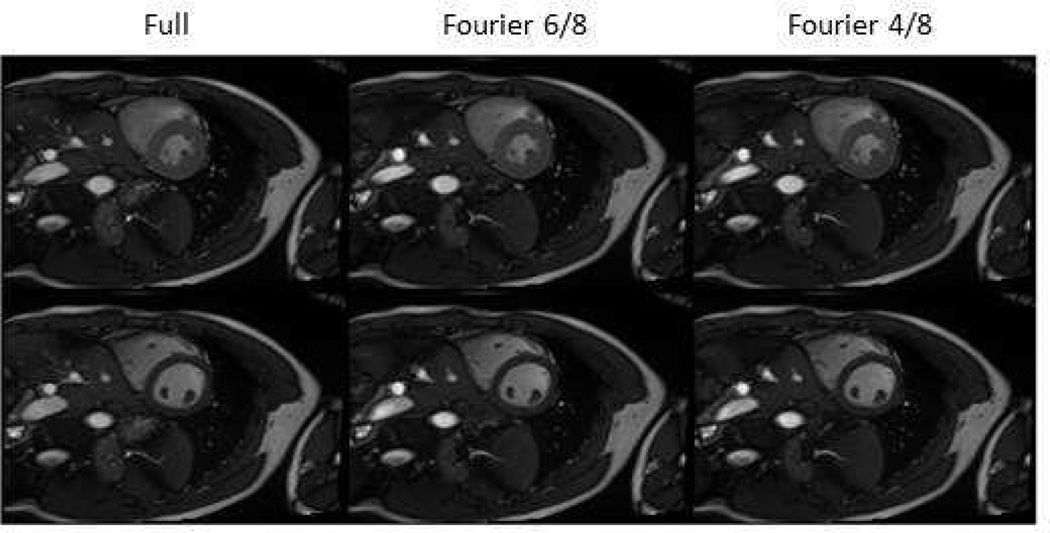
Partial acquisition can be used to shorten the acquisition time or increase the temporal resolution. In Figure 6 the scan time was reduced by partial phase encodings without visually noticeable image quality
Figure 6.
Partial phase encoding can be chosen to reduce scan time while maintaining image quality.
Key Points.
Cardiac MRI provides images with a variety of tissue contrasts, which can be exploited in the diagnosis of different cardiac conditions
A major limitation in cardiac MRI is the motion caused by the beating heart and by respiration, which further constrains the trade-offs between the image quality (such as signal-to-noise and spatial resolution) and acquisition time.
Utilization of multiple-channel coils generally provides improved image quality, and higher field strength scanners provide higher signal-to-noise ratio.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
References
- 1.Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging. 1991;10(2):154–163. doi: 10.1109/42.79473. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 2.Peters DC, Korosec FR, Grist TM, Block WF, Holden JE, Vigen KK, et al. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43(1):91–101. doi: 10.1002/(sici)1522-2594(200001)43:1<91::aid-mrm11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 3.Ahn CB, Kim JH, Cho ZH. High-speed spiral-scan echo planar NMR imaging-I. IEEE Trans Med Imaging. 1986;5(1):2–7. doi: 10.1109/TMI.1986.4307732. Epub 1986/01/01. [DOI] [PubMed] [Google Scholar]
- 4.van Vaals JJ, Brummer ME, Dixon WT, Tuithof HH, Engels H, Nelson RC, et al. "Keyhole" method for accelerating imaging of contrast agent uptake. Journal of magnetic resonance imaging : JMRI. 1993;3(4):671–5. doi: 10.1002/jmri.1880030419. Epub 1993/07/01. [DOI] [PubMed] [Google Scholar]
- 5.Korosec FR, Frayne R, Grist TM, Mistretta CA. Time-resolved contrast-enhanced 3D MR angiography. Magnetic Resonance in Medicine. 1996;36(3):345–351. doi: 10.1002/mrm.1910360304. [DOI] [PubMed] [Google Scholar]
- 6.Foo TK, Bernstein MA, Aisen AM, Hernandez RJ, Collick BD, Bernstein T. Improved ejection fraction and flow velocity estimates with use of view sharing and uniform repetition time excitation with fast cardiac techniques. Radiology. 1995;195(2):471–478. doi: 10.1148/radiology.195.2.7724769. Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- 7.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 8.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 9.Kozerke S, Tsao J, Razavi R, Boesiger P. Accelerating cardiac cine 3D imaging using k-t BLAST. Magn Reson Med. 2004;52(1):19–26. doi: 10.1002/mrm.20145. [DOI] [PubMed] [Google Scholar]
- 10.Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med. 2003;50(5):1031–1042. doi: 10.1002/mrm.10611. [DOI] [PubMed] [Google Scholar]
- 11.Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58(6):1182–1195. doi: 10.1002/mrm.21391. Epub 2007/10/31. [DOI] [PubMed] [Google Scholar]
- 12.Otazo R, Kim D, Axel L, Sodickson DK. Combination of compressed sensing and parallel imaging for highly accelerated first-pass cardiac perfusion MRI. Magn Reson Med. 2010;64(3):767–76. doi: 10.1002/mrm.22463. Epub 2010/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usman M, Prieto C, Schaeffter T, Batchelor PG. k-t group sparse: A method for accelerating dynamic MRI. Magn Reson Med. 2011 doi: 10.1002/mrm.22883. Epub 2011/03/12. [DOI] [PubMed] [Google Scholar]