Abstract
Under physiological conditions, homeostasis of inorganic phosphate (Pi) is tightly controlled by a network of increasingly more complex interactions and direct or indirect feedback loops among classical players, such as vitamin D (1,25(OH)2D3), parathyroid hormone (PTH), intestinal and renal phosphate transporters, and the recently described phosphatonins and minhibins. A series of checks and balances offsets the effects of 1,25(OH)2D3 and PTH to enable fine-tuning of intestinal and renal Pi absorptive capacity and bone resorption and mineralization. The latter include PHEX, FGF-23, MEPE, DMP1, and secreted FRP4. Despite this large number of regulatory components with complex interactions, the system has limited redundancy and is prone to dysregulation under pathophysiological conditions. This article reviews and synthesizes recent advances to present a new model of Pi homeostasis.
Keywords: phosphate homeostasis, vitamin D, parathyroid hormone, phosphatonins, minhibins
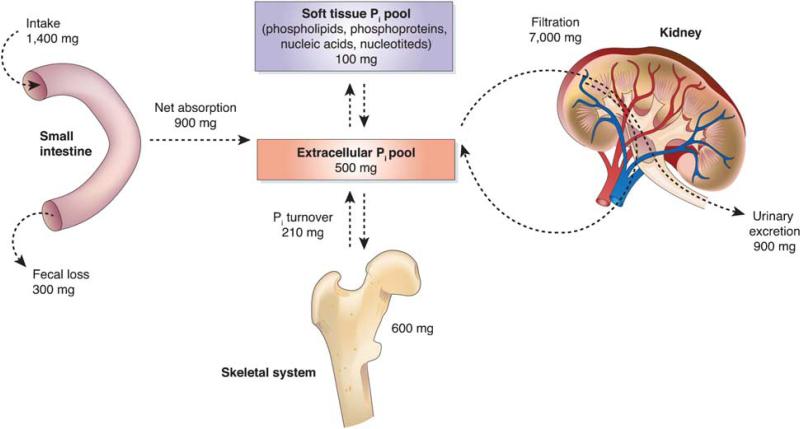
Maintenance of inorganic phosphate (Pi) homeostasis is of significant biological importance, as it relates to numerous cellular mechanisms involved in energy metabolism, cell signaling, nucleic acid synthesis, membrane function, as well as skeletal health and integrity. Pi is essential for a diverse array of biological processes, and negative Pi balance resulting from improperly regulated intestinal absorption, systemic utilization, and renal excretion (Figure 1). Thus, a spectrum of debilitating diseases manifested acutely as myopathy, cardiac dysfunction, abnormal neutrophil function, platelet dysfunction, or erythrocyte membrane fragility, and chronically an impaired bone mineralization, rickets, and osteomalacia, can result from dysregulation of Pi homeostasis. The latter are due to the fact that the rate of bone matrix formation depends equally on the availability of both calcium and Pi.
Figure 1.
Systemic phosphate balance in an adult human. On the basis of Figure 2 from a review by Berndt and Kumar, modified and used with permission.1
Classically, it was well accepted that parathyroid hormone (PTH), 1,25-dihydroxy vitamin D3, and calcium-sensing receptors coordinately regulate calcium homeostasis. This was accompanied by the view that molecules involved in calcium homeostasis passively mediate phosphate homeostasis. However, recent advances have shed new light on the mechanisms that control phosphate balance in normal and in various pathological states and suggest that the previous view is incorrect. In particular, new understanding of X-linked hypophosphatemia (XLH), autosomal dominant hypophosphatemic rickets (ADHR), and tumor-induced osteomalacia (TIO) has suggested the existence of a novel phosphate regulatory pathway that is independent of the classic mechanisms of calcium regulation. A group of factors, collectively termed phosphatonins, have emerged as major regulators of phosphate homeostasis and suggest the existence of an elaborate network of humoral interactions and feedback loops involving intestine, kidney, parathyroid gland, and bone. Thus, a series of interorgan, systemic interactions controlling and fine-tuning the systemic balance of Pi is critical. Remarkably, there is little redundancy or ability to compensate for defects. Thus, the critical role of each node in this network is exemplified by the pathophysiologies that arise from disruption of individual network components.
Pi ABSORPTION AND REABSORPTION: Slc34 SODIUM PHOSPHATE COTRANSPORTER FAMILY
The majority of the transepithelial Pi transport in the intestine and kidney is mediated by the type II family of transporters that include NaPi-lla (NPT2; SLC34A1), NaPi-llb (SLC34A2), and NaPi-llc (SLC34A3). These transporters are responsible for maintaining serum Pi concentration at 1.1 mM. NaPi-lla is primarily localized at the brush border of proximal tubular epithelial cells and is responsible for ~85% of renal Pi reabsorption.2–4 The dominant role of NaPi-IIa in renal phosphate reabsorption makes it a particularly interesting gene, as even relatively minor changes in its expression and protein activity translate into clinically significant alterations in Pi homeostasis and phosphaturia. Consistent with this, homozygous Na/Pi-IIa knockout mice exhibit increased urinary Pi excretion, decreased renal brush-border membrane Na/Pi cotransport, hypophosphatemia, an elevated serum concentration of 1,25(OH)2D3, hypercalcemia, hypercalciuria, and reduced circulating PTH.5 These features recapitulate the rare syndrome of hypophosphatemic rickets with hypocalcemia (HHRH), and as a result it was initially thought that mutations in the human ortholog SLC34a1 gene might be responsible for HHRH. However, although two allelic variants in SLC34a1 were associated with urolithiasis (Ala48Phe) and osteoporosis (Val147Met),6 they did not translate into a functional deficit,7 and no SLC34a1 mutations were identified in a cohort of extended Bedouin kindred affected by HHRH.8 Instead, genomic analysis of the Bedouin kindred mapped HRHH to the SLC34A3 gene on 9q34.9–11 SLC34A3 encodes NaPi-llc, which is expressed exclusively in the proximal tubules of deep nephrons and accounts for the residual Pi transport in Npt −/− mice.12,13 Thus, even though the contribution of NaPi-IIc is only approximately 20% of the total, the consequence of NaPi-IIc mutation demonstrates its critical contribution to Pi home-ostasis.
In contrast to NaPi-IIa and NaPi-IIc, NaPi-llb/SLC34A2 is expressed broadly, including in pulmonary alveolar type II cells,14 where it participates in Pi uptake from the alveolar fluid for surfactant production. SLC34A2 mutations have been linked with pulmonary alveolar microlithiasis, a disease characterized by the deposition of calcium phosphate microliths throughout the lungs, and possibly with a testicular form of microlithiasis.15 In the intestine, NaPi-IIb is localized to the brush border membrane (BBM) where it mediates Pi absorption from the diet,16 whereas in the liver, NaPi-IIb mediates Pi reabsorption from the primary hepatic bile ducts.
PLEIOTROPIC ROLE OF VITAMIN D IN THE CONTROL OF PHOSPHATE HOMEOSTASIS
The fact that chronic vitamin D deficiency is a universally recognized cause of rickets, osteomalacia, and osteoporosis led to the overly simplistic view of the hormone as pro-bone. The accumulated body of evidence, however, points to a more complicated role of 1,25(OH)2D3 in bone turnover as well as systemic Pi homeostasis. 1,25(OH)2D3 promotes intestinal Ca+ + and Pi absorption and, in cooperation with PTH, also induces expression of macrophage colony-stimulating factor and RANK ligand by osteoblasts (OBs), and these stimulate osteoclastogenesis, bone catabolism, and resorption. Additional layers of control to mitigate the potential phosphatemic and calcemic effects of vitamin D are therefore necessary. Partial control is provided by the negative effects of PTH on renal phosphate reabsorption, an effect mediated by acute internalization inhibition of NaPi-IIa and NaPi-IIc as the result of endocytic removal from the BBM and subsequent degradation in lysosomes.17,18 Some of the anticalcemic feedback, which also provides a break to the PTH-induced phosphaturia, is mediated by reduced PTH synthesis in the parathyroid gland as a result of 1,25(OH)2D3 action. 1,25(OH)2D3 also transcriptionally activates expression of FGF-23 in OBs.19,20 Elevated circulating FGF-23 can decrease systemic Pi by inhibiting intestinal NaPi-IIb-mediated phosphate absorption through a VDR-dependent mechanism21 and by promoting renal phosphate excretion through decreased expression and activity of renal NaPi-IIa and NaPi-IIc.22 The phosphaturic effects of FGF-23 under physiological conditions may be restricted by an FGF-23-mediated decrease in PTH synthesis. Moreover, FGF-23 regulates its own expression by inhibiting the anabolic 1a-hydroxylase (1α(OH)ase) and inducing expression of the catabolic 24-hydroxylase, thus suppressing serum levels of 1,25(OH)2D3.23 Consistent with this feedback mechanism, FGF-23 −/− mice are hyperphosphatemic with elevated serum levels of 1,25(OH)2D3.24 Genetic ablation of the vitamin D activation pathway in double-knockout (FGF-23 −/− /1α(OH)ase −/− ) mice results in amelioration of the abnormal mineral ion homeostasis and the impaired skeletogenesis seen in FGF-23 −/− mice.
FGF-23 AND KLOTHO: PARTNERS FOR (LONG) LIFE
The phosphaturic effects of FGF-23 are emphasized from studies of two clinically similar hypophosphatemic disorders with renal Pi wasting: an ADHR25 and paraneoplastic TIO.26 In the first case, FGF-23 mutations result in the production of an abnormally stable, long-lived form of FGF-23, whereas in TIO, FGF-23 is overproduced by the tumor, most commonly sarcomas. Moreover, transgenic mice overexpressing FGF-23 exhibit hypophosphatemia and hyperphosphaturia without significantly affecting serum Ca+ + levels.27 In addition to ADHR and TIO, FGF-23 is indirectly involved in the pathogenesis of three other disorders associated with hypophosphatemia: XLH, autosomal recessive hypophosphatemic rickets, and McCune–Albright syndrome. In all three disorders, FGF-23 expression is elevated, leading to phosphaturia and inhibition of renal Pi reabsorption. At the other end of the spectrum, hyperphosphatemic familial tumoral calcinosis is caused by mutations in GALNT3, which encodes UDP-N-acetyl-alpha-D-galactosamine: N-acetylgalactosaminyltransferase 3 (ppGaNTase-T3). It is at present believed that under physiological conditions, ppGaNTase-T3 mediates triple O-glycosylation of FGF-23 to render it more resistant to proteolytic processing. In the absence of functional GALNT3, defectively glycosylated FGF-23 is rapidly processed, leading to symptoms similar to those observed in FGF-23 −/− mice: hyperphosphatemia, ectopic calcification, and elevated 1,25(OH)2D3.
The hypophosphatemic effects of FGF-23 are exerted through several mechanisms. FGF-23 inhibits renal Pi reabsorption by reducing the apical expression and activity of NaPi-IIa in the proximal tubule epithelium.22,28 Under physiological conditions, FGF-23 may exert a continuous negative pressure on NaPi-IIa expression, as in FGF-23 null knockout mice, expression and activity of NaPi-IIa are abnormally elevated.24 Additionally, FGF-23 reduces intestinal absorption of dietary Pi through a VDR-dependent decrease in NaPi-IIb activity.21 As noted above, the latter phenomenon is most likely secondary to FGF-23-mediated reduction of circulating 1,25(OH)2D3 synthesis through suppression of 1α(OH)ase expression and stimulation of catabolic D-24-hydroxylase.23,29
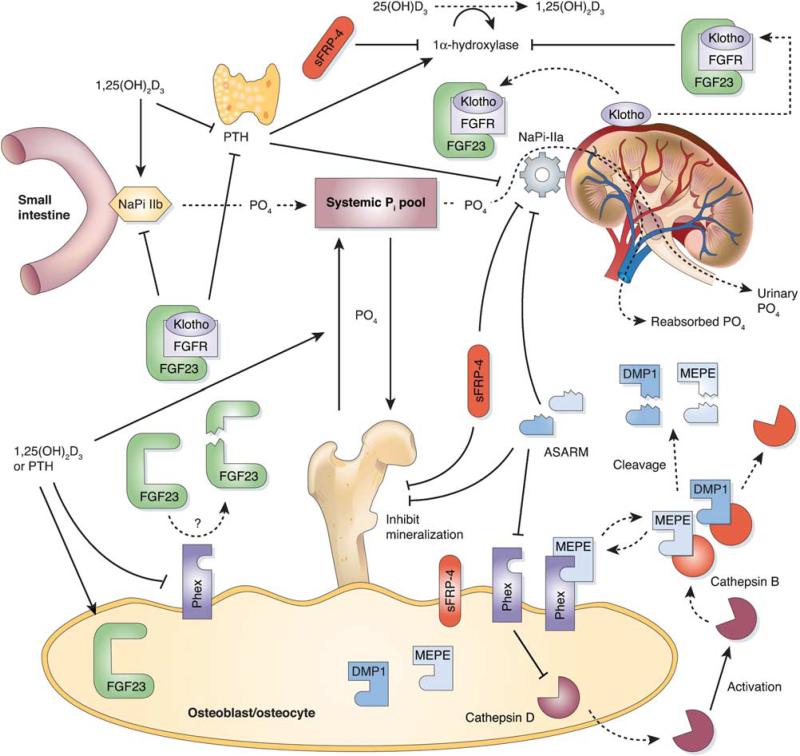
Fibroblast growth factor-23 belongs to the FGF-19 family of growth factors. One common feature of these proteins is their very weak affinity to FGF receptors in vitro, suggesting the need for a cofactor to enhance their receptor binding and to allow the initiation of downstream signaling. The incidental observation that mice lacking a protein termed Klotho are nearly identical, phenotypically, to FGF-23-deficient mice. Common features include shortened lifespan, infertility, kyphosis, atherosclerosis, soft tissue calcification, skin atrophy, muscle wasting, T-cell dysregulation, emphysema, osteopenia or osteoporosis, and abnormal mineral ion and vitamin D homeostasis. These similarities suggested that FGF-23 and Klotho might function in a common single transduction pathway. Klotho gene encodes a 130-KDa single-pass transmembrane protein with β-glucuronidase activity and is primarily expressed in the epithelial cells of renal distal tubules and in the choroid plexus. Recent studies suggest that Klotho protein binds directly to multiple FGF receptors, and the Klotho-FGFr complex binds to FGF-23 with much higher affinity than FGFr or Klotho alone.30 Thus, Klotho is an essential cofactor for FGF-23 signaling, and the lack of it renders renal cells incompetent for FGF-23 signaling.30 Of significance is that elimination or reduction of vitamin D activity from FGF-23 and Klotho mutant mice, either by dietary restriction or genetic manipulation, can rescue premature aging and ectopic calcification.31–33 Further evidence for involvement of the Klotho in bone homeostasis comes from genetic studies, which suggest that certain allelic variants of Klotho constitute one of the genetic factors influencing bone mass loss in adult males34 and post-menopausal women.35 All this evidence implies that Klotho is a multifunctional protein that regulates phosphate/calcium metabolism as well as aging. The interrelationship among phosphate, vitamin D, PTH, phosphatonins and Klotho are depicted in Figure 2.
Figure 2.
Overlay of the systemic phosphate homeostasis with the major players in the phosphate regulatory network and their functional interactions. (→stimulation; inhibition). Modified from Figure 3 by Rowe,36 with permission.
PHEX: THE CENTRAL NODE?
The PHEX (phosphate-regulating gene with homologies to Endopeptidases on the X chromosome) gene encodes a Zn-metalloendopeptidase expressed primarily in OBs and odontoblasts. Inactivating mutations in the PHEX gene result in vitamin D-resistant, X-linked hypophosphatemic rickets (XLH). This familial disorder manifests with hypophosphatemia, low circulating 1,25(OH)2D3 levels, high serum alkaline phosphatase and osteomalacia, and decreased expression and activity of NaPi-IIa in renal proximal tubules.37,38 Although the target of Phex proteolytic activity remains elusive, phenotypic changes observed in Hyp mice (a spontaneous Phex knockout model) and recently confirmed in OB/osteocyte-specific knockout mice39 suggest that Phex inactivates one or a group of phosphaturic factors, collectively termed ‘phosphatonins,’ that inhibit renal phosphate reabsorption, reduce vitamin D bioactivation, and suppress bone mineralization. Elevated circulating levels of FGF-23 in Hyp mice40 suggested that it might represent the sought-after phosphatonin. Indeed, FGF-23 knockout reversed Hypophosphatemia in Hyp mice, implying that increased plasma FGF-23 levels in Hyp mice and in XLH patients may be at least partially responsible for the phosphate imbalance.24 Initial studies seemed to confirm the Hypothesis of proteolytic degradation of FGF-23 by PHEX by demonstrating PHEX-mediated hydrolysis of FGF-23.41 This observation, however, was not confirmed in independent studies. Subsequent reports have shown that inactivating PHEX mutations result in increased expression of FGF-23 rather than result in defective proteolytic cleavage42 and that FGF-23 is cleaved by subtilisin-like proprotein convertases (furins).43 The exact mechanism of elevated FGF-23 expression in the absence of functional Phex remains unclear. The importance of the PHEX gene in bone mineralization extends beyond controlling phosphate homeostasis. Immortalized OBs isolated from Phex-deficient Hyp mice fail to mineralize under permissive conditions in vitro,44 suggesting an intrinsic defect in Hyp-derived OBs. Another report demonstrated that Hyp-derived OBs in culture have increased expression of FGF-23.42 The regulation of PHEX both in vivo and in vitro by several hormones important for skeletal homeostasis has been reported. Upregulation of Phex expression was observed after treatment with IGF1 and growth hormone45 and glucocorticoids.46 Conversely, Phex was found to be downregulated by PTH,47 PTH-related peptide,48 and vitamin D.49
While the target of PHEX proteolytic activity remains unknown, PHEX protein also binds matrix extracellular phosphoglycoprotein (MEPE) and protects it from proteolytic cleavage by cathepsin-B (also expressed in OBs). This protection is critical in preventing the proteolytic release of a small, acidic, protease-resistant ASARM peptide (acidic-serine-aspartate-rich-MEPE-associated motif),50,51 a factor inhibiting bone mineralization in vivo and in vitro, which also affects renal phosphate handling that causes phosphaturia. The role of Phex in protecting the enzymatic release of ASARM peptide goes beyond MEPE binding. Hyp mice demonstrate increased expression and proteolytic activity of cathepsin D, an upstream activator of cathepsin B, and protease inhibitors improve bone mineralization defects in Hyp mice.52 Consistent with the role of MEPE in the pathogenesis of the Hyp phenotype is also the finding that MEPE-null knockout mice exhibit marked age-dependent high bone mass density with an increased in vivo mineral deposition rate.53 Although the in vitro mineralization phenotype was corrected by crossing Hyp mice onto a MEPE-deficient background, the in vivo phenotype was not.54 It has been speculated that this may be due to the continued proteolytic degradation of the extracellular protein matrix, release of small integrin-binding ligand, N-linked glycoprotein (SIBLING) ASARM peptides (DMP1; see below), and persistent FGF-23-mediated hypophosphatemia. A feedback mechanism was also described whereby ASARM peptide as well as full-length MEPE inhibit Phex activity, elevate FGF-23 expression, and inhibit mineralization.55
Dentine matrix protein 1 (DMP1) is an acidic phosphorylated extracellular matrix protein that was originally identified from a rat incisor cDNA library and thought to have a primary function in the regulation of dentinogenesis. In 2006, Lorentz-Depiereux et al56 identified homozygous mutations in the DMP1 gene of patients diagnosed with autosomal recessive hypophosphatemic rickets (ARHR). Hypophosphatemia and skeletal abnormalities are also the phenotypic features of Dmp1-null knockout mice.57 These mice have elevated serum levels of FGF-23, and the potentially pathogenic role of this growth factor in ARHR was recently demonstrated by Liu et al58 by crossing FGF-23-deficient and Dmp1 knockout mice. These data suggest that part of the role of DMP1 in matrix mineralization is indirect and may be mediated by regulating the osteocyte production of FGF-23, which ultimately targets renal phosphate reabsorption and vitamin D metabolism. Moreover, DMP1 belongs to the same family of SIBLING proteins as MEPE and is similar to MEPE. Proteolytic degradation of DMP1 leads to the release of the ASARM peptide with potent minhibin and phosphatonin-like activity. Consistently, it was demonstrated that both DMP1 and MEPE proteins are degraded in the cultures of Phex-deficient Hyp calvarial OB cultures.59 In summary, loss of Phex function leads to elevated osteoblastic protease expression, increased MEPE expression, excessive degradation of MEPE and DMP1, and release of protease-resistant ASARM peptides from DMP1 and MEPE. The ASARM peptide inhibition of mineralization is most likely caused by a combination of direct binding to hydroxyapatite crystals and decreased expression of PHEX, whereas the phosphaturic effects are mediated by renal accumulation of ASARM peptides60 and inhibition of Pi reabsorption.61 Indeed, most recent data not only confirm that phosphorylated ASARM peptide inhibits calcium deposition by binding to hydroxyapatite, but also demonstrate that ASARM may actually be enzymatically degraded by Phex protein to prevent this effect.62
The participation of PHEX in phosphate homeostasis and bone mineralization is not an ‘all or nothing’ phenomenon. XLH is a dominant mutation with a phenotype independent of the zygosity in both Hyp mice and XLH patients. In this haploinsufficiency, the normal Phex protein produced by the wild-type allele in heterozygotes is inadequate to provide normal PHEX function. Under experimental conditions, partial antisense-mediated knockdown of PHEX in human OBs was sufficient to inhibit mineralization.63 Similar effects of antisense knockdown were reported in cultured tooth germs where partial loss of PHEX function inhibited the expression of NaPi-IIb.64 These findings further emphasize the importance of Phex as a critical player involved in the Pi balance regulation, and imply that pathophysiological conditions leading to decreased Phex expression, such as that reported for colitis and TNFα,65 will have pronounced local and systemic consequences.
SECRETED FRP-4: PHOSPHATONIN OR MINHIBIN?
Using serial analysis of gene expression (SAGE), De Beur et al66 identified frizzled-related protein 4 (FRP-4) as a gene which was elevated in oncogenic osteomalacia (OOM). Similar to FGF-23 and ASARM, soluble FRP-4 (sFRP-4) is thought to act as a phosphatonin by reducing the efficiency of renal Pi reabsorption and suppressing the synthesis of 1a,25-dihdyroxyvitamin D.67,68 Recently, sFRP-4 was shown to increase renal Pi excretion by reducing NaPi-IIa transporter abundance in the brush border of the proximal tubule through enhanced internalization of the protein.67 Moreover, sFRP-4 also acts as a minhibin to directly affect bone mineralization. Similar to other sFRPs, sFRP-4 can block both the canonical and noncanonical Wnt signaling either by acting as a decoy receptor for Wnts or by forming nonfunctional complexes with frizzled proteins69 to suppress the expansion of OB lineage. A recent study reported that transgenic mice overexpressing sFRP-4 in OB failed to induce hypophosphatemia.70 It therefore appears that OB-derived sFRP-4 does not act as a phosphaturic agent in Sfrp4 TG mice, and its primary effect is limited to the paracrine inhibition of bone formation.
INFLAMMATORY STATES AND PHOSPHATE HOMEOSTASIS
In addition to providing a microenvironment for hematopoietic stem cells, bone is also a target of the immune system, with immune cells and immune mediators influencing bone homeostasis, particularly when the immune system has been activated. These interactions are gaining wide recognition and are developing into a specific niche field of ‘osteoimmunology,’ an area of research investigating accelerated bone loss caused by inflammatory diseases, such as rheumatoid arthritis (RA) or inflammatory bowel diseases (IBDs). Although the scope of this review does not allow for more detailed description of this area of studies (for recent reviews, refer David71 and Takayanagi72), it is worthwhile to examine the limited data describing the relationship between inflammation and the key players in Pi homeostatic network.
Renal expression of Klotho has been shown to be down-regulated in the rat model of acute LPS-mediated inflammatory stress.73 Limited data also point to a role for Klotho in the immune system. It was shown that the spleen and other immune organs from Klotho knockout mice are underdeveloped and that B-cell development and differentiation are impaired.74,75 Expression of the Klotho protein was described in human CD4+ lymphocytes,76 where it was significantly decreased proportionally to advancing age, but it was also heavily suppressed in the CD4+ cells of RA patients. A similar association of Klotho in the immune cells of patients with IBD, a disease sharing many commonalities with RA, has not been investigated.
The high prevalence of hypovitaminosis D has been consistently reported in both pediatric and adult IBD patients.77 The relevance of this finding is not limited to the effects on Ca+ + and Pi balance, but also extends to the potential immunoregulatory role of 1,25(OH)2D3 in autoimmune disorders.78 Moreover, in the course of IBD, circulating proinflammatory cytokines, infiltrating lymphocytes, and other mononuclear cells provide several key factors that may influence bone metabolism by altering the balance between bone-forming OBs and bone-resorbing osteoclasts resulting in decreased bone mineral density in IBD patients. We have recently shown that inflammation, in the setting of chemically induced mouse models of colitis, results in down-regulation of the PHEX gene and protein expression.65 This decrease could be prevented by local attenuation of colitis with dietary curcumin and by neutralizing antibodies against TNFα, and it was recapitulated by parenteral administration of exogenous TNFα. In the UMR-106 OB-like cell line, TNFα profoundly inhibited deposition of mineral matrix, a phenomenon correlating with decreased expression of Phex mRNA, protein, and gene promoter activity.65 Therefore, the Phex gene, which is highly relevant to bone mineralization and Pi homeostasis, is evidently altered in the setting of colonic inflammation.
CONCLUDING REMARKS
Numerous physiological processes, including bone matrix formation by OBs, depend equally on the availability of both calcium and phosphorus. In contrast to Ca+ +, homeostasis of inorganic phosphorus (Pi) has not been extensively studied until very recently. The latest molecular and clinical findings identified numerous novel mediators and continue to unravel complex molecular interactions and intricate homeostatic mechanisms governing systemic phosphate handling. The emerging picture is that of a series of partially overlapping feedback loop mechanisms, which under physiological conditions tightly control phosphate intake, utilization, and excretion. Despite the complexity of these control mechanisms and the increasing number of recognized players, the system appears to have limited redundancy, as demonstrated by the severe phenotypic changes in knockout and transgenic mouse models, as well as in hereditary single gene disorders in humans. This limited redundancy suggests that pathophysiological events may need to target only a restricted number of the critical elements of this network to eventually lead to disturbed Pi homeostasis and phenotypic manifestations, such as osteopenia or osteoporosis. It also underscores the need for further dissection and understanding of this regulatory network as well as the factors adversely affecting the delicate Pi balance.
References
- 1.Berndt T, Kumar R. Phosphatonins and the regulation of phosphate homeostasis. Annu Rev Physiol. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 2.Murer H, Hernando N, Forster I, et al. Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol. 2003;65:531–542. doi: 10.1146/annurev.physiol.65.042902.092424. [DOI] [PubMed] [Google Scholar]
- 3.Hernando N, Biber J, Forster I, et al. Recent advances in renal phosphate transport. Ther Apher Dial. 2005;9:323–327. doi: 10.1111/j.1744-9987.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- 4.Collins JF, Ghishan FK. Molecular cloning, functional expression, tissue distribution, and in situ hybridization of the renal sodium phosphate (Na+/P(i)) transporter in the control and hypophosphatemic mouse. FASEB J. 1994;8:862–868. doi: 10.1096/fasebj.8.11.8070635. [DOI] [PubMed] [Google Scholar]
- 5.Beck L, Karaplis AC, Amizuka N, et al. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Nat Acad Sci USA. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prie D, Huart V, Bakouh N, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. New Eng J Med. 2002;347:983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 7.Virkki LV, Forster IC, Hernando N, et al. Functional characterization of two naturally occurring mutations in the human sodium-phosphate cotransporter type IIa. J Bone Miner Res. 2003;18:2135–2141. doi: 10.1359/jbmr.2003.18.12.2135. [DOI] [PubMed] [Google Scholar]
- 8.Jones A, Tzenova J, Frappier D, et al. Hereditary hypophosphatemic rickets with hypercalciuria is not caused by mutations in the Na/Pi cotransporter NPT2 gene. J Am Soc Nephrol. 2001;12:507–514. doi: 10.1681/ASN.V123507. [DOI] [PubMed] [Google Scholar]
- 9.Bergwitz C, Roslin NM, Tieder M, et al. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–192. doi: 10.1086/499409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichikawa S, Sorenson AH, Imel EA, et al. Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab. 2006;91:4022–4027. doi: 10.1210/jc.2005-2840. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Michigami T, Aranami F, et al. Hereditary hypophosphatemic rickets with hypercalciuria: a study for the phosphate transporter gene type IIc and osteoblastic function. J Bone Miner Metab. 2007;25:407–413. doi: 10.1007/s00774-007-0776-6. [DOI] [PubMed] [Google Scholar]
- 12.Madjdpour C, Bacic D, Kaissling B, et al. Segment-specific expression of sodium-phosphate cotransporters NaPi-IIa and -IIc and interacting proteins in mouse renal proximal tubules. Pflugers Arch. 2004;448:402–410. doi: 10.1007/s00424-004-1253-x. [DOI] [PubMed] [Google Scholar]
- 13.Ohkido I, Segawa H, Yanagida R, et al. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch. 2003;446:106–115. doi: 10.1007/s00424-003-1010-6. [DOI] [PubMed] [Google Scholar]
- 14.Traebert M, Hattenhauer O, Murer H, et al. Expression of type II Na-P(i) cotransporter in alveolar type II cells. Am J Physiol. 1999;277(5 Part 1):L868–L873. doi: 10.1152/ajplung.1999.277.5.L868. [DOI] [PubMed] [Google Scholar]
- 15.Corut A, Senyigit A, Ugur SA, et al. Mutations in SLC34A2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilfiker H, Hattenhauer O, Traebert M, et al. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Nat Acad Sci USA. 1998;95:14564–14569. doi: 10.1073/pnas.95.24.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacic D, Lehir M, Biber J, et al. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int. 2006;69:495–503. doi: 10.1038/sj.ki.5000148. [DOI] [PubMed] [Google Scholar]
- 18.Segawa H, Yamanaka S, Onitsuka A, et al. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol. 2007;292:F395–F403. doi: 10.1152/ajprenal.00100.2006. [DOI] [PubMed] [Google Scholar]
- 19.Kolek OI, Hines ER, Jones MD, et al. 1alpha,25-dihydroxyvitamin D3 upregulates FGF-23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 20.Barthel TK, Mathern DR, Whitfield GK, et al. 1,25-dihydroxyvitamin D(3)/VDR-mediated induction of FGF-23 as well as transcriptional control of other bone anabolic and catabolic genes that orchestrate the regulation of phosphate and calcium mineral metabolism. J Steroid Biochem Mol Biol. 2007;103:381–388. doi: 10.1016/j.jsbmb.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto K, Ito M, Kuwahata M, et al. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial. 2005;9:331–335. doi: 10.1111/j.1744-9987.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 22.Larsson T, Marsell R, Schipani E, et al. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1 (I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinol. 2004;145:3087–3094. doi: 10.1210/en.2003-1768. [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 24.Sitara D, Razzaque MS, Hesse M, et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–432. doi: 10.1016/j.matbio.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ADHRConsortium Autosomal dominant hypophosphataemic rickets is associated withmutations in FGF-23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 26.Shimada T, Mizutani S, Muto T, et al. Cloning and characterization of FGF-23 as a causative factor of tumor-induced osteomalacia. Proc Nat Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada T, Urakawa I, Yamazaki Y, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type I Ia. Biochem Biophys Res Comm. 2004;314:409–414. doi: 10.1016/j.bbrc.2003.12.102. [DOI] [PubMed] [Google Scholar]
- 28.Baum M, Schiavi S, Dwarakanath V, et al. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68:1148–1153. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 29.Perwad F, Zhang MY, Tenenhouse HS, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 30.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF-23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 31.Razzaque MS, Sitara D, Taguchi T, et al. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–722. doi: 10.1096/fj.05-5432fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razzaque MS, Lanske B. Hypervitaminosis D and premature aging: lessons learned from FGF-23 and Klotho mutant mice. Trends Mol Med. 2006;12:298–305. doi: 10.1016/j.molmed.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Hesse M, Frohlich LF, Zeitz U, et al. Ablation of vitamin D signaling rescues bone, mineral, and glucose homeostasis in Fgf-23 deficient mice. Matrix Biol. 2007;26:75–84. doi: 10.1016/j.matbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Zarrabeitia MT, Hernandez JL, Valero C, et al. Klotho gene polymorphism and male bone mass. Calcified Tissue Int. 2007;80:10–14. doi: 10.1007/s00223-006-0233-x. [DOI] [PubMed] [Google Scholar]
- 35.Riancho JA, Valero C, Hernandez JL, et al. Association of the F352V variant of the Klotho gene with bone mineral density. Bio Gerontol. 2007;8:121–127. doi: 10.1007/s10522-006-9039-5. [DOI] [PubMed] [Google Scholar]
- 36.Rowe PS. The wrickkened pathways of FGF23, MEPE and PHEX. Crit Rev Oral Biol Med. 2004;15:264–281. doi: 10.1177/154411130401500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drezner MK. PHEX gene and hypophosphatemia. Kidney Int. 2000;57:9–18. doi: 10.1046/j.1523-1755.2000.00807.x. [DOI] [PubMed] [Google Scholar]
- 38.Dixon PH, Christie PT, Wooding C, et al. Mutational analysis of PHEX gene in X-linked hypophosphatemia. J Clin Endocrinol Metabol. 1998;83:3615–3623. doi: 10.1210/jcem.83.10.5180. [DOI] [PubMed] [Google Scholar]
- 39.Yuan B, Takaiwa M, Clemens TL, et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J Clin Invest. 2008;118:722–734. doi: 10.1172/JCI32702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu S, Zhou J, Tang W, et al. Pathogenic role of FGF-23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–E49. doi: 10.1152/ajpendo.00008.2006. [DOI] [PubMed] [Google Scholar]
- 41.Bowe AE, Finnegan R, Jan de Beur SM, et al. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Com. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Guo R, Simpson LG, et al. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- 43.Benet-Pages A, Lorenz-Depiereux B, Zischka H, et al. FGF-23 is processed by proprotein convertases but not by PHEX. Bone. 2004;35:455–462. doi: 10.1016/j.bone.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Xiao ZS, Crenshaw M, Guo R, et al. Intrinsic mineralization defect in Hyp mouse osteoblasts. Am J Physiol. 1998;275(4 Part 1):E700–E708. doi: 10.1152/ajpendo.1998.275.4.E700. [DOI] [PubMed] [Google Scholar]
- 45.Zoidis E, Gosteli-Peter M, Ghirlanda-Keller C, et al. IGF-I and GH stimulate Phex mRNA expression in lungs and bones and 1,25-dihydroxyvitamin D(3) production in hypophysectom ized rats. Eur J Endocrinol. 2002;146:97–105. doi: 10.1530/eje.0.1460097. [DOI] [PubMed] [Google Scholar]
- 46.Hines ER, Collins JF, Jones MD, et al. Glucocorticoid regulation of the murine PHEX gene. Am J Physiol Renal Physiol. 2002;283:F356–F363. doi: 10.1152/ajprenal.00357.2001. [DOI] [PubMed] [Google Scholar]
- 47.Alos N, Ecarot B. Downregulation of osteoblast Phex expression by PTH. Bone. 2005;37:589–598. doi: 10.1016/j.bone.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Vargas MA, St-Louis M, Desgroseillers L, et al. Parathyroid hormone-related protein(1–34) regulates Phex expression in osteoblasts through the protein kinase A pathway. Endocrinol. 2003;144:4876–4885. doi: 10.1210/en.2003-0253. [DOI] [PubMed] [Google Scholar]
- 49.Hines ER, Kolek OI, Jones MD, et al. 1,25-dihydroxyvitamin D3 down-regulation of PHEX gene expression is mediated by apparent repression of a 110 kDa transfactor that binds to a polyadenine element in the promoter. J Biol Chem. 2004;279:46406–46414. doi: 10.1074/jbc.M404278200. [DOI] [PubMed] [Google Scholar]
- 50.Guo R, Rowe PS, Liu S, et al. Inhibition of MEPE cleavage by Phex. Biochem Biophys Res Com. 2002;297:38–45. doi: 10.1016/s0006-291x(02)02125-3. [DOI] [PubMed] [Google Scholar]
- 51.Rowe PS, Garrett IR, Schwarz PM, et al. Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP). Bone. 2005;36:33–46. doi: 10.1016/j.bone.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rowe PS, Matsumoto N, Jo OD, et al. Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone. 2006;39:773–786. doi: 10.1016/j.bone.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gowen LC, Petersen DN, Mansolf AL, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Brown TA, Zhou J, et al. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol. 2005;16:1645–1653. doi: 10.1681/ASN.2004121060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S, Rowe PS, Vierthaler L, et al. Phosphorylated acidic serineaspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192:261–267. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lorenz-Depiereux B, Bastepe M, Benet-Pages A, et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 2006;38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye L, Mishina Y, Chen D, et al. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005;280:6197–6203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S, Zhou J, Tang W, et al. Pathogenic role of Fgf23 in Dmp1 null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin A, David V, Laurence JS, et al. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (Minhibins): ASARM peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bresler D, Bruder J, Mohnike K, et al. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–R9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rowe PS, Kumagai Y, Gutierrez G, et al. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Addison W, Nakano Y, Loisel T, et al. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite—an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 63.Shih NR, Jo OD, Yanagawa N. Effects of PHEX antisense in human osteoblast cells. J Am Soc Nephrol. 2002;13:394–399. doi: 10.1681/ASN.V132394. [DOI] [PubMed] [Google Scholar]
- 64.Onishi T, Okawa R, Ogawa T, et al. Phex mutation causes the reduction of npt2b mRNA in teeth. J Dent Res. 2007;86:158–162. doi: 10.1177/154405910708600210. [DOI] [PubMed] [Google Scholar]
- 65.Uno JK, Kolek OI, Hines ER, et al. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology. 2006;131:497–509. doi: 10.1053/j.gastro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 66.De Beur SM, Finnegan RB, Vassiliadis J, et al. Tumorsm associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- 67.Berndt TJ, Bielesz B, Craig TA, et al. Secreted frizzled-related protein-4 reduces sodium-phosphate co-transporter abundance and activity in proximal tubule cells. Pflugers Arch. 2006;451:579–587. doi: 10.1007/s00424-005-1495-2. [DOI] [PubMed] [Google Scholar]
- 68.Berndt T, Craig TA, Bowe AE, et al. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116(Part 13):2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 70.Nakanishi R, Akiyama H, Kimura H, et al. Osteoblast-targeted expression of Sfrp4 in mice results in low bone mass. J Bone Miner Res. 2008;23:271–277. doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- 71.David JP. Osteoimmunology: a view from the bone. Adv Immunol. 2007;95:149–165. doi: 10.1016/S0065-2776(07)95005-1. [DOI] [PubMed] [Google Scholar]
- 72.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 73.Ohyama Y, Kurabayashi M, Masuda H, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Com. 1998;251:920–925. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 74.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 75.Okada S, Yoshida T, Hong Z, et al. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol. 2000;12:861–871. doi: 10.1093/intimm/12.6.861. [DOI] [PubMed] [Google Scholar]
- 76.Witkowski JM, Soroczynska-Cybula M, Bryl E, et al. Klotho—a common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J Immunol. 2007;178:771–777. doi: 10.4049/jimmunol.178.2.771. [DOI] [PubMed] [Google Scholar]
- 77.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162–1174. doi: 10.1097/01.mib.0000236929.74040.b0. [DOI] [PubMed] [Google Scholar]
- 78.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66:1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]