Abstract
The prevalence and severity of pediatric obesity have dramatically increased since the late 1980s, raising concerns about a subsequent increase in cardiovascular outcomes. Strong evidence, particularly from autopsy studies, supports the concept that precursors of adult cardiovascular disease (CVD) begin in childhood, and that pediatric obesity has an important influence on overall CVD risk. Lifestyle patterns also begin early and impact CVD risk. In addition, obesity and other CVD risk factors tend to persist over time. However, whether childhood obesity causes adult CVD directly, or does so by persisting as adult obesity, or both, is less clear. Regardless, sufficient data exist to warrant early implementation of both obesity prevention and treatment in youth and adults. In this Review, we examine the evidence supporting the impact of childhood obesity on adult obesity, surrogate markers of CVD, components of the metabolic syndrome, and the development of CVD. We also evaluate how obesity treatment strategies can improve risk factors and, ultimately, adverse clinical outcomes.
Introduction
The prevalence and severity of obesity, defined as BMI >95th percentile for age,1 has increased dramatically for all age groups over the past 30 years.2 Obesity prevalence rose from approximately 5% to 17% in children and adoles cents over this time period,2 and has subsequently been linked with increasing prevalence of type 2 diabetes mellitus (T2D), hypertension, and atherosclerosis.3–6 This trend in childhood obesity raises concerns about accelerated development of cardiovascular disease (CVD), and the need to prevent such adverse outcomes. Few longitudinal studies specifically link childhood obesity to CVD outcomes in adulthood, as the gradual athero sclerotic process takes decades to evaluate. Nevertheless, three major lines of evidence support the relationship between childhood obesity and adverse outcomes later in life. First, childhood obesity is likely to persist into adulthood, parti cularly when the child is older at the time of evaluation, severely obese, or has a family history of obesity (Table 1). Persistence of obesity is important because adult obesity is clearly associated with increased rates of CVD.7 Secondly, a cross-sectional association exists between childhood obesity and risk factors for CVD, such as insulin resistance, hypertension, dyslipidemia, hepatic and visceral adi posity, and inflammation, and between childhood obesity and intermediate surrogate markers of CVD progression such as vascular imaging and measures of vascular stiffness. These variables also tend to persist over time, suggest ing that high-risk status, once reached, is maintained. This line of evidence is bolstered by research demonstrating that the absence of risk factors confers substantial lifetime protection from CVD. Moreover, in most studies, weight loss not only favorably modifies CVD risk, but some reversibility of early onset vascular wall changes can be demonstrated.8,9 The third, more-direct line of evidence comes from studies providing longitudinal views of the relationship between childhood obesity and actual adverse CVD outcomes in adulthood.10–12 In this Review, we examine the evidence supporting the impact of childhood obesity on CVD development and explore the metabolic consequences of pediatric obesity, indicating mechanisms that increase CVD risk (Figure 1). We also evaluate the effects of strategies for the treatment of obesity on risk factors for CVD and, ultimately, adverse clinical outcomes.
Table 1.
Longitudinal studies demonstrating persistence of adiposity from childhood to adulthood
| Study | Baseline year; study size (n); participant age | Follow-up year; study size (n); participant age | Association between childhood obesity and adulthood obesity | Unique data or comments |
|---|---|---|---|---|
| Follow-up of the Harvard Growth Study12 | 1922–1935; 508; 13–18 years | 1988; 425; 72 ± 1 years | 52% of survivors who were obese in youth remained obese in old age; adolescent BMI predicted adverse outcomes independent of adult BMI | 55 year follow-up; all-cause and CHD mortality in men; weight unknown in subjects deceased at follow-up (32%) |
| Bogalusa Heart Study14,17,21,22,24,55 | Multiple; 16,000 overall; 0–17 years | Multiple; variable; Up to 38 years | Baseline BMI and adiposity correlated with adult BMI (r=0.66); 62% in highest childhood BMI quartile persisted to highest BMI quartile in adulthood; highest childhood BMI and age most likely to persist to high adult BMI; even BMI at 2–5 years correlated with adult BMI | NHW and AA, community-based study |
| NHLBI Growth and Health Study50 | 1986; 2,379 girls; 9–10 years | 1998–2001; 2,054; yearly until 21–23 years | Overweight girls were 11–30-times more likely to be obese adults; relationship between CVD risk factors and weight already present at 9 years of age | NHW and AA, females; large cohort; good retention |
| Minneapolis Children's Blood Pressure Study78 | 1977; 1,207; 7.7 years | 1993–1995; 679; 23.6 years | Childhood and adulthood BMI and weight correlated; childhood weight gain related to adult CVD risk factors | Data on IR |
| Cardiovascular Risk in Young Finns Study128,129 | 1980; 3,596; 3–18 years | 2001; 2,283; 24–39 years | Childhood BMI correlated with adult BMI (r=0.45, P<0.0001); obesity persisted from youth to adulthood | Large cohort; good retention; >20 years of follow up |
| Muscatine Coronary Risk Factor Project130,131 | 1971; 8,909; 9–18 years | 1981; 2,631; 23, 28, and 33 years | Correlation between child and adult obesity quintiles: r=0.51–0.88 for weight; r=0.58–0.91 for BMI; 49–70% and 48–75% remained in upper quintiles; 31% from upper/lower quintiles changed groups | Population based; 15–24 years of follow up |
| Copenhagen Male Draftees Study132 | 1937–1963; 93,800; 7 years | 1988; 1,400; time of military draft | Compared 429 severely obese young adults to 1% random sample of draftees; risk of adult obesity increased exponentially over childhood BMI range | Large population-based cohort; males |
| Australian Schools Health and Fitness Survey133 | 1985; 8,498; 7–15 years | 2001–2005; 4,571; 24–34 years | RR=4.7 for obese child to become obese adult; proportion of adult obesity attributed to childhood obesity 6.4% in males and 12.6% in females | Australian cohort |
| 1958 British Birth Cohort134 | 1958; 17,378; birth | 1965–1991; 11,407; 7, 11, 16, 23, and 33 years | Correlation between child (7 years) and adult (33 years) BMI (r=0.33 male, r=0.37 female), but only 17–18% of obese 33-year-olds had been obese at 7 years. Having two obese parents increased risk of adult obesity | British cohort |
| New Delhi Birth Cohort135 | 1969–1972; 2,584; birth | 1998–2002; 1,492; 26–32 years | Greater childhood BMI gain from 5–14 years associated with MetS, IGT, DM, but none obese at 12 years | Indian cohort |
| Newton Girls Study136 | 1965; 793, 8–9 years | 1998; 448, 42.1 years | Premenarchal weight status main influence on adult weight status | Longitudinal data on females |
Abbreviations: AA, African American; CVD, cardiovascular disease; DM, diabetes mellitus; IGT, impaired glucose tolerance; IR, insulin resistance; MetS, metabolic syndrome; NHLBI, National Heart, Lung and Blood Institute, NHW, non-Hispanic white; RR, relative risk.
Figure 1.
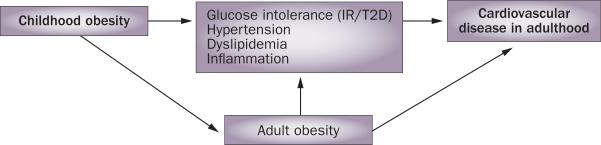
Temporal association between cardiovascular disease and childhood and adulthood obesity, and potential mechanisms that increase cardiovascular risk in adults. Abbreviations: IR, insulin resistance; T2D, type 2 diabetes mellitus.
A predictor of adult obesity
Numerous longitudinal studies conducted worldwide (Table 1) demonstrate that obesity persists from childhood into adulthood; the risk of adult obesity increases with rising severity of childhood obesity and age.12 However, data indicate that childhood obesity can be reversible.13 Although lean youths have a lower risk of becoming obese as adults than obese youths, the current high prevalence of adult obesity means that all young people are currently at risk for adult obesity,2 which indicates that population-wide public health initiatives and targeted risk-factor-based approaches toward preventing adult obesity are needed. The independent association between childhood obesity and adult CVD is less clear, however, as adverse adult CVD profiles could result from persistence of obesity over many years rather than from the independent effects of childhood obesity.14
Birth weight
Various cut-off points for ‘high’ and ‘low’ birth weight have been used in practice. However, importantly, this variable is a continuum and we have not referred to exact cut-off points to emphasize the continuous nature of birth weight as a risk factor. High birth weight, parti cularly in association with maternal gestational diabetes, is associated with obesity in childhood and adulthood (Box 1). For example, among 252,961 Danish youths born between 1936 and 1983, the proportion of individuals who were overweight at ages 6–13 years increased consistently with each increase in birth-weight category.15 In the Bogalusa Heart Study, the offspring of parents with T2D, especially black females, had significantly higher birth weight, weight at all ages, and (independent of adult weight) higher levels of insulin, glucose, tri glycerides, VLDL cholesterol, and LDL chol esterol in adulthood.16 Among 506 indivi duals from South India, high ponderal index (kg/m3) was associated with T2D at ages 39–60 years.17 Whereas high birth weight seems to predict pediatric and adult obesity, low birth weight and thinness during infancy are linked to reduced lean body mass and to adulthood visceral adiposity, hypertension, CVD, and T2D, potentially owing to permanent changes in hormone secretion or sensitivity.18 Thus, a ‘u-shaped’ curve for the relationship between birth weight and later obesity seems to exist. In the Bogalusa Heart Study, birth weight was inversely associated with systolic blood pressure (SBP) and diastolic blood pressure (DBP).19 Determinants of infant body composition such as fat mass, lean mass, and visceral and hepatic adi posity, in addition to birth weight alone, will allow more-subtle dissection of these relationships. Further research is required on the relationship between potentially avoidable preconception and prenatal maternal factors, such as maternal diet, lipemia, glycemia, and activity levels that impact birth weight, length, and body composition, and how they influence the risk of obesity and CVD in adulthood.
Patterns of weight change
Studies of birth weight support a relationship between prenatal factors and later obesity. However, the results of the Danish study discussed above also argue for early post-natal environmental influences because the obesity rates increased even though the birth rate distribution remained stable over the study period.15 In some studies, an early postinfancy BMI rebound (age <5.5 years for boys and <5 years for girls) correlated with increased adult BMI, and could be an important modifiable, postnatal factor.20,21 In a longitudinal study of 8,760 people born in Helsinki, Finland between 1934 and 1944, both low weight gain between birth and 1 year of age, and early BMI rebound, predicted later adult obesity and T2D.22 However, Freedman et al. reported that the association between BMI rebound and adult obesity was not independent of childhood BMI in the Bogalusa Heart Study;23 therefore, the importance of BMI rebound remains controversial. Another modifiable early postnatal factor that has not yet been studied and is deserving of research is whether the standard high-calorie supplementation in infants and toddlers of low BMI or low birth weight is beneficial or could actually promote later obesity and CVD.
Physical activity
Another factor affecting adult weight is physical activity level in childhood. In a cohort of 1,319 youths who were followed-up for 21 years in the Young Finns study, both decreasing activity level and persistent inactivity over time predicted adult obesity.24 Physical inactivity persisted more strongly than physical activity, and constantly active individuals had a better coronary risk profile than those who were constantly inactive.25 Maintaining a high level of physical activity from youth to adulthood was independently protective of abdominal obesity in women, but not in men.24 These findings suggest that declining physical activity over time could contribute to the development of abdominal obesity in women. Maximal exercise capacity is reduced in obese youths, and even more substantially in those who also have T2D.26 However, whether obesity or diabetes reduce the ability to exercise, or whether intrinsic abnormalities limiting ability to exercise increase risk for obesity and hyperglycemia, is yet to be determined.
Links with surrogate CVD markers
CVD events remain rare in pediatric studies unless large cohorts are followed for more than 30–40 years. Although such cohorts are invaluable, and are required for definitive conclusions, practicality and cost often mandate the initial use of surrogate CVD markers as alternatives to actual CVD events. In adults, CVD biomarkers are useful at multiple points along the pathophysio logical pathway as indicators of disease traits, disease state, rate of progression, or as surrogate end points for treatment trials.27 Examples of commonly used CVD surrogate bio markers include anatomical measures (carotid intima–media thickness [CIMT] and coronary artery calcification [CAC]), physio logical measures (blood pressure, endothelial function, and vascular stiffness) and soluble biomarkers (lipids and inflammatory markers). In addition, a growing body of research suggests that pediatric cardiometabolic risk factors are in fact associated with noninvasive measures of vascular morphology and function.28,29 To be useful in pediatrics, the early presence of surrogate markers must predict later CVD. Potential shortcomings of surro gate markers in children include the facts that surrogates do not cause disease, are only part of a multipathway process, can be inappropriately sensitive or responsive to inter vention, could measure effects independent of inter ventions or often lack methodological standardization.28
In 2009, the AHA published recommendations on the use of surrogate markers of CVD—including CIMT, CAC, endothelial function, and arterial stiffness—in pediatric patients.29 The primary conclusion from this scientific statement was that risk factors associated with CVD develop ment are increasingly prevalent in youth, necessitating develop ment and standardization of non invasive methods of CVD risk profiling to target the highest- risk youths for the most-aggressive interventions.29 In particular, an urgent need was identified for normative data specific for age, race, and sex, longitudinal studies to determine normal age-related and puberty-related changes in the measures, correlations to better-established pediatric intermediate target-organ end points (left ventricular hypertrophy, microalbuminuria), and studies to evaluate improvement in vascular function with treatment of cardiovascular risk factors.29
Anatomical and physiological surrogates
Early atherosclerosis can involve the endothelia of many arteries. The peripheral arteries can be measured noninvasively (a requisite for pediatric studies) and are, therefore, used as surrogates for the coronary arteries. CIMT is a marker of preclinical atherosclerosis associated with both the severity and extent of coronary artery disease (CAD) and predicts the likelihood of CVD events in adults.30
In the Bogalusa Heart Study, participants were examined at least twice during childhood with an average follow-up period of 26.4 years (final age 25–44 years).31–33 Significant predictors of increased CIMT in adulthood were childhood plasma triglyceride levels in white males; LDL-cholesterol levels in white and black females; SBP in white females and black males; and BMI in black females.31 Overall, childhood LDL-cholesterol level and BMI, cumulative LDL-cholesterol and HDL-cholesterol burden, and adult LDL-cholesterol and HDL-cholesterol levels, BMI, and SBP were associated with adult CIMT.32,33 The impact of childhood BMI on adult CIMT persisted even after adjustment for adult BMI.33 Similarly, in the Young Finns Study of children aged 3–18 years assessed in 1980, childhood BMI, LDL-cholesterol level, SBP, and smoking, and adult BMI, SBP, and smoking were associated with CIMT measured 21 years later.34 The associations between childhood risk factors and adult atherosclerosis were stronger in men than in women, consistent with observed earlier CVD development in men.34 The Muscatine study investigators examined adults aged 33–42 years, who had been followed since the age of 8–18 years. Multivariate analysis demon strated that childhood BMI in women, and levels of total cholesterol and LDL cholesterol in childhood as well as adulthood age, in both sexes, predicted adult CIMT.35 In a model incorporating CVD risk over time, childhood BMI and levels of LDL cholesterol and triglycerides were predictive of CVD in women, and DBP and levels of LDL cholesterol and HDL cholesterol were predictive in men.35 In the Muscatine study, aortic intima– media thickness was found to correlate more strongly with CVD risk factors in adolescents than did CIMT in young adults.36
Another method for estimating atherosclerosis is electron- beam CT or spiral or helical CT imaging. Electron-beam CT is highly sensitive in defining the location and extent of CAC, which predicts CAD, and future coronary events.37 Mahoney et al. demonstrated a significant relationship between levels of coronary risk factors measured during childhood and young adulthood and the presence of CAC in young adulthood.38 Pediatric experience with CAC assessment is extremely limited. Gidding et al. demonstrated significant CAC in seven of 29 adolescents and young adults with heterozygous familial hypercholesterolemia, and that obesity increased the likelihood of CAC.39 However, radiation exposure associated with CT currently limits its utility in most young people.
Brachial artery reactivity assessment by ultrasonography, known as flow-mediated dilation (FMD), is the most well-established technique to evaluate arterial function in adults. Impaired FMD correlates with CVD risk factors in adults and independently predicts CVD events.40 Studies have shown that obese youths have reduced FMD,41 even when compared with normal-weight control indivi duals matched for blood pressure, chol esterol, and glucose, and that FMD correlated with BMI.42 Thus, FMD appears to be a good predictor of CVD events in adults, and obesity already appears to be impacting FMD in youth.
Arterial stiffness is another noninvasive surrogate marker of vascular health. In the Bogalusa Heart Study, decreased brachial distensibility was associated with greater adiposity, hypertension, and dyslipidemia in adults aged 19–37 years.43 This measure has also been associated with increased CAC.44 In children, brachial distensibility correlated inversely with total cholesterol level,45 adiposity, DBP, inflammation, and insulin resistance.46 In the Young Finns study, both childhood and adolescent CVD risk factors predicted abnormal pulse wave velocity in adulthood.9 Therefore, childhood CVD risk factors, including BMI (especially in females), are associated with anatomical surrogates of CVD in adults. Additional data are needed on the predictive ability of anatomical surrogates used in youth on later adult CVD.
Metabolic syndrome
The metabolic syndrome (MetS) is a constellation of related factors known to increase the risk of developing CVD and T2D,47 and is also (sometimes inappropriately) used as a surrogate for CVD. Owing to the dynamic effects of growth and puberty on cardiometabolic factors, the exact definition of pediatric MetS remains controversial, but its general components include obesity, insulin resistance (hyperinsulinemia, hyperglycemia), hypertension, dyslipidemia (low HDL-cholesterol and high triglyceride levels), hepatic and visceral adiposity, and inflammation.48 Few studies follow these factors from youth to adulthood; therefore, the extent to which pediatric MetS predicts adult CVD is unclear. In addition, components of childhood MetS are specific, but not sensitive, in predicting adult MetS.
As the prevalence and severity of pediatric obesity increases, so does the prevalence of childhood MetS, which occurs in 38.7% of moderately obese and 49.7% of severely obese youths.49 In the National Heart, Lung, and Blood Institute Growth and Health study,50 children who were overweight had significantly increased waist circumference, SBP and DBP, and triglycerides levels, and lower levels of HDL cholesterol. Pediatric obesity is also associated with increased MetS prevalence in adulthood. In the Princeton Lipid Clinics Follow-up study,51 adult MetS risk increased by 24% for every 10-point increase in childhood BMI percentile.51 Childhood BMI was the strongest predictor of adult MetS in the Muscatine study.52 Adults with MetS in the Fels longitudinal study53 had detectable differences in BMI by the age of 8 years in boys and by 13 years in girls, and in waist circumference by 6 years of age in boys and by 13 years in girls. Therefore, despite difficulties in defining key components of the MetS, strong evidence supports obesity, especially central, as an essential correlate of cardiometabolic risk.53
In the Bogalusa Heart Study, clustering of MetS variables persisted over 8 years, more strongly than did individ ual MetS components, and the degree of clustering increased with increasing baseline age, BMI (unadjusted or adjusted for insulin levels), and change in weight over time.54–56 Similarly, combined data from the Fels longitudinal, Muscatine, and Princeton-Lipid Research Clinic Cohort studies demonstrated that clustering of MetS variables,57 and the addition of family history58 increased the likelihood of MetS components continuing into adulthood. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score (comprised of sex, age, lipoprotein levels, and the presence of hypertension, obesity and hyperglycemia) predicted adult atherosclerosis as measured by CIMT in the Young Finns study,58 and 10–15 year follow-up CAC scores in the Coronary Artery Risk Development in Young Adults (CARDIA) study.59 Thus, pediatric obesity increases the likelihood of MetS variable clustering, which, along with severity, persist over time and predict atherosclerosis as measured by CIMT and CAC. In the following sections, we review the evidence for the contribution of childhood obesity to the risk of each individual MetS component in adulthood.
Insulin resistance
Insulin resistance is considered to be an essential component of the MetS, but its direct measurement with the hyperinsulinemic-euglycemic clamp method is relatively invasive and technically difficult to perform. Therefore, estimates such as fasting insulin and homeo-static model assessment for insulin resistance (HOMAIR) are frequently employed as alternatives to direct measurement of insulin resistance. Limitations of these estimates include the lack of a normal range for insulin levels in children, poorly standardized insulin assays, loss of validity if the patient is not fasting, and failure to consider the impact of insulin clearance.60 Fasting hyperinsulinemia, as a surro gate for insulin resistance, is associated with athero sclerosis and cardiovascular morbidity.61 Evidence suggests that hyperinsulinemia itself might directly promote CVD62 or, alternatively, that insulin is antiatherogenic, arguing that a lack of insulin action causes CVD.63
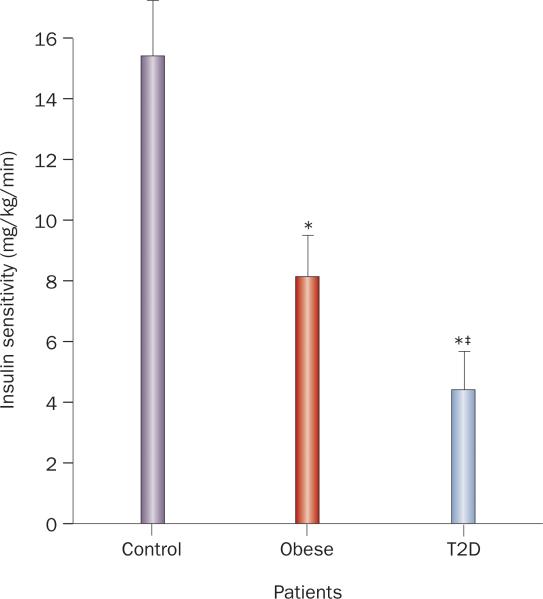
In general, childhood obesity correlates with insulin resistance, as demonstrated by a hyperinsulinemic-euglycemic clamp study in adolescents (Figure 2), in which glucose disposal rate expressed per kg of body weight or per kg of fat-free mass was significantly lower in obese adolescents than in lean, control adolescents.26 Similarly, BMI was the best predictor of insulin resistance by intravenous glucose tolerance testing in 97 healthy indivi duals aged 9.7–14.5 years.64 In addition, among 243 obese children aged 6–14 years, insulin level and HOMA-IR were higher than in normal-weight controls.65 However, obesity is not synonymous with insulin resistance; obese youths with T2D are significantly more insulin resistant than youths without diabetes matched for age, sex, and BMI (Figure 2).66 Insulin resistance in obese youth varies by ethnicity,67 and many studies show effects of insulin resistance on CVD outcomes, independent of BMI.68–71 In addition, cardiometabolic risk varies markedly in obese adults with the degree of insulin resistance.72
Figure 2.
Insulin resistance in adolescents with obesity and T2D. Insulin sensitivity in mg/kg/min as determined by hyperinsulinemic-euglycemic clamp in adolescents (aged 12–19 years): normal weight/control (n = 12), obese (n = 13), and those with T2D (n = 14). All three groups were similar in age, pubertal stage, and activity level; the obese and T2D groups were similar in BMI. *P <0.001 versus control. ‡P <0.02 versus obese.26 Abbreviation: T2D, type 2 diabetes mellitus.
In children aged 5–17 years from Bogalusa, LA, USA, BMI and adiposity correlated with fasting insulin, glucose, and insulin:glucose ratio.68,73–76 Overweight youths had higher adult insulin and glucose levels,55 and the prevalence of obesity was increased by 36-fold among those whose insulin levels were consistently in the highest quartile68 when compared with those consistently in the lowest quartile. In a cohort from Minneapolis, MN, USA, BMI in 13 year-olds was highly correlated with BMI and hyperinsulinemic-euglycemic clamp-derived glucose utiliza tion at age 22 years.77 In addition, the rate of weight gain, starting at 7.7 years, correlated with fasting insulin levels in young adulthood (23.6 ± 0.2 years) in another cohort from Minneapolis.78 Weight gain is frequently ascribed to hyperinsulinemia; however, obesity was instead shown to lead to hyperinsulinemia in three longi tudinal cohorts (427 children, baseline age 5–7 years; 674 adolescents, baseline age 12–14 years; and 396 young adults, baseline age 20–24 years).79 Moreover, another longitudinal cohort (n = 111, baseline age 9.7–14.5 years) demonstrated that children who are more insulin resistant during puberty have decreased subcutaneous fat gain over puberty when compared with those who are less insulin resistant.80
The relationship between plasma glucose and insulin levels and traditional CVD risk factors, even in children without diabetes, suggests the importance of subtle abnormalities in carbohydrate metabolism early in the natural history of CVD. Progression to T2D confers an even higher risk of CVD than obesity alone and is increasingly prevalent among young people, in parallel with the obesity epidemic, heralding a potential rise in the prevalence of early cardiovascular complications.81 In a series of youths with T2D,82 mean BMI ranged from 26–38 kg/m2; thus, obesity is nearly universal in pediatric patients with T2D and is likely to contribute to future CVD. In pediatric hyperinsulinemic-euglycemic clamp studies, both adi posity and insulin resistance independently predicted CVD risk factors, but adiposity and insulin resistance combined predicted greater CVD risk than the sum of individual risks.70
Hypertension
Cross-sectional and longitudinal studies link pediatric obesity to hypertension, a MetS component. In the Fels longitudinal cohort, childhood blood pressure mediated the effects of childhood BMI on adult hypertension, but increased BMI, not increased blood pressure, explained trends in adult hypertension.83 Among 23,191 male and 3,789 female adolescents from the Metabolic Lifestyle and Nutrition Assessment in Young Adults cohort, BMI at the age of 17 years was strongly and independently associated with adult hypertension, even when adjusted for blood pressure at age 17 years, particularly in males.84 BMI at age 30 years partially, but not completely, attenuated this association, particularly in girls.84 In the Bogalusa Heart Study, childhood BMI and age of obesity onset predicted adult hypertension, but was attributable to the strong persis tence of weight status from childhood to adulthood.14 Thus, additional data are needed to assess the independent relationship between childhood weight status and adult hypertension. Regardless, pediatric hypertension associated with obesity cannot be ignored, as hypertension in cross-sectional studies of obese youths with T2D is documented to co-exist with left ventricular hypertrophy as early as adolescence.26
Dyslipidemia
Dyslipidemia (high levels of triglycerides and low levels of HDL cholesterol) are additional MetS constituents contributing to CVD risk. In an analysis of data from the Young Finns, Bogalusa Heart, and Childhood Determinants of Adult Health (CDAH) studies, Magnussen et al. concluded that adolescent dyslipidemia is more strongly associ ated with adult CIMT than change in lipid levels over time, and that dyslipidemia combined with obesity is most strongly associated with increased CIMT.85 In the Young Finns Study, participants with type IIb dyslipidemia (high LDL-cholesterol and triglyceride levels) more commonly had obesity and MetS than a family history of early-onset CHD, suggesting that lifestyle, rather than genetic influences, predominate in the atherogenic type IIb dyslipidemia phenotype.86
Data from the Bogalusa Heart Study suggest that childhood non-HDL-cholesterol levels persist and best predict adult dyslipidemia and other CVD risks.14 Change in BMI over time was the next best predictor of adult dyslipi demia,87 but was not independent of adult obesity.14 Combined data from the CDAH, Young Finns, and Bogalusa Heart studies, indicated that childhood BMI was the best predictor of adult HDL-cholesterol levels.88 Of note, failure to account for expected fluctuations in lipid metabolism related to age, sex, and puberty could account for variability in some longitudinal data.
Hepatic and visceral adiposity
Hepatic and visceral adiposity are highly correlated with obesity and, independently, with all MetS components. In a large pediatric autopsy study, the prevalence of non-alcoholic fatty liver disease (NAFLD) was four times higher in obese youth than in normal-weight youth, after adjustment for age, sex, and ethnicity.89 Elevated amino transferase levels have been reported in 10–25% of obese youth and in half of young people with T2D, suggesting hepatic inflammation.90 The histological pattern of pediatric NAFLD differs from adults, and fibrosis is reported more commonly in children,91 supporting the idea that unique mechanisms might underlie pediatric NAFLD.
In the longitudinal West of Scotland coronary prevention study,92 NAFLD independently increased the risk of T2D and CVD. This association is likely to be caused by hepatic insulin resistance and overproduction of glucose, VLDL cholesterol, C-reactive protein (CRP), and coagulation factors.93,94 Thus, NAFLD is considered to be the hepatic component of the MetS. In the Bogalusa Heart Study, children who had persistently elevated amino-transferase levels at 12 years of age had abnormal BMI, insulin resistance index, LDL-cholesterol levels, and MetS variables.95 Therefore, NAFLD and visceral adiposity are tightly tied to pediatric obesity and insulin resistance and, although often clinically silent in youth, appear to be a warning sign for future T2D and CVD.
Inflammation
Inflammatory cytokines are associated with CAD in adults, one of the most sensitive being CRP (high sensitivity test; hsCRP), hence its frequent use as a CVD biomarker.96 By contrast, the adipokine adiponectin is negatively correlated with CAD risk in adults, and could be a cardioprotective factor.97 Including hsCRP as a MetS component has been proposed because inflammation might be necessary for full manifestation of MetS.98 In a multivariate analysis of 1,083 individuals in their mid-thirties from the Bogalusa Heart Study population, obesity, not estimated insulin resistance, was the major contributor to increased hsCRP levels.99 Similarly, in 342 healthy youths aged 10–16 years from Minneapolis, hsCRP level was most-strongly related to adiposity.100 After adjustment for adiposity, hsCRP differed by race (higher in black children than in white children), but was not significantly associated with fasting insulin, insulin resistance measured by hyperinsulinemic-euglycemic clamp, or other MetS criteria.100 Finally, adiposity also appears to be the major determinant of hsCRP levels in pubertal children, with a small, independent effect of physical fitness.101 Therefore, obesity appears to be indepen dently related to hsCRP from a very young age, but little longitudinal data exist for the stability of hsCRP over time or its predictive power for adult CVD when elevated in youth.
Childhood obesity and CVD
Theoretical data
In 2007, adolescent obesity in the USA was projected to increase obesity prevalence at age 35 years from 25% to 30–37% in men and from 32% to 34–44% in women by 2020.102 These increases in obesity were projected to subsequently raise the prevalence of CHD by 5–16%.102 Such projections underscore the potential importance of worsening childhood obesity on future CHD103 and declining life expectancy,104 especially since they could underestimate the currently predicted future prevalence of CHD by not accounting for concurrent rising adult obesity rates or early atherogenesis caused by pediatric-onset obesity.
Epidemiological data
Mortality data from American Indian youths—a population particularly plagued by obesity and its complications—also indicate that children should be the focus of CVD mortality prevention. For example, among 4,857 American Indian children born between 1945 and 1984, endogenous death rates in the highest BMI quartile were 2.3 times as high as those in the lowest BMI quartile.105 Similarly, in a British study of 2,399 children aged 2–14 years, an associ ation between childhood BMI and death from all causes and from ischemic heart disease was reported.10 In a Danish study of 276,835 individuals, the risk of nonfatal and fatal CHD events in adulthood was associated with BMI at 7–13 years of age in males and BMI at 10–13 years of age in females.11 The associations were linear for each age, and risk increased across the entire BMI distribution and with increasing childhood age.11 Differences between the sexes were also noted in the Harvard growth study, where overweight adolescents had an increased risk of CHD and atherosclerosis-associated morbidity in adulthood.12 However, CHD and all-cause mortality was only increased among men who had been overweight in youth.12 Childhood BMI was the only signifi cant predictor of adult left ventricular dilatation among participants in the Bogalusa Heart Study.106 Finally, among a cohort of 37,674 prospectively followed Israeli male military personnel, elevated BMI at age 17 years was a significant predictor of T2D (hazard ratio [HR] for the lowest to highest decile 2.76, 95% CI 2.11–3.58) and of angiographically proven CHD (HR 5.43, 95% CI 2.77–10.62).107 When controlling for current BMI, T2D was no longer associated with adoles cent BMI, but the associ ation with CHD was strengthened (HR 6.85, 95% CI 3.3–14.21), suggesting that the processes contributing to CHD begin earlier and more gradually that those causing T2D.107
Not all studies show positive associations between childhood BMI and CVD or mortality in adults, however. An investigation of 11,106 Scottish children (mean age 4.9 years)108 and a meta-analysis of three studies (age range of participants 2–22 years)109 did not detect effects of childhood BMI on CHD and ischemic heart disease in adulthood. Possible explanations for the negative findings were lack of statistical power, cohorts formed before the current obesity pandemic, or younger ages of children at entry where BMI might have reduced prognostic power. Moreover, high childhood BMI might be predictive only because it persisted as high adult BMI, rather than exerting independent effects (Figure 1). However, the result of the Harvard Growth Study refute this argument, as adolescent BMI independently and more-powerfully predicted CHD and overall morbidity and mortality than adulthood BMI.12
Autopsy data
CVD risk factors and surrogate markers are already identifi able in overweight children and appear to be amplified by obesity. However, the ultimate question is whether excess childhood weight has an impact on the hard outcomes of directly measured atherosclerosis, cardiovascular events, and death. Two early studies suggested that heavier American110 and Swedish111 children had greater risk for CVD110,111 and mortality.111 Autopsy analyses from the Bogalusa Heart4 and PDAY5 studies further demonstrated that the extent of early aortic and coronary atherosclerosis is directly associated with the severity of obesity in childhood and adolescence. In the PDAY study particularly, the findings of autopsy specimens from individuals aged 15–34 years asserted the importance of nonlipid CVD risk factors in youth with a favorable lipoprotein profile112 and that coronary atherosclerosis is significantly associated with BMI and visceral adiposity in young men.5
Treatment of childhood obesity
Effects on obesity
In 2004, Atlatis et al. published a systematic review of pediatric randomized physical activity trials that included 14 studies with a total of 481 overweight youths.113 The investigators concluded that 155–180 min per week of moderate-to-high intensity aerobic exercise reduces body fat in youth, but with inconclusive effects on weight and central adiposity.113 A more-recent Cochrane meta-analysis included 64 randomized, controlled primary pediatric obesity trials (n = 5,230), with at least 6 months of follow-up, that were published between 1985 and 2008.114 Trials included lifestyle (six dietary, 12 physical activity, 36 behavioral) or drug (10 metformin, orlistat, or sibutramine) interventions versus standard care or self help. No surgical intervention studies met the inclusion criteria. The researchers concluded that lifestyle interventions improve weight at 6–12 months in children and adolescents, that orlistat or sibutramine (if tolerated) might be helpful additions in adolescents, and that future research should focus on psychosocial determinants of behavior change, strategies to improve clinician–family interaction, and cost-effective primary care and community programs.114 In 2010, Whitlock et al. reviewed 2,786 abstracts and 369 full-text articles and found 15 “fair-quality to good-quality” short-term obesity trials of individuals aged 4–18 years.13 Comprehensive medium-to-high-intensity behavioral interventions were determined the most effective (1.9–3.3 kg/m2 difference in BMI favoring intervention at 12 months), whereas combined interventions (behavioral change plus medication) resulted in small (orlistat 0.85 kg/m2) or moderate (sibutramine 2.6 kg/m2) BMI reductions in adolescents while on medication, but with a greater incidence of adverse effects.13 Sibutramine was removed from the market in Europe and the USA on 8 October 2010 after clinical trials showed an increased risk of heart attacks and strokes among users.115 A meta-analysis by Brown and Summerbell of controlled school-based lifestyle interventions (minimum duration 12 weeks) included 38 studies published in 2006–2007.116 Approximately 39% of school-based studies showed a short-term reduction in BMI, although insufficient evidence existed regarding long-term efficacy of pediatric dietary interventions. The investigators concluded that, although inconsistent, overall findings suggest that school-based physical activity interventions, particularly among girls, help prevent pediatric obesity in the short-term.116
Despite the large number of pediatric weight-loss studies, data are lacking on the long-term effectiveness of such interventions. Additional areas for research in young people might include combinations of short-term interventions (calorie restriction, appetite suppressants, or both) for initial weight loss, followed by long-term exercise and lifestyle change for weight maintenance. Further research is also needed on screening tools and genetic predictors of response to calorie restriction, exercise, and appetite suppressant interventions, to allow a more cost-effective focus on those young people who are most likely to respond to particular interventions.
Although surgical approaches to weight control are also being used in limited numbers of severely obese youths with substantial comorbidities, current studies are limited in size and number. Further study is required regarding the safety and long-term effectiveness of obesity surgery in youth. Regardless, neither currently available appetite suppressants nor surgery are adequately safe, realistic, or cost-effective approaches for the majority of currently obese youths.
Effects on metabolic syndrome components
MetS components are modifiable determinants of adult CVD risk, and are suitable targets for interventions. In adults, weight loss of 10–15% of body weight improves glucose tolerance, and reduces waist circumference, visceral and hepatic fat, blood pressure, and insulin resistance.117 The addition of physical activity increases HDL-cholesterol levels, and further reduces waist circumference and hepatic fat.117 Exercise training improves insulin resistance and endothelial function beyond the benefits of glycemic and blood pressure control in children.118 Among 126 children aged 6–14 years who reduced their standard deviation score of BMI (SDS-BMI) in response to a 1-year intervention (physical exercise, behavioral therapy, and education on nutrition), fasting insulin levels and HOMA-IR improved significantly and were sustained 1 year after the intervention.65 In a study by Reinehr and colleagues, a similar decrease in SDS-BMI was observed among obese children aged 10–14 years who received a 1-year lifestyle intervention (n = 124) or a 6-week inpatient intervention (n = 119); however, only the longer, outpatient intervention reduced fasting insulin levels.119 During a 1-year obesity intervention program, BMI-SDS change was associated with change in HOMA-IR among 57 obese children aged 6–14 years.120 In addition, the results of a randomized, controlled trial showed that a 1-year intensive family-based program including exercise, nutrition, and behavior modification improved BMI, HOMA-IR, and blood pressure for up to 12 months in obese indivi duals aged 8–16 years.121
Weight loss decreases the prevalence of pediatric NAFLD in a dose–response manner.122 Nobili et al. showed that a 12-month program of diet and physical exercise advice in youth (age 3.0–18.8 years) significantly decreased BMI, ultrasonographic steatosis, and fasting levels of glucose, insulin, lipids, and aminotransferases.123 In small studies, metformin has been used effectively in adolescents with T2D to decrease BMI and improve glucose tolerance124 and to improve aminotransferases, steatosis, and estimated insulin resistance in youths with NALFD.125 Levels of hsCRP and adiponectin also tend to improve with pediatric interventions that result in weight loss of ~5%, regardless of the type or length of intervention.126 Thus, although success rates of pediatric weight-loss programs are variable, clear improvements in surrogate markers of CVD occur when weight loss is actually achieved.
Effect on intermediate markers of CVD
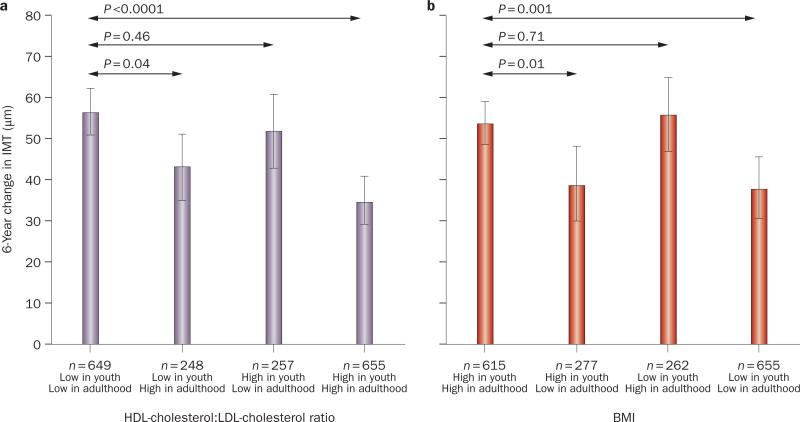
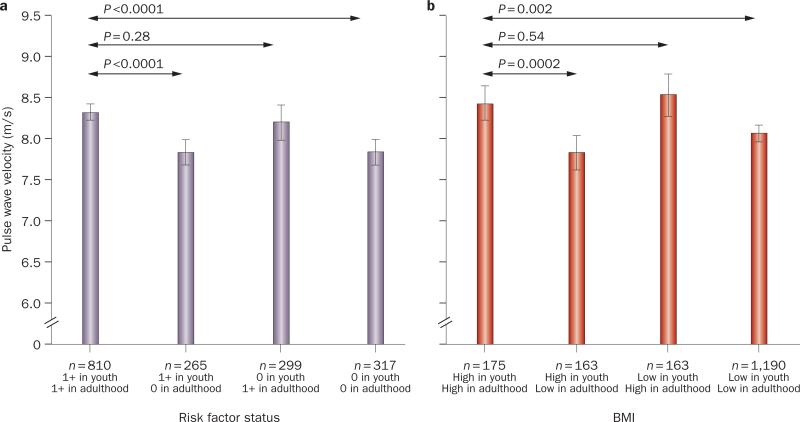
An important public health question addressed by the Young Finns study8 is whether improvement in CVD risk factors in young adulthood can reverse the effect of childhood risk factors on future atherosclerosis. Improvement in HDL-cholesterol:LDL-cholesterol ratio and obesity from childhood to adulthood was associated with reduced CIMT progression (Figure 3). However, CIMT still progressed more than in participants who had normal baseline and follow-up HDL-cholesterol:LDL-cholesterol ratio and BMI.8 Frequent fruit intake and physical activity in childhood were also associated with lower adult CIMT.8 Similarly, the number of CVD risk factors in childhood and adulthood in the Young Finns Study were associated with increased pulse wave velocity in adulthood, but reduction in the number of risk factors and improvement in BMI over time were associated with lower pulse wave velocity in adulthood (Figure 4).9 Therefore, although pediatric lifestyle remains important, these data also suggest that vascular changes in childhood can at least be improved with appropriate improvement in CVD risk factors during young adulthood.
Figure 3.
Relationship between childhood and adulthood cardiovascular risk factors and adult carotid IMT. a Relationships between HDL and LDL cholesterol in childhood (ages 3–18 years) and adulthood (ages 24–45 years) with IMT progression in adulthood (mean [95% CI]). A cut-point of 50th percentile was used in classifying the HDL-cholesterol:LDL-cholesterol ratio as low or high. b Relationships between BMI in childhood and adulthood with IMT progression in adulthood. A cut-point of 50th percentile was used in classifying BMI as high or low. P values are from t-tests. Abbreviation: IMT, intimal–medial thickness. Reproduced from Juonala, M. et al. Lifetime risk factors and progression of carotid atherosclerosis in young adults: the Cardiovascular Risk in Young Finns study. Eur. Heart J. (2010) 31 (14), 1745–1751 by permission of Oxford University Press.
Figure 4.
Relationship between childhood and adulthood cardiovascular risk factors and adult pulse wave velocity. a Relationship between risk score in childhood (ages 3–18 years) and adulthood (ages 24–45 years) with pulse wave velocity in adulthood. b Relationship between BMI in childhood and adulthood with pulse wave velocity in adulthood. Permission obtained from Wolters Kluwer Health © Aatola, H. et al. Lifetime Risk Factors and Arterial Pulse Wave Velocity in Adulthood: The Cardiovascular Risk in Young Finns Study. Hypertension 55 (3), 806–811 (2010).
Conclusions
Strong evidence supports the concept that precursors of adult CVD begin in childhood, with obesity as an important correlate of overall CVD risk. The clearest evidence comes from autopsy studies showing that coronary athero-sclerotic lesions occur in early life and are strongly associated with pediatric obesity, hypertension, and dyslipi demia. Obesity and CVD risk factors tend to persist over time. Observations from pediatric epidemi ology studies over the past several decades further document that obesity, atherosclerosis, and associated risk factors begin in childhood. Lifestyle patterns, such as poor eating behavior, also begin early and influence CVD risk. The question of whether childhood obesity leads to increased adulthood CVD via increased CVD risk factors in childhood or via persistence into adulthood obesity, or both, remains unclear. However, sufficient data exist to warrant both obesity prevention and reduction in youth and adults.
Clearly, individuals who are both obese and have underlying risk factors for CVD, and their families, deserve the most-intensive interventions as these people are at high risk for CVD events and are most-likely to benefit metabolically from weight loss. However, little evidence exists to suggest that adipose triglyceride deposition itself has a defined, independent role in CVD, which has led some to argue that young people with ‘isolated obesity’ should merely be monitored for development of other CVD risk factors, because interventions in this lower-risk population might not be cost-effective. Although a better understanding of these obese, insulin-sensitive children is required, lifestyle interventions should still be considered in youths with isolated obesity owing to the likelihood that they will continue to be obese as adults and will develop the morbidities associated with adult obesity.
Normal-weight children who become obese as adults are also at increased risk of adult morbidity, and under-weight children who become obese adults could be at even higher risk of adult hypertension and renal disease than youths with higher weights.110 Thus, excessive weight gain has negative consequences in all young people. Data show that established obesity is difficult to treat; therefore, early prevention efforts to avoid unhealthy lifestyles and encourage adoption of healthy behaviors are critical in all children and adolescents, not just those who are already obese. The pervasive nature of adult CVD indicates that new strategies for population-based approaches are also needed.
Several areas in pediatric obesity and CVD remain in need of research. Genetic abnormalities that cause severe early obesity have been identified. Affected genes include LEPR (leptin receptor), MC4R (melanocortin 4 receptor), and POMC (proopiomelanocortin).127 However, these gene defects are rare and the remainder of the genome appears to have a minor role in the current obesity epidemic. Genes conferring increased risk for CVD have also been identi fied, but how these genes interact with obesity, or how obesity-associated genes interact with athero sclerosis is, as yet, unclear. Thus, we lack genetic insight into the relationships between obesity, MetS, and CVD. We also lack sufficient understanding of the inter mediate players and biomarkers in the relationship between obesity and CVD. A better understanding is required of the impact of elevated fasting glucose and glycated hemoglobin levels, how they distribute in youth (especially during puberty), and their predictive value for long-term CVD outcomes. A limitation of our current approach to pediatric obesity research is the reliance on surrogate markers, as we do not know how well abnormalities in surrogates such as CIMT or pulse wave velocity persist over time. Further research is required on biomarkers of insulin resistance in youth, their genetic basis, and their persistence over time. Longitudinal studies, with large cohorts of youths, are now required to answer many of these areas of deficiency in our knowledge. Finally, better tools for prevention and treatment of childhood obesity are required, as none of the current methods are particularly successful or cost-effective.
Key points.
■ The prevalence and severity of pediatric obesity have dramatically increased since the late 1980s
■ Precursors of adult cardiovascular disease begin in childhood, with obesity as an important correlate of overall cardiovascular risk
■ Lifestyle patterns begin early in childhood and influence cardiovascular risk
■ Obesity and other cardiovascular risk factors persist over time
■ Obesity prevention and reduction should begin early in childhood to prevent adult cardiovascular disease
■ The pervasive nature of adult cardiovascular disease translates to an urgent need for new population-based obesity-prevention strategies
Box 1 Relationship between early anthropomorphic factors and later outcomes.
Parental diabetes
Associated with increased birth weight,62,65 increased childhood BMI,62 increased adult BMI,62 increased adult risk factors for CVD (increased levels of insulin, glucose, triglycerides, VLDL cholesterol, and LDL cholesterol) independent of adult weight62
Parental obesity
Associated with increased birth weight, but childhood and adult BMI unaffected63
High birth weight
Associated with increases in childhood and adult BMI64 and adult T2D65
Low birth weight
Associated with lower adult lean body mass, increased adult visceral adiposity, and hypertension and T2D,66 and increased adult CHD risk28,29,66
Short birth length
Associated with increased adult CHD risk in females29
Low infancy weight gain
Associated with increased adult BMI and increased adult risk of T2D and CHD74
Early BMI rebound
Associated with increased adult BMI,72–74 but not independent of childhood BMI,75 and increased adult risk of T2D74 and CHD in males of low birth weight28,29
Catch-up height gain
Associated with increased adult risk of CHD in previously short females29
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; T2D, type 2 diabetes mellitus.
Review criteria.
Articles for this Review were selected from the PubMed database, starting with a search for large longitudinal cohort studies (such as the Bogalusa Heart Study, Pathobiologic Determinants of Atherosclerosis in Youth, etc). Next, a search using the following key words was performed: “arterial stiffness”, “atherosclerosis”, “brachial artery ultrasound”, “carotid intima–media thickness”, “cardiovascular disease”, “coronary artery calcification”, “endothelial function”, “heart attack”, “metabolic syndrome” (and its components), “myocardial infarction”, “obesity”, “obesity tracking”, “obesity treatment”, “pediatric”, and “weight loss”. No limit was placed on year of publication and English language was specified for searches as was full-text papers. Reference lists of identified papers were searched for additional relevant papers.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors contributed equally to discussion of content, writing, and reviewing/editing the manuscript before submission and after peer-review. K. J. Nadeau and D. M. Maahs also researched data for the article.
Contributor Information
Kristen J. Nadeau, The Children's Hospital, Department of Pediatrics, University of Colorado School of Medicine, Building A, 13123 East 16th Avenue, Aurora, CO 80045, USA.
David M. Maahs, Barbara Davis Center for Childhood Diabetes, Department of Pediatrics, University of Colorado School of Medicine, 1775 Aurora Court, Aurora, CO 80045, USA.
Stephen R. Daniels, The Children's Hospital, Department of Pediatrics, University of Colorado School of Medicine, Building A, 13123 East 16th Avenue, Aurora, CO 80045, USA.
Robert H. Eckel, Department of Medicine, University of Colorado School of Medicine, 12801 East 17th Avenue, Aurora, CO 80045, USA.
References
- 1.Centers for Disease Control and Prevention Growth charts [online] 2010 http://www.cdc.gov/growthcharts.
- 2.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J. Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 4.Berenson GS, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 5.McGill HC, Jr, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 6.McGill HC, Jr, et al. Association of coronary heart disease risk factors with microscopic qualities of coronary atherosclerosis in youth. Circulation. 2000;102:374–379. doi: 10.1161/01.cir.102.4.374. [DOI] [PubMed] [Google Scholar]
- 7.Flint AJ, et al. Excess weight and the risk of incident coronary heart disease among men and women. Obesity (Silver Spring) 2010;18:377–383. doi: 10.1038/oby.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juonala M, et al. Life-time risk factors and progression of carotid atherosclerosis in young adults: the Cardiovascular Risk in Young Finns study. Eur. Heart J. 2010;31:1745–1751. doi: 10.1093/eurheartj/ehq141. [DOI] [PubMed] [Google Scholar]
- 9.Aatola H, et al. Lifetime risk factors and arterial pulse wave velocity in adulthood: the cardiovascular risk in young Finns study. Hypertension. 2010;55:806–811. doi: 10.1161/HYPERTENSIONAHA.109.145102. [DOI] [PubMed] [Google Scholar]
- 10.Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey Smith G. Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am. J. Clin. Nutr. 1998;67:1111–1118. doi: 10.1093/ajcn/67.6.1111. [DOI] [PubMed] [Google Scholar]
- 11.Baker JL, Olsen LW, Sørensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N. Engl. J. Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N. Engl. J. Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 13.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125:e396–e418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 15.Rugholm S, et al. Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obes. Res. 2005;13:2187–2194. doi: 10.1038/oby.2005.271. [DOI] [PubMed] [Google Scholar]
- 16.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. The relation of parental cardiovascular disease to risk factors in children and young adults. The Bogalusa Heart Study. Circulation. 1995;91:365–371. doi: 10.1161/01.cir.91.2.365. [DOI] [PubMed] [Google Scholar]
- 17.Fall CH, et al. Size at birth, maternal weight, and type 2 diabetes in South India. Diabet. Med. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 18.Barker DJ. Outcome of low birthweight. Horm. Res. 1994;42:223–230. doi: 10.1159/000184197. [DOI] [PubMed] [Google Scholar]
- 19.Mzayek F, et al. The association of birth weight with developmental trends in blood pressure from childhood through mid-adulthood: the Bogalusa Heart study. Am. J. Epidemiol. 2007;166:413–420. doi: 10.1093/aje/kwm098. [DOI] [PubMed] [Google Scholar]
- 20.Prokopec M, Bellisle F. Adiposity in Czech children followed from 1 month of age to adulthood: analysis of individual BMI patterns. Ann. Hum. Biol. 1993;20:517–525. doi: 10.1080/03014469300002922. [DOI] [PubMed] [Google Scholar]
- 21.Williams SM, Goulding A. Patterns of growth associated with the timing of adiposity rebound. Obesity (Silver Spring) 2009;17:335–341. doi: 10.1038/oby.2008.547. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of type 2 diabetes in adult life. Diabetologia. 2003;46:190–194. doi: 10.1007/s00125-002-1012-5. [DOI] [PubMed] [Google Scholar]
- 23.Freedman DS, Kettel Khan L, Serdula MK, Srinivasan SR, Berenson GS. BMI rebound, childhood height and obesity among adults: the Bogalusa Heart Study. Int. J. Obes. Relat. Metab. Disord. 2001;25:543–549. doi: 10.1038/sj.ijo.0801581. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Telama R, Viikari J, Raitakari OT. Risk of obesity in relation to physical activity tracking from youth to adulthood. Med. Sci. Sports Exerc. 2006;38:919–925. doi: 10.1249/01.mss.0000218121.19703.f7. [DOI] [PubMed] [Google Scholar]
- 25.Raitakari OT, et al. Effects of persistent physical activity and inactivity on coronary risk factors in children and young adults. The Cardiovascular Risk in Young Finns Study. Am. J. Epidemiol. 1994;140:195–205. doi: 10.1093/oxfordjournals.aje.a117239. [DOI] [PubMed] [Google Scholar]
- 26.Nadeau KJ, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J. Clin. Endocrinol. Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 28.Tardif JC, Heinonen T, Orloff D, Libby P. Vascular biomarkers and surrogates in cardiovascular disease. Circulation. 2006;113:2936–2942. doi: 10.1161/CIRCULATIONAHA.105.598987. [DOI] [PubMed] [Google Scholar]
- 29.Urbina EM, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 30.Greenland P, et al. Prevention Conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention: noninvasive tests of atherosclerotic burden: Writing Group III. Circulation. 2000;101:E16–E22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 31.Li S, et al. Race (black-white) and gender divergences in the relationship of childhood cardiovascular risk factors to carotid artery intima–media thickness in adulthood: the Bogalusa Heart Study. Atherosclerosis. 2007;194:421–425. doi: 10.1016/j.atherosclerosis.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Li S, et al. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290:2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 33.Freedman DS, et al. The contribution of childhood obesity to adult carotid intima–media thickness: the Bogalusa Heart Study. Int. J. Obes. (Lond.) 2008;32:749–756. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 34.Raitakari OT, et al. Cardiovascular risk factors in childhood and carotid artery intima–media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA. 2003;290:2277–2283. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 35.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal–medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation. 2001;104:2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 36.Dawson JD, Sonka M, Blecha MB, Lin W, Davis PH. Risk factors associated with aortic and carotid intima–media thickness in adolescents and young adults: the Muscatine Offspring Study. J. Am. Coll. Cardiol. 2009;53:2273–2279. doi: 10.1016/j.jacc.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, et al. for the MESA Study Investigators. Coronary artery calcification and myocardial perfusion in asymptomatic adults: the MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2006;48:1018–1026. doi: 10.1016/j.jacc.2006.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahoney LT, et al. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J. Am. Coll. Cardiol. 1996;27:277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 39.Gidding SS, Bookstein LC, Chomka EV. Usefulness of electron beam tomography in adolescents and young adults with heterozygous familial hypercholesterolemia. Circulation. 1998;98:2580–2583. doi: 10.1161/01.cir.98.23.2580. [DOI] [PubMed] [Google Scholar]
- 40.Chan SY, et al. The prognostic importance of endothelial dysfunction and carotid atheroma burden in patients with coronary artery disease. J. Am. Coll. Cardiol. 2003;42:1037–1043. doi: 10.1016/s0735-1097(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 41.Aggoun Y, et al. Arterial mechanical changes in children with familial hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2000;20:2070–2075. doi: 10.1161/01.atv.20.9.2070. [DOI] [PubMed] [Google Scholar]
- 42.Woo KS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima–media thickening. Int. J. Obes. Relat. Metab. Disord. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 43.Urbina EM, Kieltkya L, Tsai J, Srinivasan SR, Berenson GS. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: the Bogalusa Heart Study. Am. J. Hypertens. 2005;18:767–771. doi: 10.1016/j.amjhyper.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 44.Budoff MJ, et al. Measures of brachial artery distensibility in relation to coronary calcification. Am. J. Hypertens. 2003;16:350–355. doi: 10.1016/s0895-7061(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 45.Leeson CP, et al. Cholesterol and arterial distensibility in the first decade of life: a population-based study. Circulation. 2000;101:1533–1538. doi: 10.1161/01.cir.101.13.1533. [DOI] [PubMed] [Google Scholar]
- 46.Whincup PH, et al. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112:1789–1797. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 47.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 48.Steinberger J, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 49.Weiss R, et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004;350:2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 50.Thompson DR, et al. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J. Pediatr. 2007;150:18–25. doi: 10.1016/j.jpeds.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 52.Burns TL, Letuchy EM, Paulos R, Witt J. Childhood predictors of the metabolic syndrome in middle-aged adults: the Muscatine Study. J. Pediatr. 2009;155:S5.e17–S5. e26. doi: 10.1016/j.jpeds.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun SS, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J. Pediatr. 2008;152:191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bao W, Srinivasan SR, Wattigney WA, Berenson GS. Persistence of multiple cardiovascular risk clustering related to syndrome X from childhood to young adulthood. The Bogalusa Heart Study. Arch. Intern. Med. 1994;154:1842–1847. [PubMed] [Google Scholar]
- 55.Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45:235–240. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- 56.Chen W, Srinivasan SR, Li S, Xu J, Berenson GS. Clustering of long-term trends in metabolic syndrome variables from childhood to adulthood in Blacks and Whites: the Bogalusa Heart Study. Am. J. Epidemiol. 2007;166:527–533. doi: 10.1093/aje/kwm105. [DOI] [PubMed] [Google Scholar]
- 57.Schubert CM, Sun SS, Burns TL, Morrison JA, Huang TT. Predictive ability of childhood metabolic components for adult metabolic syndrome and type 2 diabetes. J. Pediatr. 2009;155:S6.e1–S6.e7. doi: 10.1016/j.jpeds.2009.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schubert CM, Cook S, Sun SS, Huang TT. Additive utility of family history and waist circumference to body mass index in childhood for predicting metabolic syndrome in adulthood. J. Pediatr. 2009;155:S6.e9–S6.e13. doi: 10.1016/j.jpeds.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gidding SS, et al. Prediction of coronary artery calcium in young adults using the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score: the CARDIA study. Arch. Intern. Med. 2006;166:2341–2347. doi: 10.1001/archinte.166.21.2341. [DOI] [PubMed] [Google Scholar]
- 60.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 61.Despres JP, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N. Engl. J. Med. 1996;334:952–957. doi: 10.1056/NEJM199604113341504. [DOI] [PubMed] [Google Scholar]
- 62.Goalstone ML, et al. Insulin potentiates platelet-derived growth factor action in vascular smooth muscle cells. Endocrinology. 1998;139:4067–4072. doi: 10.1210/endo.139.10.6270. [DOI] [PubMed] [Google Scholar]
- 63.Aljada A, Ghanim H, Mohanty P, Kapur N, Dandona P. Insulin inhibits the pro-inflammatory transcription factor early growth response gene-1 (Egr)-1 expression in mononuclear cells (MNC) and reduces plasma tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) concentrations. J. Clin. Endocrinol. Metab. 2002;87:1419–1422. doi: 10.1210/jcem.87.3.8462. [DOI] [PubMed] [Google Scholar]
- 64.Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J. Clin. Endocrinol. Metab. 1995;80:172–178. doi: 10.1210/jcem.80.1.7829608. [DOI] [PubMed] [Google Scholar]
- 65.Reinehr T, de Sousa G, Toschke AM, Andler W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am. J. Clin. Nutr. 2006;84:490–496. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- 66.Nadeau KJ, et al. Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J. Clin. Endocrinol. Metab. 2009;94:3687–3695. doi: 10.1210/jc.2008-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J. Clin. Endocrinol. Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 68.Bao W, Srinivasan SR, Berenson GS. Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults. The Bogalusa Heart Study. Circulation. 1996;93:54–59. doi: 10.1161/01.cir.93.1.54. [DOI] [PubMed] [Google Scholar]
- 69.Taittonen L, et al. Insulin and blood pressure among healthy children. Cardiovascular risk in young Finns. Am. J. Hypertens. 1996;9:194–199. doi: 10.1016/0895-7061(95)00345-2. [DOI] [PubMed] [Google Scholar]
- 70.Sinaiko AR, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–1991. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 71.Raitakari OT, et al. The role of insulin in clustering of serum lipids and blood pressure in children and adolescents. The Cardiovascular Risk in Young Finns Study. Diabetologia. 1995;38:1042–1050. doi: 10.1007/BF00402173. [DOI] [PubMed] [Google Scholar]
- 72.McLaughlin T, Abbasi F, Lamendola C, Reaven G. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch. Intern. Med. 2007;167:642–648. doi: 10.1001/archinte.167.7.642. [DOI] [PubMed] [Google Scholar]
- 73.Burke GL, et al. Fasting plasma glucose and insulin levels and their relationship to cardiovascular risk factors in children: Bogalusa Heart Study. Metabolism. 1986;35:441–446. doi: 10.1016/0026-0495(86)90135-6. [DOI] [PubMed] [Google Scholar]
- 74.Kikuchi DA, et al. Relation of serum lipoprotein lipids and apolipoproteins to obesity in children: the Bogalusa Heart Study. Prev. Med. 1992;21:177–190. doi: 10.1016/0091-7435(92)90017-c. [DOI] [PubMed] [Google Scholar]
- 75.Jiang X, Srinivasan SR, Berenson GS. Relation of obesity to insulin secretion and clearance in adolescents: the Bogalusa Heart Study. Int. J. Obes. Relat. Metab. Disord. 1996;20:951–956. [PubMed] [Google Scholar]
- 76.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103:1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 77.Steinberger J, Moran A, Hong CP, Jacobs DR, Jr., Sinaiko AR. Adiposity in childhood predicts obesity and insulin resistance in young adulthood. J. Pediatr. 2001;138:469–473. doi: 10.1067/mpd.2001.112658. [DOI] [PubMed] [Google Scholar]
- 78.Sinaiko AR, Donahue RP, Jacobs DR, Jr., Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children's Blood Pressure Study. Circulation. 1999;99:1471–1476. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 79.Srinivasan SR, Myers L, Berenson GS. Temporal association between obesity and hyperinsulinemia in children, adolescents, and young adults: the Bogalusa Heart Study. Metabolism. 1999;48:928–934. doi: 10.1016/s0026-0495(99)90231-7. [DOI] [PubMed] [Google Scholar]
- 80.Travers SH, Jeffers BW, Eckel RH. Insulin resistance during puberty and future fat accumulation. J. Clin. Endocrinol. Metab. 2000;87:3814–3818. doi: 10.1210/jcem.87.8.8765. [DOI] [PubMed] [Google Scholar]
- 81.Pinhas-Hamiel O, Zeitler P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet. 2007;369:1823–1831. doi: 10.1016/S0140-6736(07)60821-6. [DOI] [PubMed] [Google Scholar]
- 83.Fagot-Campagna A, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J. Pediatr. 2000;136:664–672. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 83.Choha AC, et al. Secular trends in blood pressure during early-to-middle adulthood: the Fels Longitudinal Study. J. Hypertens. 2011;29:838–845. doi: 10.1097/HJH.0b013e328344da30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tirosh A, et al. Progression of normotensive adolescents to hypertensive adults: a study of 26,980 teenagers. Hypertension. 2010;56:203–209. doi: 10.1161/HYPERTENSIONAHA.109.146415. [DOI] [PubMed] [Google Scholar]
- 85.Magnussen CG, et al. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima–media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J. Am. Coll. Cardiol. 2009;53:860–869. doi: 10.1016/j.jacc.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Juonala M, et al. Associations of dyslipidemias from childhood to adulthood with carotid intima–media thickness, elasticity, and brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler. Thromb. Vasc. Biol. 2008;28:1012–1017. doi: 10.1161/ATVBAHA.108.163329. [DOI] [PubMed] [Google Scholar]
- 87.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. Risk factors and adult body mass index among overweight children: the Bogalusa Heart Study. Pediatrics. 2009;123:750–757. doi: 10.1542/peds.2008-1284. [DOI] [PubMed] [Google Scholar]
- 88.Magnussen CG, et al. Utility of currently recommended pediatric dyslipidemia classifications in predicting dyslipidemia in adulthood: evidence from the Childhood Determinants of Adult Health (CDAH) study, Cardiovascular Risk in Young Finns Study, and Bogalusa Heart Study. Circulation. 2008;117:32–42. doi: 10.1161/CIRCULATIONAHA.107.718981. [DOI] [PubMed] [Google Scholar]
- 89.Schwimmer JB. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 90.Nadeau KJ, Klingensmith G, Zeitler P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J. Pediatr. Gastroenterol. Nutr. 2005;41:94–98. doi: 10.1097/01.mpg.0000164698.03164.e5. [DOI] [PubMed] [Google Scholar]
- 91.Schwimmer JB, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 92.Sattar N, et al. Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the west of Scotland coronary prevention study. Diabetes. 2004;53:2855–2860. doi: 10.2337/diabetes.53.11.2855. [DOI] [PubMed] [Google Scholar]
- 93.Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr. Pharm. Des. 16:1941–1951. doi: 10.2174/138161210791208875. [DOI] [PubMed] [Google Scholar]
- 94.Targher G, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 95.Patel DA, Srinivasan SR, Xu JH, Chen W, Berenson GS. Persistent elevation of liver function enzymes within the reference range is associated with increased cardiovascular risk in young adults: the Bogalusa Heart Study. Metabolism. 2007;56:792–798. doi: 10.1016/j.metabol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 96.Pearson TA, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 97.Maahs DM, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–2825. doi: 10.1161/01.CIR.0000132467.45278.59. [DOI] [PubMed] [Google Scholar]
- 99.Patel DA, et al. Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism. 2006;55:699–705. doi: 10.1016/j.metabol.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 100.Moran A, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care. 2005;28:1763–1768. doi: 10.2337/diacare.28.7.1763. [DOI] [PubMed] [Google Scholar]
- 101.Cook DG, et al. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 102.Bibbins-Domingo K, Coxson P, Pletcher MJ, Lightwood J, Goldman L. Adolescent overweight and future adult coronary heart disease. N. Engl. J. Med. 2007;357:2371–2379. doi: 10.1056/NEJMsa073166. [DOI] [PubMed] [Google Scholar]
- 103.Ludwig DS. Childhood obesity—the shape of things to come. N. Engl. J. Med. 2007;357:2325–2327. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]
- 104.Olshansky SJ, et al. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 105.Franks PW. Childhood obesity, other cardiovascular risk factors, and premature death. N. Engl. J. Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haji SA, et al. Predictors of left ventricular dilatation in young adults (from the Bogalusa Heart Study). Am. J. Cardiol. 2006;98:1234–1237. doi: 10.1016/j.amjcard.2006.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tirosh A, et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N. Engl. J. Med. 2011;364:1315–1325. doi: 10.1056/NEJMoa1006992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lawlor DA, Leon DA. Association of body mass index and obesity measured in early childhood with risk of coronary heart disease and stroke in middle age: findings from the aberdeen children of the 1950s prospective cohort study. Circulation. 2005;111:1891–1896. doi: 10.1161/01.CIR.0000161798.45728.4D. [DOI] [PubMed] [Google Scholar]
- 109.Lawlor DA, et al. Association of body mass index measured in childhood, adolescence, and young adulthood with risk of ischemic heart disease and stroke: findings from 3 historical cohort studies. Am. J. Clin. Nutr. 2006;83:767–773. doi: 10.1093/ajcn/83.4.767. [DOI] [PubMed] [Google Scholar]
- 110.Abraham S, Collins G, Nordsieck M. Relationship of childhood weight status to morbidity in adults. HSMHA Health Rep. 1971;86:273–284. [PMC free article] [PubMed] [Google Scholar]
- 111.DiPietro L, Mossberg HO, Stunkard AJ. A 40-year history of overweight children in Stockholm: life-time overweight, morbidity, and mortality. Int. J. Obes. Relat. Metab. Disord. 1994;18:585–590. [PubMed] [Google Scholar]
- 112.McGill HC, et al. Effects of nonlipid risk factors on atherosclerosis in youth with a favorable lipoprotein profile. Circulation. 2001;103:1546–1550. doi: 10.1161/01.cir.103.11.1546. [DOI] [PubMed] [Google Scholar]
- 113.Atlantis E, Barnes EH, Singh MA. Efficacy of exercise for treating overweight in children and adolescents: a systematic review. Int. J. Obes. (Lond.) 2006;30:1027–1040. doi: 10.1038/sj.ijo.0803286. [DOI] [PubMed] [Google Scholar]
- 114.Oude Luttikhuis H, et al. Interventions for treating obesity in children. Cochrane Database of Systematic Reviews Issue 1. 2009 doi: 10.1002/14651858.CD001872.pub2. Art. No.: CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 115.US FDA News release. Abbott Laboratories agrees to withdraw its obesity drug Meridia [online] 2010 http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm228812.htm.
- 116.Brown T, Summerbell C. Systematic review of school-based interventions that focus on changing dietary intake and physical activity levels to prevent childhood obesity: an update to the obesity guidance produced by the National Institute for Health and Clinical Excellence. Obes. Rev. 2009;10:110–141. doi: 10.1111/j.1467-789X.2008.00515.x. [DOI] [PubMed] [Google Scholar]
- 117.Goodpaster BH, et al. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: a randomized trial. JAMA. 2010;304:1795–1802. doi: 10.1001/jama.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stewart KJ. Exercise training and the cardiovascular consequences of type 2 diabetes and hypertension: plausible mechanisms for improving cardiovascular health. JAMA. 2002;288:1622–1631. doi: 10.1001/jama.288.13.1622. [DOI] [PubMed] [Google Scholar]
- 119.Reinehr T, de Sousa G, Wabitsch M. Changes of cardiovascular risk factors in obese children effects of inpatient and outpatient interventions. J. Pediatr. Gastroenterol. Nutr. 2006;43:506–511. doi: 10.1097/01.mpg.0000235752.29735.31. [DOI] [PubMed] [Google Scholar]
- 120.Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114:1569–1573. doi: 10.1542/peds.2003-0649-F. [DOI] [PubMed] [Google Scholar]
- 121.Savoye M, et al. Effects of a weight management program on body composition and metabolic parameters in overweight children: a randomized controlled trial. JAMA. 2007;297:2697–2704. doi: 10.1001/jama.297.24.2697. [DOI] [PubMed] [Google Scholar]
- 122.Reinehr T, Schmidt C, Toschke AM, Andler W. Lifestyle intervention in obese children with non-alcoholic fatty liver disease: 2-year follow-up study. Arch. Dis. Child. 2009;94:437–442. doi: 10.1136/adc.2008.143594. [DOI] [PubMed] [Google Scholar]
- 123.Nobili V, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 124.Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2002;25:89–94. doi: 10.2337/diacare.25.1.89. [DOI] [PubMed] [Google Scholar]
- 125.Nadeau KJ, Ehlers LB, Zeitler PS, Love-Osborne K. Treatment of non-alcoholic fatty liver disease with metformin versus lifestyle intervention in insulin-resistant adolescents. Pediatr. Diabetes. 2008;10:5–13. doi: 10.1111/j.1399-5448.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 126.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes. Rev. 2010;11:118–126. doi: 10.1111/j.1467-789X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 127.Herrera BM, Lindgren CM. The genetics of obesity. Curr. Diab. Rep. 2010;10:498–505. doi: 10.1007/s11892-010-0153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang X, et al. Testing a model of physical activity and obesity tracking from youth to adulthood: the cardiovascular risk in young Finns study. Int. J. Obes. (Lond.) 2007;31:521–527. doi: 10.1038/sj.ijo.0803459. [DOI] [PubMed] [Google Scholar]
- 129.McMahan CA, et al. Association of Pathobiologic Determinants of Atherosclerosis in Youth risk score and 15-year change in risk score with carotid artery intima–media thickness in young adults (from the Cardiovascular Risk in Young Finns Study). Am. J. Cardiol. 2007;100:1124–1129. doi: 10.1016/j.amjcard.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clarke WR, Schrott HG, Leaverton PE, Connor WE, Lauer RM. Tracking of blood lipids and blood pressures in school age children: the Muscatine study. Circulation. 1978;58:626–634. doi: 10.1161/01.cir.58.4.626. [DOI] [PubMed] [Google Scholar]
- 131.Clarke WR, Lauer RM. Does childhood obesity track into adulthood? Crit. Rev. Food Sci. Nutr. 1993;33:423–430. doi: 10.1080/10408399309527641. [DOI] [PubMed] [Google Scholar]
- 132.Sørensen TI, Sonne-Holm S. Risk in childhood of development of severe adult obesity: retrospective, population-based case-cohort study. Am. J. Epidemiol. 1988;127:104–113. doi: 10.1093/oxfordjournals.aje.a114770. [DOI] [PubMed] [Google Scholar]
- 133.Venn AJ, et al. Overweight and obesity from childhood to adulthood: a follow-up of participants in the 1985 Australian Schools Health and Fitness Survey. Med. J. Aust. 2007;186:458–460. doi: 10.5694/j.1326-5377.2007.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 134.Power C, Lake JK, Cole TJ. Body mass index and height from childhood to adulthood in the 1958 British born cohort. Am. J. Clin. Nutr. 1997;66:1094–1101. doi: 10.1093/ajcn/66.5.1094. [DOI] [PubMed] [Google Scholar]
- 135.Sachdev HP, et al. Predicting adult metabolic syndrome from childhood body mass index: follow-up of the New Delhi birth cohort. Arch. Dis. Child. 2009;94:768–774. doi: 10.1136/adc.2008.140905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Must A. Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton Girls Study and its follow-up. Pediatrics. 2005;116:620–627. doi: 10.1542/peds.2004-1604. [DOI] [PubMed] [Google Scholar]