Abstract
We have developed a computer system, MITOMASTER, to make analysis of human mitochondrial DNA (mtDNA) sequences efficient, accurate, and easily available. From imported sequences, the system identifies nucleotide variants, determines the haplogroup, rules out possible pseudogene contamination, identifies novel DNA sequence variants, and evaluates the potential biological significance of each variant. This system should be beneficial for mtDNA analyses of biomedical physicians and investigators, population biologists and forensic scientists. MITOMASTER can be accessed at http://mammag.web.uci.edu/twiki/bin/view/Mitomaster.
Keywords: mitochondria, mitochrondrial DNA, mtDNA, variation, bioinformatics, clinical analysis, MITOMASTER
Introduction
There is a rapidly growing requirement for analyzing human mitochondrial DNA (mtDNA) variation for a wide variety of applications including: determining its contribution to rare and common genetic diseases; identification and interpretation of acquired variants in cancer, aging, and age-related diseases; analyzing variation for lineage and populations studies; making individual identifications in forensics; etc. (Coskun, et al., 2003;Wallace, 2005b; Wallace, 2005a). Consequently, large quantities of mtDNA sequence data are being acquired using automated sequencing (Sarzi, et al., 2007), heteroduplex screening using denaturing gradient gels (Wong, et al., 2002) and Surveyor Endonuclease digestions (Bannwarth, et al., 2005), and chip-based sequencing (Maitra, et al., 2004; van Eijsden, et al., 2006).
The 16,569 nucleotide (nt) circular mtDNA encodes a 12S and 16S rRNA, 22 tRNAs, 13 polypeptides, and a 1,121 nt control region. The 13 polypeptides encompass critical components of the mitochondrial energy production pathway, oxidative phosphorylation (OXPHOS): seven subunits (ND1, 2, 3, 4L, 4, 5, 6) of complex I, one subunit (cytochrome b, cytb) of complex III, three subunits (COI, II, III) of complex IV, and two subunits (ATP6 & 8) of complex V (Wallace, 2007).
The maternally inherited mtDNA is present in thousands of copies per cell and has a very high mutation rate. New mutations arise in cells mixed among normal mtDNAs (heteroplasmy), and segregate randomly during cytokinesis. Therefore, clinical phenotypes are determined by the type and severity of the mutations and the percentage of heteroplasmy (Wallace, 2005a; Wallace, et al., 2007).
The mtDNA nucleotide positions are numbered according to the modified Cambridge Reference Sequence (mCRS) (accession: AC_000021.2 gi:115315570) (Anderson, et al., 1981; Howell, et al., 1992; Ruiz-Pesini, et al., 2007). Hence, a new sequence can be described relative to the mCRS by specifying only the nucleotide position and deviant base. Deviant bases fall into four categories: recent pathogenic mutations, ancient polymorphisms, age-related somatic mutations, or altered bases in nuclear DNA (nDNA)-encoded mtDNA pseuodgenes.
New pathogenic mtDNA mutations have been estimated at a frequency of 1 in 5000 individuals (Schaefer, et al., 2004; Wallace, et al., 2007). Hundreds of putative pathogenic mtDNA variants have been identified (Wallace, et al., 2007). Mild pathogenic mutations cause disease when homoplasmic, while severe mutations can be pathogenic when heteroplasmic (Wallace, et al., 2007).
Ancient mtDNA polymorphisms are not only common in the mtDNA, but occur in discrete groups of related haplotypes, known as haplogroups. This is because the mtDNA is exclusively maternally inherited, so that the mtDNAs can only change by the sequential accumulation of mutations (Cann, et al., 1987; Ingman, et al., 2000; Johnson, et al., 1983; Merriwether, et al., 1991; Mishmar, et al., 2003; Wallace, et al., 1999). The geographical association of mtDNA haplogroups is a consequence of the presence of adaptive variants that permitted the associated mtDNAs to become established in specific environments (Mishmar, et al., 2003; Ruiz-Pesini, et al., 2004; Ruiz-Pesini and Wallace, 2006). Today, these same variants can influence longevity and predisposition to disease (Baudouin, et al., 2005; Chagnon, et al., 1999; De Benedictis, et al., 1999; Ivanova, et al., 1998; McMahon, et al., 2000; Niemi, et al., 2003; Ross, et al., 2001; van der Walt, et al., 2004; van der Walt, et al., 2003).
Mutations in the mtDNA also arise in somatic tissues as a component of the aging process (Michikawa, et al., 1999; Murdock, et al., 2000; Wang, et al., 2001; Zhang, et al., 2003) and have been proposed to be the aging clock (Coskun, et al., 2004; Wallace, 2005a). Somatic variants also arise in cancerous cells (Brandon, et al., 2006; Petros, et al., 2005).
Finally, throughout animal cellular evolution, fragments of the mtDNA sequence have been transferred to the nuclear DNA to generate nuclear mitochondrial DNA (NUMTs) pseudogene sequences (Mishmar, et al., 2004; Wallace, 2007). These can be mistaken for “heteroplasmic” pathogenic mtDNA variants (Davis and Parker, 1998; Davis, et al., 1997; Hirano, et al., 1997; Wallace, et al., 1997).
All of these considerations make interpreting the biological significance of mtDNA sequence variants particularly challenging. Not only must a large and diverse literature be known and employed in the analysis, but multiple interacting factors must be considered. For a clinical sequence, not only is it necessary to identify putative new mutations, relative to the mCRS, the background mtDNA variation must be identified and the functional significance of previously observed, as well as novel, variants must be evaluated. Potentially spurious NUMT variants must also be identified and the contribution of mtDNA variants evaluated.
Therefore, simple catalogues, such as all known pathogenic mtDNA mutations and common haplogroup polymorphisms, are no longer adequate for interpreting mtDNA sequence variation (www.mitomap.org). Such databases must now be extended to include intelligent analytical systems that can immediately bring to bear other relevant information on the interpretation of new mtDNA sequences. This is the purpose of the analytical system, MITOMASTER (http://mammag.web.uci.edu/twiki/bin/view/Mitomaster), described in this paper.
Results and Procedures
Logic of the MITOMASTER mtDNA Sequence Analysis System
MITOMASTER permits the automatic evaluation of mtDNA sequence variation through comparisons of a series of complementary data sets, each encapsulating the known information about one of the classes of mtDNA variants: reference sequence, functional domains, haplogroup variants, pathogenic mutations, pseudogenes, etc.
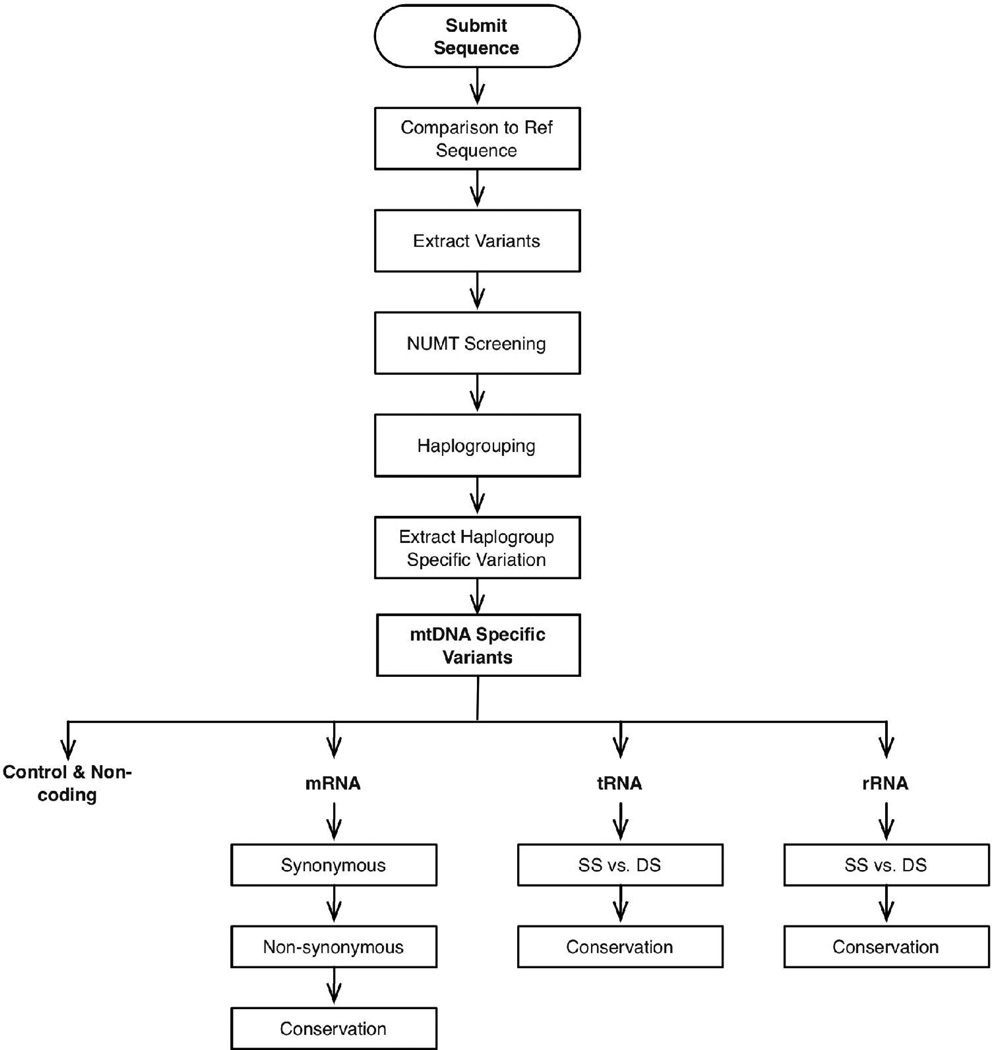
First, the mtDNA sequence is aligned with the mCRS to identify all deviant nucleotides specified by nucleotide numbers (Figure 1). We use the mCRS, instead of a nodal African sequence from haplogroup L0, because current human mtDNA sequence numbers are based on he original CRS and small insertion-deletion mutants in another sequence could misalign the MITOMASTER sequence analyses relative to the literature. The sequence variants are then compared to our library of 247 NUMTs to determine if the variant might be the product of a contaminating nuclear pseudogene sequence. The sequence variants are also compared to our library of haplogroups. This can be done in two ways: (1) recognition of haplogroup-specific polymorphisms or (2) comparison of the sequence to the 2452 mtDNA sequences in our mtDNA phylogenetic tree and identifying the most similar sequence and its haplotype (Ruiz-Pesini, et al., 2007). Once the haplogroup has been determined, the ancient haplogroup-specific mtDNA sequence variants are subtracted. The remaining sequence variants must be recent, and thus potentially pathogenic. Each of the recent variants is then classified according to the region of the mtDNA in which it occurs: polypeptide, tRNA, rRNA, control region. Protein coding gene variants are further characterized by codon position, the affect on the amino acid sequence, and the interspecific species conservation index (CI) of the mutated amino acid (Mishmar, et al., 2003). Similarly, tRNA and rRNA variants are localized within the secondary structural models of RNAs (Ruiz-Pesini and Wallace, 2006) and the CI of the affected base calculated.
Figure 1.
The algorithm used by biologist, and implemented within MITOMASTER, to identify possible clinical variants within a patient's mtDNA sequence. Variants are extracted by alignment with a reference sequence; the group of extracted variants is used to determine the haplogroup of the sequence and each individual variant is checked within the database and analyzed to determine its biological effect; variation within coding loci is further analyzed to determine its coding effect and inter-species rate of conservation.
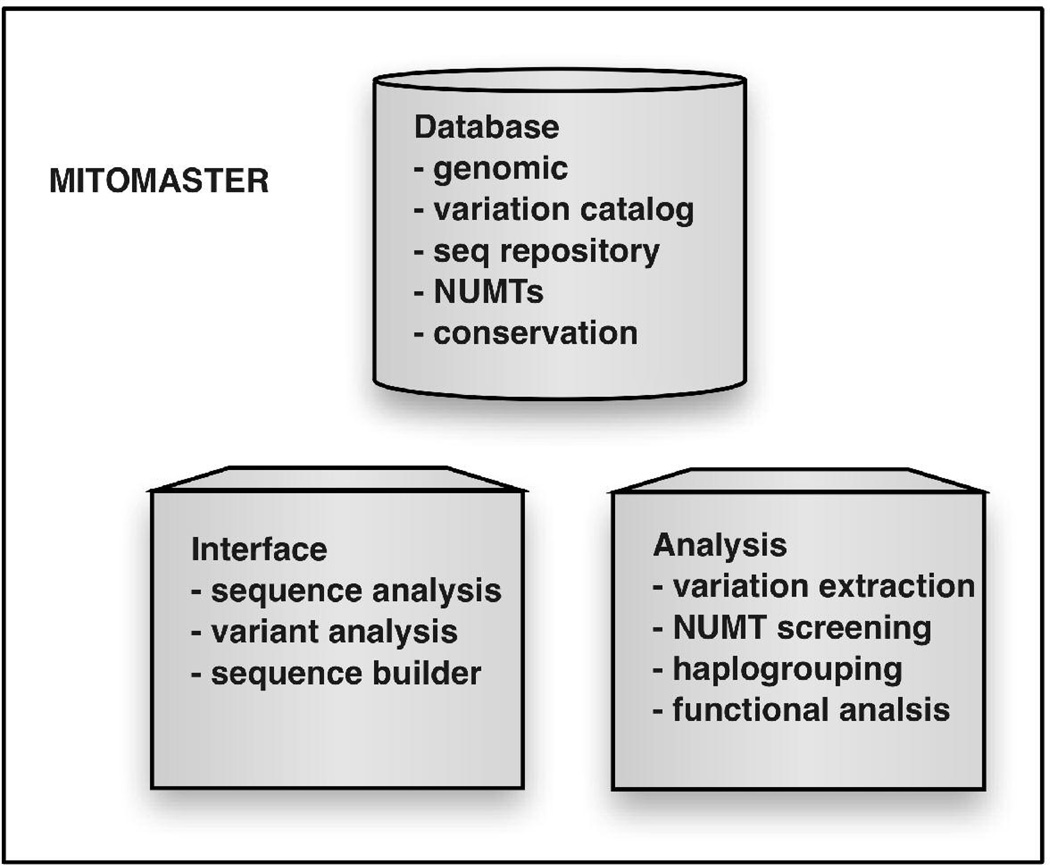
MITOMASTER interaction of three elements: database, analysis, and interface (Figure 2). Though these parts are generally intended to be used together as an online tool, the database and code library that implements the analyses can function independent of the interface. As such, they provide an extensible framework for bioinformaticians to issue queries directly to the database or write problem-specific programs. Each of the components is discussed in more detail below.
Figure 2.
MITOMASTER's functional components. A website interface allows users to submit either files of whole mtDNA sequences or individual variants for analysis. Programs within the analysis component compare the submission with the mCRS, extract the variants found within submitted sequences, perform NUMT screening on the variants extracted, assign the sequences to a haplogroup, and determine the predicted functional effect of each variant.
MITOMASTER Database
The primary data set is the 16,569 nucleotide (nt) mCRS, a haplogroup H subject (Anderson, et al., 1981) that has been corrected for errors, but with insertion-deletion errors maintained to sustain the original nucleotide numbering system (Howell, et al., 1992). Hence, each mCRS nucleotide number is a unique identifier permitting indexing of nucleotides. The sequence has been annotated to encompass all of the known mtDNA functional domains from the MITOMAP database (http://www.mitomap.org).
The second data set encompasses the 247 known human NUMT region sequences (Mishmar, et al., 2004). These NUMTs have been aligned with the reference sequence to determine the area spanned and the nt position and altered base for all deviants relative to mCRS. Since there is an overlap between mtDNA variants found in legitimate mtDNA sequences and those found in certain NUMTs, we have elected to provide the user with an accounting of the proportion of NUMTs that align with the query sequence region that also harbor the variant of interest.
The third data set encompasses the curated mtDNA sequences of 2452 human subjects from around the globe. These sequences have been pre-aligned with the mCRS, and tables generated that list all of the observed nucleotide differences. The current collection of mtDNA sequences encompasses 1920 complete mtDNA sequences plus 532 coding region mtDNA sequences. These sequences encompass 3512 total population variants including 453 non-coding, 2563 mRNA, 216 tRNA, and 280 rRNA variant sites (Brandon, et al., 2003). Each of the sequences has also been assigned to an mtDNA haplogroup.
The fourth data set is the information or pathogenic mtDNA mutations that have been accumulated over the past 14 years in our MITOMAP database. This is a curated database to assure the most internally consistent and accurate data possible. Currently, MITOMAP encompasses 271 nt variants that have been documented as potentially pathogenic mutations, plus a list of over 800 unpublished mtDNA sequence variants submitted to the MITOMAP website.
The fifth data set encompasses multiple amino acid sequence alignments of the various mtDNA polypeptide genes from an array of species. The current default number that we query includes 39 species. This comparison permits calculation of the interspecific conservation index (CI) of any mutant amino acid.
The final data set involves the structure and common polymorphisms of the human mtDNA tRNAs and rRNAs genes (Ruiz-Pesini and Wallace, 2006). This data set permits interspecies tRNA and rRNA sequence alignments for functional analysis and calculation of the conservation index, with the current default of 17 species.
mtDNA Sequence Data Analysis
The algorithms supporting the analysis are implemented within an object-oriented class library written in PERL. The goal is to create a reusable code base, tightly integrated with the database, and having a flexible range of functions for analyzing mitochondrial data.
Analysis by the MITOMASTER system is initiated by the submission of a new mtDNA sequence under investigation. The sequence is optimally aligned to the mCRS and the variants are found. These variants are assigned position numbers based upon commonly accepted conventions, and the collection of variants is used to assign the sequence to a haplogroup.
The NUMT sequences encompassed by the patient sequence are then searched for the same sequence variants. If the variant is found, the number and region encompassed by the NUMT are listed for further evaluation.
If NUMTs are eliminated, then the effect of the variant on the mtDNA gene structure and function is investigated. For polypeptide variants, the codon change is identified and its amino acid and CI are provided. For alterations of tRNAs or an rRNA, the specific structural location affected is marked and displayed upon the appropriate diagram.
Once the variant nucleotides have been identified and the characteristics of the variants ascertained, then the variant nucleotide is compared to lists of known pathogenic mtDNA mutations and neutral or adaptive polymorphisms.
MITOMASTER's Interface
MITOMASTER provides an interactive interface by which users submit an mtDNA sequence, and the sequence variants are returned with links to the relevant information about the evaluation. The web page interface is distributable to anyone with a web browser and can be used to submit or retrieve mtDNA sequences, to perform clinical analyses, and query the significance of single mtDNA nucleotide changes.
Because each submitted sequence undergoes a lengthy preprocessing that includes alignment, haplogrouping, and simulated transcription and translation, users must wait following submission of their sequence until the initial processing is completed. The user is then notified by email when the system has finished preprocessing. Afterwards the user can access the various outputs by making specific inquiries.
The MITOMASTER system also uses an application programming interface (API) which facilitates the implementation of more specialized analyses. The class API facilitates the extension and development of software that can use MITOMASTER's database for new types of analyses.
Database and Algorithm Design
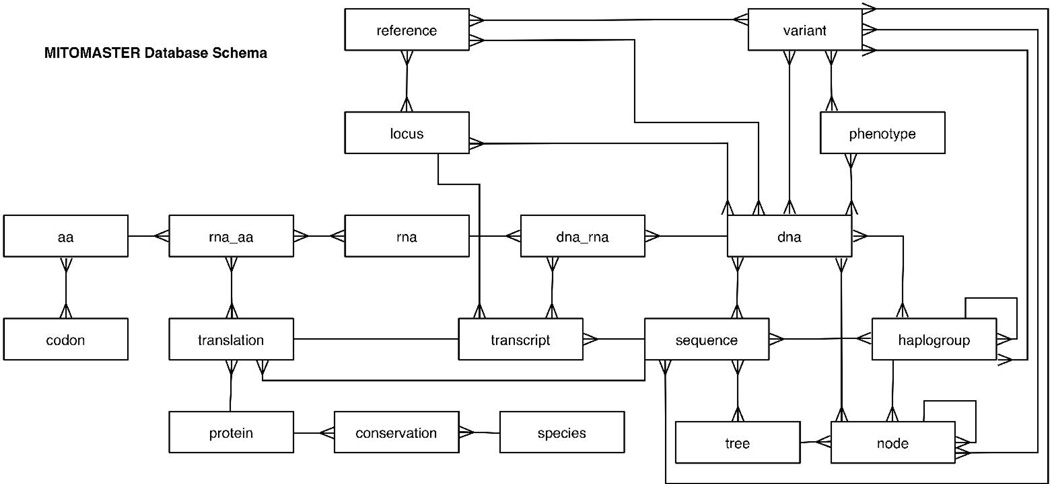
Unlike typical sequence databases, MITOMASTER models its data at the individual nucleotide level (Figure 3). When this level of granularity is combined with the structured query language (SQL), many types of biological analyses become possible simply by querying the database. One such example is the computation of the average number of amino acid variants found within the cytochrome oxidase I locus for all the sequences stored in the database (Table 1).
Figure 3.
Simplified schema diagram for MITOMASTER's database. DNA sequences are decomposed into their constituent nucleic acids and entries made in the dna table. During simulated transcription and translation processes, all variation (relative to the mCRS) is entered into the rna and aa tables, respectively. Some variation involves multiple base alterations and these cases are grouped in the “variant” table. The aggregated variants that define the known haplogroups are represented in the haplogroups data set, the nodes are the branch points of the mtDNA phylogenetic tree.
TABLE 1.
MITOMASTER SQL query that computes the average number of amino acid variants found within the cytochrome oxidase I locus
| SELECT AVG(pepcount) |
| FROM ( |
| SELECT seqid, COUNT(peptide) AS pepcount |
| FROM seqpep |
| WHERE locusid = 16 |
| GROUP BY seqid |
| UNION |
| SELECT sequence.id, 0 AS pepcount |
| FROM sequence |
| WHERE |
| sequence.id > 1 AND |
| sequence.id NOT IN |
| (SELECT DISTINCT seqid |
| FROM seqpep |
| WHERE locusid = 16) ) AS FOO |
This query computes an average from two sets of data, those sequences with an amino acid substitution and those without. The top query from “SELECT seqid, COUNT(peptide) AS pepcount” through “GROUP BY seqid” identify all of the sequences in the database that have a variant amino acid. However, some sequences do not have a variant amino acid, so these need to be assigned a zero value, which is accomplished by the query from “SELECT sequence.id, 0 AS pepcount” to “WHERE locusid = 16)) AS FOO.” These two datsets are combined by “UNION” and averaged by the “SELECT AVG(pepcount)” function.
Though powerful, implementing relational models to capture all the nuances of biological systems can make them complicated. MITOMASTER uses virtual tables, i.e. "views", to create a simplified meta-layer to the data model when needed. Views are conceptualizations of the set of data output from queries. MITOMASTER's views facilitate easy retrieval of data about many different aspects of the biology of each mtDNA nucleotide: eg. reference sequence nucleotide, somatic mutation, pathological mutation, population polymorphism, etc.
Data on mtDNA sequence variation has been collected under the auspices of the MITOMAP curatorial team using Excel spreadsheets, and is available at the MITOMAP website (http://www.mitomap.org). Data from spreadsheets is transferred into PostgreSQL tables using the Perl module Spreadsheet::ParseExcel. Submitted sequences can be analyzed from fasta (complete nucleotide sequence) formatted text files. Data is then extracted (parsed) using bioperl (Stajich, et al., 2002).
MITOMASTER is implemented on a dual-processor, 2 GHz Pentium IV linux server running Apache 2.2 and PostgreSQL 8.1. Web interfaces (HTML pages) are enhanced using javascript and cascading style sheets to make the users interaction with MITOMASTER more dynamic. The HTML::Mason Perl module is used to embed sub-routines into MITOMASTER that will instantaneously provide up-dated values for changing information.
Web server programs are implemented with mod_perl 2.0 to increase efficiency and security. Use of the CGI Perl module facilitates efficient application development, while use of the DBI Perl module provides database connectivity.
Discussion
In this report, we describe a new computational tool, MITOMASTER, to assist in the management and analysis of the increasing quantity and complexity of mtDNA data of relevance to mitochondrial medicine, human population genetics and forensics. MITOMASTER is designed to accept new human mtDNA sequences and to then apply to the sequence analysis a sequential array of analytical functions with domain-specific factual information. Through this strategy, the program follows a similar logic process that might be used by an expert investigator to draw on the extensive data embedded in the literature for interpreting a new mtDNA sequence. As a result, the non-mitochondrial expert can interpret the occasional mtDNA sequence with confidence.
Because the initial steps in analysis of DNA sequences are often the same, the basic architecture of MITOMASTER should be readily extendable for use in the analysis of other types of sequence data. Therefore, it is our hope that the architecture we have developed using the human mtDNA as a model system will pave the way for the development of similar sequence knowledge bases for other genomic regions.
Acknowledgments
This work was supported by an NIH training grant to P.F.B and D.C.W. This work as supported by NIH grants NS21328, AG24373, DK73691, and AG13154 awarded to D.C.W.
References
- Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bannwarth S, Procaccio V, Paquis-Flucklinger V. Surveyor Nuclease: a new strategy for a rapid identification of heteroplasmic mitochondrial DNA mutations in patients with respiratory chain defects. Human Mutation. 2005;25(6):575–582. doi: 10.1002/humu.20177. [DOI] [PubMed] [Google Scholar]
- Baudouin SV, Saunders D, Tiangyou W, Elson JL, Poynter J, Pyle A, Keers S, Turnbull DM, Howell N, Chinnery PF. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005;366(9503):2118–2121. doi: 10.1016/S0140-6736(05)67890-7. [DOI] [PubMed] [Google Scholar]
- Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncogene. 2006;25(34):4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- Brandon MC, Baldi P, Navathe SB, Lott MT, Wallace DC. Mitomaster - tool for diagnosing genetic diseases. Proceedings of the IEEE Computer Society Bioinformatics Conference. 2003 [Google Scholar]
- Cann RL, Stoneking M, Wilson AC. Mitochondrial DNA and human evolution. Nature. 1987;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Chagnon P, Gee M, Filion M, Robitaille Y, Belouchi M, Gauvreau D. Phylogenetic analysis of the mitochondrial genome indicates significant differences between patients with Alzheimer disease and controls in a French-Canadian founder population. American Journal of Medical Genetics. 1999;85(1):20–30. doi: 10.1002/(sici)1096-8628(19990702)85:1<20::aid-ajmg6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(29):10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskun PE, Ruiz-Pesini EE, Wallace DC. Control region mtDNA variants: longevity, climatic adaptation and a forensic conundrum. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2174–2176. doi: 10.1073/pnas.0630589100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JN, II, Parker WD., Jr Evidence that two reports of mtDNA cytochrome c oxidase 'mutations' in Alzheimer's disease are based on nDNA pseudogenes of recent evolutionary origin. Biochemical and Biophysical Research Communications. 1998;244(3):877–883. doi: 10.1006/bbrc.1998.8353. [DOI] [PubMed] [Google Scholar]
- Davis RE, Miller S, Herrnstadt C, Ghosh SS, Fahy E, Shinobu LA, Galasko D, Thal LJ, Beal MF, Howell N, et al. Mutations in mitochondrial cytochrome c oxidase genes segregate with late-onset Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4526–4531. doi: 10.1073/pnas.94.9.4526. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- De Benedictis G, Rose G, Carrieri G, De Luca M, Falcone E, Passarino G, Bonafe M, Monti D, Baggio G, Bertolini S, et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. FASEB Journal. 1999;13(12):1532–1536. doi: 10.1096/fasebj.13.12.1532. [DOI] [PubMed] [Google Scholar]
- Hirano M, Shtilbans A, Mayeux R, Davidson MM, DiMauro S, Knowles JA, Schon EA. Apparent mtDNA heteroplasmy in Alzheimer's disease patients and in normals due to PCR amplification of nucleus-embedded mtDNA pseudogenes. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14894–14899. doi: 10.1073/pnas.94.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N, McCullough DA, Kubacka I, Halvorson S, Mackey D. The sequence of human mtDNA: the question of errors versus polymorphisms. American Journal of Human Genetics. 1992;50(6):1333–1340. [PMC free article] [PubMed] [Google Scholar]
- Ingman M, Kaessmann H, Paabo S, Gyllensten U. Mitochondrial genome variation and the origin of modern humans. Nature. 2000;408(6813):708–713. doi: 10.1038/35047064. [DOI] [PubMed] [Google Scholar]
- Ivanova R, Lepage V, Charron D, Schachter F. Mitochondrial genotype associated with French Caucasian centenarians. Gerontology. 1998;44(6):349. doi: 10.1159/000022041. [DOI] [PubMed] [Google Scholar]
- Johnson MJ, Wallace DC, Ferris SD, Rattazzi MC, Cavalli-Sforza LL. Radiation of human mitochondria DNA types analyzed by restriction endonuclease cleavage patterns. Journal of Molecular Evolution. 1983;19(3–4):255–271. doi: 10.1007/BF02099973. [DOI] [PubMed] [Google Scholar]
- Maitra A, Cohen Y, Gillespie SE, Mambo E, Fukushima N, Hoque MO, Shah N, Goggins M, Califano J, Sidransky D, et al. The Human MitoChip: a high-throughput sequencing microarray for mitochondrial mutation detection. Genome Research. 2004;14(5):812–819. doi: 10.1101/gr.2228504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon FJ, Chen YS, Patel S, Kokoszka J, Brown MD, Torroni A, DePaulo JR, Wallace DC. Mitochondrial DNA sequence diversity in bipolar affective disorder. American Journal of Psychiatry. 2000;157(7):1058–1064. doi: 10.1176/appi.ajp.157.7.1058. [DOI] [PubMed] [Google Scholar]
- Merriwether DA, Clark AG, Ballinger SW, Schurr TG, Soodyall H, Jenkins T, Sherry ST, Wallace DC. The structure of human mitochondrial DNA variation. Journal of Molecular Evolution. 1991;33(6):543–555. doi: 10.1007/BF02102807. [DOI] [PubMed] [Google Scholar]
- Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286(5440):774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- Mishmar D, Ruiz-Pesini E, Brandon M, Wallace DC. Mitochondrial DNA-like sequences in the nucleus (NUMTs): insights into our African origins and the mechanism of foreign DNA integration. Human Mutation. 2004;23(2):125–133. doi: 10.1002/humu.10304. [DOI] [PubMed] [Google Scholar]
- Mishmar D, Ruiz-Pesini EE, Golik P, Macaulay V, Clark AG, Hosseini S, Brandon M, Easley K, Chen E, Brown MD, et al. Natural selection shaped regional mtDNA variation in humans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):171–176. doi: 10.1073/pnas.0136972100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock DG, Christacos NC, Wallace DC. The age-related accumulation of a mitochondrial DNA control region mutation in muscle, but not brain, detected by a sensitive PNA-directed PCR clamping based method. Nucleic Acids Research. 2000;28(21):4350–4355. doi: 10.1093/nar/28.21.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi AK, Hervonen A, Hurme M, Karhunen PJ, Jylha M, Majamaa K. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Human Genetics. 2003;112(1):29–33. doi: 10.1007/s00439-002-0843-y. [DOI] [PubMed] [Google Scholar]
- Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross OA, McCormack R, Curran MD, Duguid RA, Barnett YA, Rea IM, Middleton D. Mitochondrial DNA polymorphism: its role in longevity of the Irish population. Experimental Gerontology. 2001;36(7):1161–1178. doi: 10.1016/s0531-5565(01)00094-8. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lott MT, Procaccio V, Poole J, Brandon MC, Mishmar D, Yi C, Kreuziger J, Baldi P, Wallace DC. An enhanced MITOMAP with a global mtDNA mutational phylogeny. Nucleic Acids Research. 2007;35(Database issue):D823–D828. doi: 10.1093/nar/gkl927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303(5655):223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Wallace DC. Evidence for adaptive selection acting on the tRNA and rRNA genes of the human mitochondrial DNA. Human Mutation. 2006;27(11):1072–1081. doi: 10.1002/humu.20378. [DOI] [PubMed] [Google Scholar]
- Sarzi E, Brown M, Lebon S, Chretien D, Munnich A, Rotig A, Procaccio V. A novel recurrent mitochondrial DNA mutation in ND3 gene is associated with isolated complex I deficiency causing Leigh syndrome and dystonia. American Journal of Medical Genetics. 2007;143A(1):33–41. doi: 10.1002/ajmg.a.31565. [DOI] [PubMed] [Google Scholar]
- Schaefer AM, Taylor RW, Turnbull DM, Chinnery PF. The epidemiology of mitochondrial disorders--past, present and future. Biochimica et Biophysica Acta. 2004;1659(2–3):115–120. doi: 10.1016/j.bbabio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Stajich JE, Block D, Boulez K, Brenner SE, Chervitz SA, Dagdigian C, Fuellen G, Gilbert JG, Korf I, Lapp H, et al. The Bioperl toolkit: Perl modules for the life sciences. Genome Res. 2002;12(10):1611–1618. doi: 10.1101/gr.361602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt JM, Dementieva YA, Martin ER, Scott WK, Nicodemus KK, Kroner CC, Welsh-Bohmer KA, Saunders AM, Roses AD, Small GW, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neuroscience Letters. 2004;365(1):28–32. doi: 10.1016/j.neulet.2004.04.051. [DOI] [PubMed] [Google Scholar]
- van der Walt JM, Nicodemus KK, Martin ER, Scott WK, Nance MA, Watts RL, Hubble JP, Haines JL, Koller WC, Lyons K, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. American Journal of Human Genetics. 2003;72(4):804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijsden RG, Gerards M, Eijssen LM, Hendrickx AT, Jongbloed RJ, Wokke JH, Hintzen RQ, Rubio-Gozalbo ME, De Coo IF, Briem E, et al. Chip-based mtDNA mutation screening enables fast and reliable genetic diagnosis of OXPHOS patients. Genetics in Medicine. 2006;8(10):620–627. doi: 10.1097/01.gim.0000237782.94878.05. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annual Review of Genetics. 2005a;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. The mitochondrial genome in human adaptive radiation and disease: On the road to therapeutics and performance enhancement. Gene. 2005b;354:169–180. doi: 10.1016/j.gene.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Why do we have a maternally inherited mitochondrial DNA? Insights from Evolutionary Medicine. Annual Review of Biochemistry. 2007 Jul;76 doi: 10.1146/annurev.biochem.76.081205.150955. 2007. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Brown MD, Lott MT. Mitochondrial DNA variation in human evolution and disease. Gene. 1999;238(1):211–230. doi: 10.1016/s0378-1119(99)00295-4. [DOI] [PubMed] [Google Scholar]
- Wallace DC, Lott MT, Procaccio V. Mitochondrial Genes in Degenerative Diseases, Cancer and Aging. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery and Rimoin's Principles and Practice of Medical Genetics. 5th Edition. Philadelphia, PA: Churchill Livingstone Elsevier; 2007. pp. 194–298. [Google Scholar]
- Wallace DC, Stugard C, Murdock D, Schurr T, Brown MD. Ancient mtDNA sequences in the human nuclear genome: a potential source of errors in identifying pathogenic mutations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14900–14905. doi: 10.1073/pnas.94.26.14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Michikawa Y, Mallidis C, Bai Y, Woodhouse L, Yarasheski KE, Miller CA, Askanas V, Engel WK, Bhasin S, et al. Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4022–4027. doi: 10.1073/pnas.061013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LJ, Liang MH, Kwon H, Park J, Bai RK, Tan DJ. Comprehensive scanning of the entire mitochondrial genome for mutations. Clinical Chemistry. 2002;48(11):1901–1912. [PubMed] [Google Scholar]
- Zhang J, Asin-Cayuela J, Fish J, Michikawa Y, Bonafe M, Olivieri F, Passarino G, De Benedictis G, Franceschi C, Attardi G. Strikingly higher frequency in centenarians and twins of mtDNA mutation causing remodeling of replication origin in leukocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1116–1121. doi: 10.1073/pnas.242719399. [DOI] [PMC free article] [PubMed] [Google Scholar]