Abstract
Ovarian cancer is the second most common gynecological cancer and the five-year survival rate is only about 40%. High-grade serous carcinoma is the pre-dominant histotype associated with hereditary ovarian cancer and women with inherited mutations in BRCA1 have a lifetime risk of 40–60%. BRCA1 and its isoform BRCA1a are multifunctional proteins that are the most evolutionary conserved of all the other splice variants. Our group has previously reported that BRCA1/1a proteins, unlike K109R and C61G mutants, suppress growth of ovarian cancer cells by tethering Ubc9. In this study we found wild type BRCA1/1a proteins to induce expression of caveolin-1, a tumor suppressor in BRCA1-mutant serous epithelial ovarian cancer (SEOC) cells by immunofluorescence analysis. The K109R and C61G disease associated mutant BRCA1 proteins that do not bind Ubc9 were not as efficient in up-regulation of caveolin-1 expression in SEOC cells. Additionally, immunofluorescence analysis showed BRCA1/1a proteins to induce redistribution of Caveolin-1 from cytoplasm and nucleus to the cell membrane. This is the first study demonstrating the physiological link between loss of Ubc9 binding, loss of growth suppression and loss of Caveolin-1 induction of disease-associated mutant BRCA1 proteins in SEOC cells. Decreased Caveolin-1 expression combined with elevated Ubc9 expression can in the future be used as an early biomarker for BRCA1 mutant SEOC.
Keywords: BRCA1, BRCA1a, Ubc9, Serous Epithelial Ovarian Cancer, Caveolin-1, Protein-protein
Introduction
Ovarian cancer is the second most common gynecological cancer and over 95 percent of malignant tumors are of the epithelial type. Epithelial cancer of the ovary is a malignant transformation of the epithelium of the surface of the ovary, peritoneum, or uterine tube [1]. Approximately 10% of the epithelial ovarian cancers (EOC) are caused by mutations in the tumor suppressor gene BRCA1 [2,3]. In sporadic EOC, BRCA1 mutations are rare, but reduced expression or aberrant subcellular localization of BRCA1 is common [4–6]. The majority of epithelial ovarian cancers (EOC) are of the serous subtype and they are further subdivided into high-grade and low-grade tumors. High-grade serous carcinoma is the predominant histotype associated with hereditary ovarian cancer and women with inherited mutations of BRCA1 have a lifetime risk of 40–60 % [7] African-American women are less likely to receive recommended surgery and chemotherapy for advanced epithelial ovarian cancer. Incomplete treatment is correlated with decreased survival and, between 1975 and 2005, the 5-year survival rate for United States white women with advanced ovarian cancer improved from 37% to 45% but declined for black women from 43% to 38% [8].
Our lab has identified and cloned two major isoforms of BRCA1, namely BRCA1a/p110 and BRCA1b/p100 [9,10], which are the most evolutionary conserved of all the isoforms and expressed at reduced levels in ovarian cancers compared to normal cells [11–14]. We found BRCA1a protein to induce apoptosis and inhibit in vivo tumor growth of hormone-independent ES-2 ovarian cancer cells, but the mechanism of tumor suppression is not known [15,16]. BRCA1 and its splice variants are nuclear proteins that have several functional domains, an N-terminal RING finger domain that interacts with several proteins and two BRCA1 C-terminal domains. We have found BRCA1, BRCA1a and BRCA1b proteins to be localized in the mitochondria, and their nuclear-cytoplasmic shuttling to be a regulated process [9,14,17]. BRCA1 nuclear import and export is mediated by the action of nuclear localization signal (NLS) and nuclear export signals (NES) located in the RING domain that mediates nuclear export via association with BARD1 [18]. The BRCA1 delta 11 isoform, which lacks NLS, also enters the nucleus via the RING-domain mediated BARD1 import pathway [19]. The RING domain of BRCA1, in complex with BARD1, mediates an E3 Ubiquitin ligase activity on ER-α in-vitro [20,21]. Recent studies using an Ubiquitin ligase-deficient BRCA1 I26A mutant suggested that the Ubiquitin ligase activity is dispensable for both genomic stability as well as homology-directed repair of double-strand DNA breaks, but is required for inhibition of ER-α activity [22,23]. Post-translational modification of proteins is reversible and normal cells use this mechanism to regulate cellular proliferation [24]. SUMO (Small Ubiquitin-like modifier) modification of proteins affects several functions like stability, localization, protein-protein interactions and transcriptional regulation (reviewed by [25–27]). The SUMO modification pathway was shown to be involved in BRCA1 response to DNA damage and transcriptional repression [28,29]. We have shown that the amino-terminal domain of BRCA1, BRCA1a and BRCA1b proteins bind to SUMO-E2-conjugating enzyme Ubc9 and regulate ER-α activity by promoting its degradation in vivo [30]. This work suggested a relationship between the SUMO and Ubiquitin pathways, similar to the Ubiquitin ligase RNF4, by highlighting the biochemical function of BRCA1 as a putative SUMO-1 and Ubc9-dependent E3 Ubiquitin ligase for ER-α SUMO conjugates [31,32].
Ubc9 binding site mutations, as well as disease associated mutation in the BRCA1 RING domain (C61G), disrupted the ability to regulate Ubc9-mediated estrogen-induced ER-α transcriptional activity in breast cancer cells [30] but did not disrupt SUMO-1 binding [28] nor auto ubiquitination activity of BRCA1 [30]. Both BRCA1/BRCA1a K109R and disease associated C61G mutants, which are localized mainly in the cytoplasm, fail to inhibit the growth of breast and ovarian cancer cells [33,34]. Ubc9 has been shown to play an important role in both cancer progression and resistance to chemotherapy [35– 38]. In fact, Ubc9 was found to act as both a positive and negative regulator of proliferation and transformation of HMGA1 proteins [37].
Caveolae are invaginations in the plasma membrane 60–80nm in diameter associated with various caveolin proteins for endocytosis, signal transduction, and vesicular transport. Caveolin-1 and 2 are most highly expressed throughout the body in endothelial cells, adipocytes, smooth muscle cells, and fibroblasts, while caveolin-3 appears to be the only form found in skeletal and cardiac muscle [41,42]. Caveolin-1 can directly regulate the activity of signaling molecules within caveolae. Interaction with caveolin-1 leads to the inhibition of the basal activity of signaling molecules and their downstream pathways. When stimulated, inhibition of these molecules facilitated by caveolin-1 is halted, allowing signal propagation. Many of the proteins that interact with, transcriptionally repress, or are inhibited by caveolin-1 fall under the pro-proliferative, oncogenic, and anti-apoptotic category of molecules. These molecules include G-protein coupled receptors, protein kinase C, and receptor tyrosine kinase. Studies using caveolin-1- null cells have demonstrated that caveolin-1 inhibits cell proliferation and cell-cycle progression. Caveolin-1-null mouse embryo fibroblasts display increased proliferation rates and cell cycle progression [43]. Caveolin-1 also has genetic characteristics that may contribute to its ability to affect proliferation rates. The human caveolin-1 locus revealed that it maps to 7q31.1, adjacent to the marker D7S522, a fragile site with deletions in many tumors in cancers such as breast, prostate, and ovarian [44]. Hence, caveolin-1 is thought of as a putative tumor suppressor of which its decreased expression allows for cancer progression. Down-regulated expression of caveolin-1 is seen in metastatic breast cancer cells. Using a model of spontaneous breast metastasis, caveolin-1 appeared to be expressed in low and non-metastatic primary tumors and to be expressed at much lower levels in highly metastatic tumors [45]. Thus, in metastatic tumors, the role of caveolin-1 as a tumor suppressor is absent, allowing the tumor to spread. In a study of caveolin-1 in ovarian cancer, immunohistochemical analysis of caveolin-1 shows normal expression of caveolin-1 in the surface epithelium and in the underlying stroma of normal ovary. A similar staining is apparent in the epithelial lining of a serous tumor, although loss of the membrane-associated localization and down-regulation can be observed in a grade 1 serous carcinoma. A complete loss of caveolin-1 expression is seen in high-grade serous epithelial carcinoma [46]. BRCA1 has been shown to induce the transcriptional activation of the caveolin-1 gene in mouse embryo fibroblast cells [47]. Here, we have further investigated these findings and have studied the effect of wild type BRCA1, BRCA1a and altered Ubc9 binding BRCA1a mutants on the expression of caveolin-1 in a physiologically relevant BRCA1 mutant SEOC cells.
Materials and Methods
Expression Constructs
Full length BRCA1a, BRCA1a Mut #1 and BRCA1a Mut #4, were cloned into pCDNA3 vectors as described previously [30]. Point mutations were generated as described previously [30].
Cell Culture
UWB1.289 and UWB1.289 BRCA1 cells were obtained from American Type Culture Collection (Rockville, MD, USA) and cultivated as described previously [48].
Antibodies and Reagents
The antibodies used in this study were MS110 ascites (Ab1, EMD Millipore), polyclonal rabbit anti-caveolin-1 antibody (Santa Cruz Biotechnology).
Immunoflourescence Analysis
To analyze the subcellular localization UWB1.289 and UWB1.289+BRCA1 cells were seeded into 6-well plates a day before transfection with pcDNA3 BRCA1a and their respective mutant plasmids. Cells were fixed in methanol 24 hrs after transfection and blocked with 10% BSA, followed by primary Monoclonal Mouse anti-caveolin-1 antibody 1:150 dilute for 1hr and Alexa488 goat anti-mouse (Molecular Probes) for 50 min, in combination with staining with DAPI dye or Hoechst. The cells were visualized under a fluorescent microscope (Olympus, 100× oil lens) as described previously [34] or LSM 700 Confocal Microscope (Carl Zeiss, 63× oil lens).
Results
BRCA1 and Caveolin-1 Expression is Reduced Significantly in BRCA1 Mutant SEOC Cells
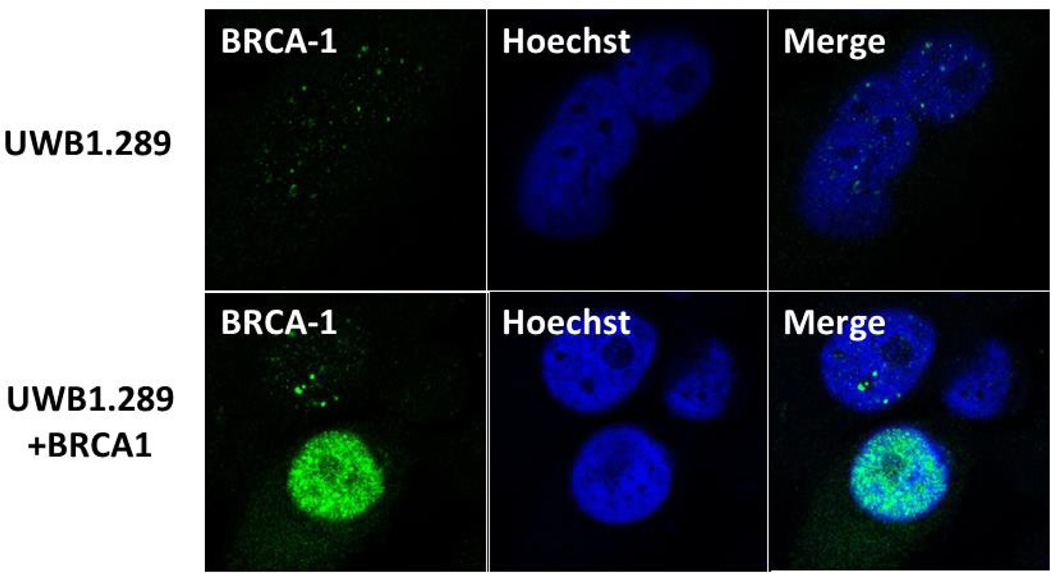
Caveolin-1 is a major structural component of caveolae and participates in many physiological functions [49–51]. Immunohistochemistry revealed expression of caveolin-1 in normal and benign ovarian epithelial cells, but loss of expression in serous ovarian carcinomas [46]. We therefore, initially examined the expression of BRCA1 and caveolin-1 in a BRCA1-mutant human ovarian cancer cell line, UWB1.289. UWB1.289 is a BRCA1-null ovarian cancer cell line obtained from a papillary serous tumor [48]. This cell line carries a germline BRCA1 mutation within exon 11 and has a deletion of the wild-type allele. Immunoflourescence analysis using BRCA1 antibody showed very low levels of expression of BRCA1 in UWB1.289 cells compared to UWB1.289 BRCA1 cells (Figure1). Similarly immunoflourescence analysis using caveolin-1 antibody revealed low level expression of caveolin-1 in UWB1.289 cells (Figure 2). These results suggest that loss of BRCA1 in UWB1.289 can down regulate caveolin-1 expression similar to what was observed previously in serous ovarian carcinomas [48].
Figure 1.
Loss of BRCA1 expression in BRCA1 mutant SEOC cell line UWB1.289 cells by immunoflourescence analysis. UWB1.289 and UWB1.289+BRCA1 cells were seeded into six-well plates and after 24 hours the nuclei were visualized with DNA staining dye Hoechst. Cells were fixed in icy methanol and probed with BRCA1 antibody (EMD Millipore, Ab1 1/100 followed by Alexa Fluor 488 labeled secondary antibody (Invitrogen, 1/200) as described previously [34]. The nuclei were visualized by Hoechst staining. The images were taken using LSM 700 Confocal Microscope 63× oil lense, Carl Zeiss).
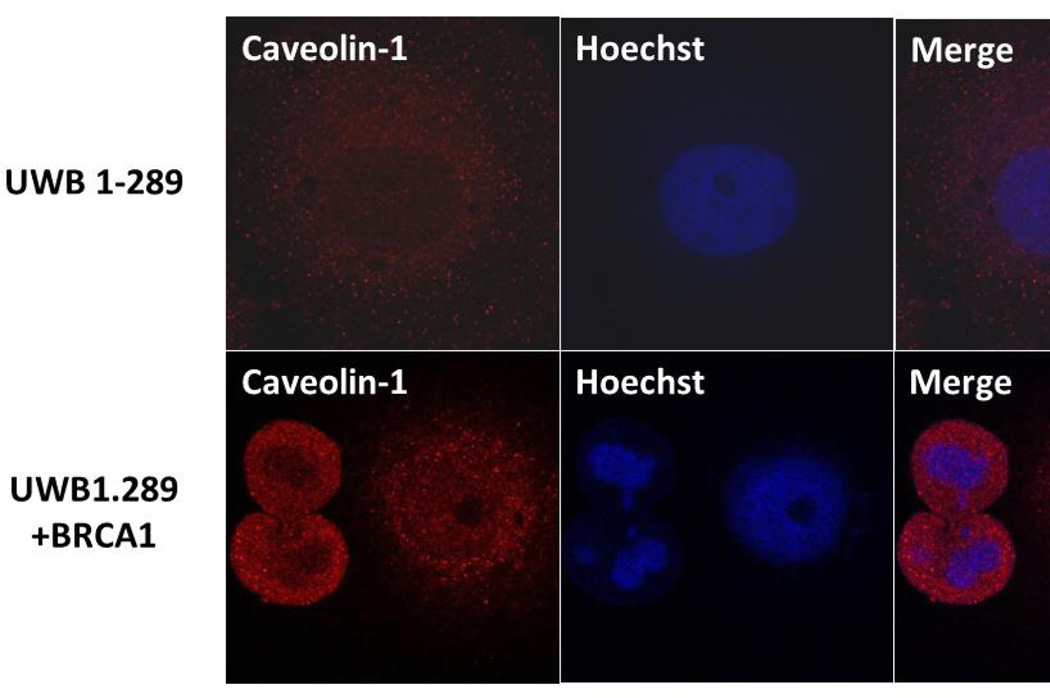
Figure 2.
Caveolin-1 is expressed at very low levels in BRCA1 mutant SEOC cells UWB1.289 cells and BRCA1 induces caveolin-1 expression in UWB1.289 cells as detected by immunofluorescence analysis. UWB1.289 and UWB1.289+BRCA1 cells were seeded into six-well plates. The nuclei were visualized using DNA staining dye Hoechst. Cells were fixed in ice cold methanol and probed with caveolin-1 antibody (Santa Cruz, Caveolin-1 1/250) followed by Alexa Fluor 568 labeled secondary antibody (Invitrogen, 1/200) as described previously [34]. The nuclei were visualized by Hoechst staining. The images were taken using LSM 700 Confocal Microscope 63× oil lense, Carl Zeiss).
Wild Type BRCA1 Protein Induces Caveolin-1 Expression in BRCA1 Mutant SEOC Cells
Since we observed low levels of expression of caveolin-1 in BRCA1 mutant UWB1.289 cells and if this is due to loss of BRCA1 then introducing wild type BRCA1 into these cells should induce expression of caveolin-1. We studied the expression of caveolin-1 in UWB1.289 and UWB1.289 BRCA1 cells by immunoflourescence analysis using caveolin-1 antibodies. We observed high levels of expression of caveolin-1 in UWB1.289 BRCA1 cells compared to parental UWB1.289 cells (Figure 2). We also observed a more concentrated membrane staining in UWB1.289 BRCA1 cells (Figure 2). These results are consistent with what was observed previously by Wang et al. [47] in BRCA1+/+ MEF cells.
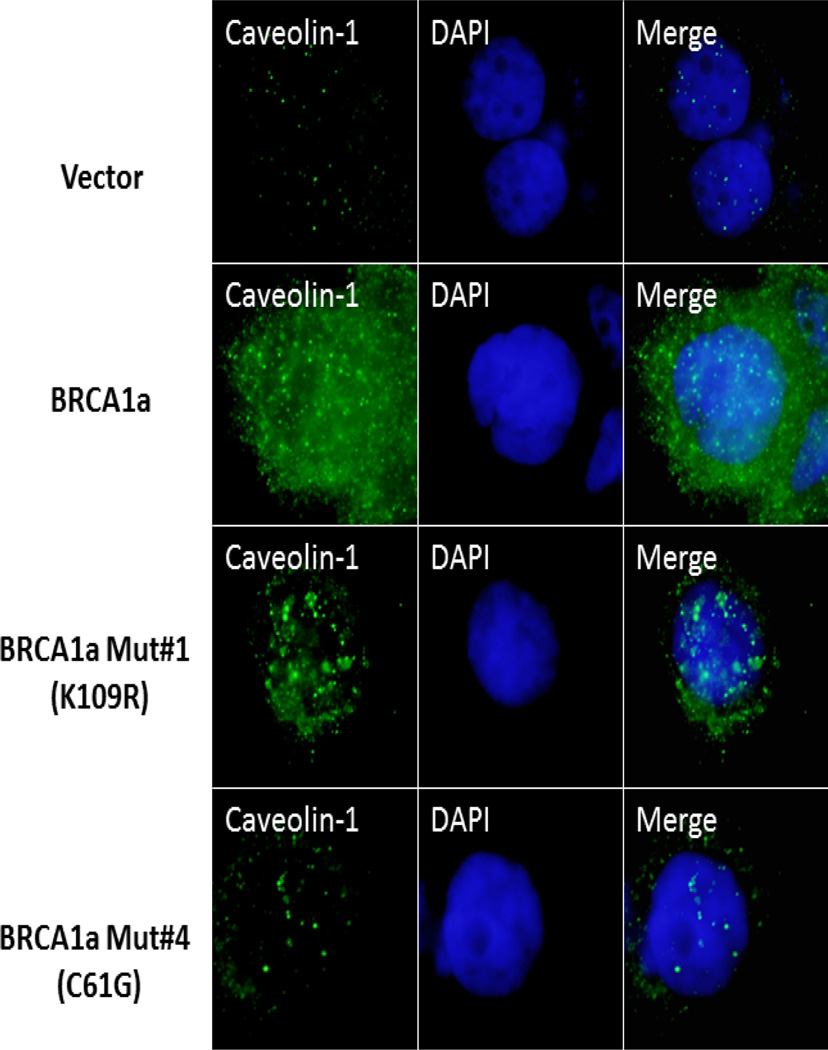
BRCA1a but not Ubc9 Binding BRCA1a Mutants are Able to Induce Caveolin-1 Expression in BRCA1 Mutant SEOC Cells
BRCA1 and BRCA1a proteins inhibit the growth of human breast and ovarian cancer cells [16,52–56]. By subjecting BRCA1a, their corresponding Mut#1 K109R, and cancer-predisposing Mut#4 C61G to colony suppression assays using ovarian cancer cells, we were able to show the requirement of Ubc9 binding on the growth suppressor function of BRCA1a proteins in ovarian cancer cells [16]. We wanted to test whether Ubc9 binding by BRCA1 proteins has anything to do with inducing caveolin-1 expression in UWB1.289 cells. We transfected UWB1.289 cells with BRCA1a, BRCA1a Mut#1 and BRCA1a MUT#4 and studied the expression of caveolin-1 by immunoflourescence analysis using caveolin-1 antibodies. We observed high expression of caveolin-1 in BRCA1a transfected UWB1.289 cells compared to BRCA1a Mut#1 and BRCA1a Mut#4 transfected cells (Figure 3). Furthermore we also observed redistribution of caveolin-1 to the plasma membrane unlike the two BRCA1a mutants. These results are consistent with the notion that a direct association of BRCA1a proteins with Ubc9 is critical for inducing the expression of caveolin-1 in SEOC cells. These results also suggest that a direct interaction of BRCA1 with Ubc9 may be needed for growth/tumor suppression by BRCA1 /1a proteins and lack of binding results in deregulated Ubc9 levels causing SEOC (Figure 4).
Figure 3.
Wild type BRCA1a but not the Ubc9 binding mutants induce caveolin-1 expression in BRCA1 mutant SEOC cells, UWB1.289 by immunofluorescence analysis. UWB1.289 cells were seeded into six-well plates and transfected with pcDNA3 or pcDNA3 BRCA1a or pcDNA3 BRCA1a Mut#1 or pcDNA3 BRCA1a Mut#4 using X-tremeGENE 9 DNA transfection reagent (Roche). The nuclei were visualized with DNA staining dye DAPI 24 hours after transfection. Cells were fixed in ice cold methanol and probed with caveolin-1 antibody (Santa Cruz, caveolin-1 1/250) followed by Alexa Fluor 488 labeled secondary antibody (Invitrogen, 1/200) staining as described [34]. The nuclei were visualized by 4, 6-Diamidino-2-Phenylindole (DAPI) staining. The images were taken using fluorescent microscope (100×, oil Olympus).
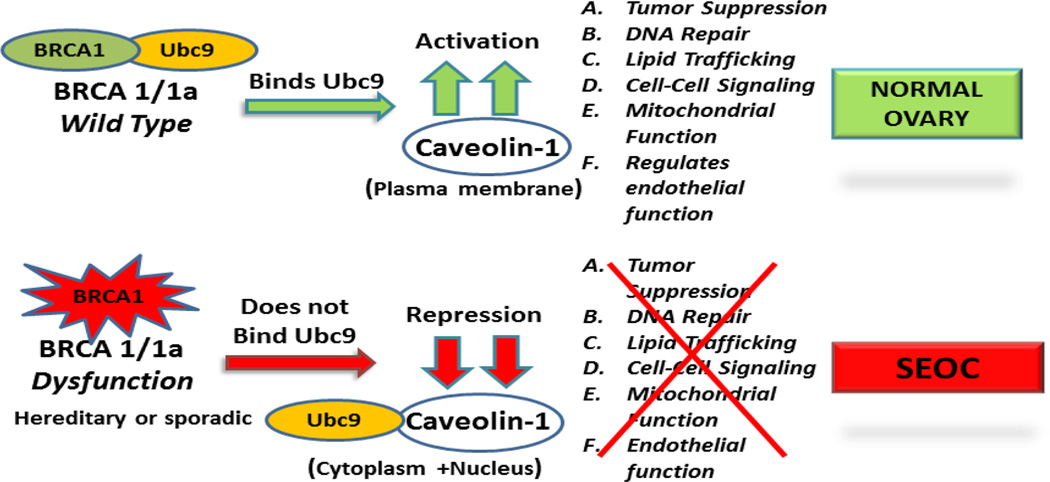
Figure 4.
Working hypothetical model showing how BRCA1/1a binding to Ubc9 regulates caveolin-1 expression and function in normal ovaries. In SEOC with BRCA1 dysfunction, Ubc9 is unleashed which inhibits caveolin-1 expression causing loss of DNA repair, lipid trafficking, cellular signaling, endothelial and mitochondrial function resulting in SEOC.
Discussions
Women who have a mutation in the BRCA1 gene have an increased risk of developing EOC. High grade serous carcinoma (HGSC) is the most common and lethal histotype associated with germ line BRCA1 mutation. Recent evidence suggests that the fallopian tube could be the most likely tissue of origin of HGSC [7]. Investigating the functional significance of loss of BRCA1 in women with ovarian cancer is critical to understanding how BRCA1 dysfunction results in ovarian cancer. Caveolin-1, a tumor suppressor is the major structural protein of caveolae and plays a critical role in the regulation of various physiological [47,49–51] and pathological processes such as cardiovascular diseases, cancers and neurological disorders. Immunohistochemistry demonstrated expression of caveolin-1 in normal and benign ovarian epithelial cells, but loss of expression of caveolin-1 was seen in SEOC [46]. BRCA1 up regulates caveolin-1 mRNA levels via tethering caveolin-1 promoter in MEF’s cells [47].
For the first time, we are demonstrating low levels of expression of caveolin-1 in the UWB1.289 cells and high levels of caveolin-1 in UWB1.289 BRCA1 cells using immunofluorescence analysis. These results are in agreement with work done by others in MEF cells [47]. We also observed up regulation of caveolin-1 in UWB1.289 cells that have been transfected with BRCA1a and very low levels in BRCA1a K109R and disease associated C61G mutant proteins. Additionally BRCA1/BRCA1a expression led to the distribution of caveolin-1 more to the plasma membrane unlike the Ubc9 binding BRCA1a Mut#1 and BRCA1a Mut#4. As demonstrated by us earlier, the Ubc9 binding mutants fail to bind Ubc9, lack E3 Ubiquitin ligase activity, fail to suppress the growth of ovarian cancer cells and are mislocalized in the cytoplasm of ovarian cancer cells [34]. These results demonstrate for the first time that BRCA1/BRCA1a proteins need to tether Ubc9 in order to induce expression of caveolin-1 in SEOC cells. BRCA1 dysfunction as seen in sporadic SEOC could unleash Ubc9 resulting in down regulation of caveolin-1 expression causing loss of multiple physiological functions of caveolin-1 (like tumor suppression, DNA repair, lipid trafficking, cellular signaling, endothelial and mitochondrial function) resulting in tilting the balance towards ovarian cancer (Figure 4). Since caveolin-1 was found to translocate to the plasma membrane in the presence of BRCA1/BRCA1a proteins, we can speculate that this may provide an important mechanism for regulating the tumor suppression function in sporadic ovarian cancers where somatic mutations in BRCA1 are rarely found. Future efforts will be directed towards understanding whether BRCA1/BRCA1a proteins use Ubc9 binding as a “switch” to control caveolin-1 expression enabling rapid and tight regulation of ovarian cell growth. This work could help in identifying biomarkers to detect ovarian cancer earlier thus reducing the mortality associated with high grade SEOC.
Acknowledgements
We thank all the members of Drs. Rao and Reddy labs for their help. We thank Mr. Abramson, research media services, Ms Wimes and Mr.Hill for editorial assistance and RCMI core facilities at Morehouse School of Medicine, for their assistance. This work was supported in part by Georgia Cancer Coalition Distinguished Cancer Scholar award, NIH-NCRR-RCMI grant G-12-RR003034, U54 RR02613, 5P20RR11104, NIHMD research endowment grant 2S21MD000101 and U54 CA118638 to V.N.R. Georgia Cancer Coalition Distinguished Cancer Scholar award to E.S.P.R. V.N.R’s lab was also supported in part by funds from the ING foundation and a summer research stipend to Stephanie Agyemang and Mercedes Giles by NIH/NIMHD grant 5P20MD006881.
Reference
- 1.Arpita D, Xu J, Aysola K, Qin Y, Okoli C, et al. Epithelial Ovarian Cancer: An Overview. World J Transl Med. 2014;3:1–8. doi: 10.5528/wjtm.v3.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 3.Berchuck A, Heron KA, Carney ME, Lancaster JM, Fraser EG, et al. Frequency of germline and somatic BRCA1 mutations in ovarian cancer. Clin Cancer Res. 1998;4:2433–2437. [PubMed] [Google Scholar]
- 4.Merajver SD, Pham TM, Caduff RF, Chen M, Poy EL, et al. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995;9:439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- 5.Clark-Knowles KV, O'Brien AM, Weberpals JI. BRCA1 as a Therapeutic Target in Sporadic Epithelial Ovarian Cancer. J Oncol. 2010;2010:891059. doi: 10.1155/2010/891059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguewa PA, Fuertes MA, Cepeda V, Alonso C, Quevedo C, et al. Poly(ADP-ribose) polymerase-1 inhibitor 3-aminobenzamide enhances apoptosis induction by platinum complexes in cisplatin-resistant tumor cells. Med Chem. 2006;2:47–53. doi: 10.2174/157340606775197697. [DOI] [PubMed] [Google Scholar]
- 7.George SH, Shaw P. BRCA and Early Events in the Development of Serous Ovarian Cancer. Front Oncol. 2014;4:5. doi: 10.3389/fonc.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell EA, Egorova N, Hayes MP, Wisnivesky J, Franco R, et al. Racial Disparities in the Treatment of Advanced Epithelial Ovarian Cancer. Obstet Gynecol. 2013;122:1025–1032. doi: 10.1097/AOG.0b013e3182a92011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Shao N, Ding OM, Cui J, Reddy ESP, et al. BRCA1 proteins are transported to the nucleus in the absence of serum and splice variants BRCA1a, BRCA1b are tyrosine phophoprotein that associate with E2F, cyclin and cyclin dependent kinases. Oncogene. 1997;15:143–157. doi: 10.1038/sj.onc.1201252. [DOI] [PubMed] [Google Scholar]
- 10.Chai YL, Cui J, Chipitsyna G, Liao B, Lui S, et al. C-Fos oncogene regulator Elk-1 interacts with BRCA1 splice variants BRCA1a/1b and enhances BRCA1a/1b mediated growth suppression in breast cancer cells. Oncogene. 2001;20:1357–1367. doi: 10.1038/sj.onc.1204256. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CA, Payton MN, Elliott ES, Buaas FW, Cajulis EE, et al. Differential subcellular localization, expression and biological toxicity of BRCA1 and the splice variant BRCA1-delta 11b. Oncogene. 1997;14:1–16. doi: 10.1038/sj.onc.1200924. [DOI] [PubMed] [Google Scholar]
- 12.Lu M, Conzen SD, Cole CN, Arrick B. Characterizations of functional messenger RNA splice variants of BRCA1 expressed in non malignant and tumor-derived breast cells. Cancer Res. 1996;56:4578–4581. [PubMed] [Google Scholar]
- 13.Orban TI, Olah E. Expression profiles of BRCA1 splice variants in a synchronous and in G1/S synchronized tumor cell lines. Biochem Biophys Res Commun. 2001;280:32–38. doi: 10.1006/bbrc.2000.4068. [DOI] [PubMed] [Google Scholar]
- 14.Maniccia A, Lewis C, Begum N, Xu J, Cui J, et al. Mitochondrial localization, ELK-1 transcriptional regulation and Growth inhibitory functions of BRCA1, BRCA1a and BRCA1b proteins. J Cell Physiol. 2009;219:634–641. doi: 10.1002/jcp.21708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shao N, Chai YL, Reddy ESP, Rao VN. Induction of apoptosis by the tumor suppressor protein BRCA1. Oncogene. 1996;13:1–7. [PubMed] [Google Scholar]
- 16.Chai Y, Shao N, Lee L, Reddy V, Gabriela O, et al. BRCA1a has antitumor activity in Triple-negative breast and ovarian and prostate cancer cells. Oncogene. 2007;26:6031–6037. doi: 10.1038/sj.onc.1210420. [DOI] [PubMed] [Google Scholar]
- 17.Coene ED, Hollinshead MS, Waeytens AA, Schelfhout VR, Eechaute WP, et al. Phosphorylated BRCA1 is predominantly located in the nucleus and mitochondria. Mol Biol Cell. 2005;16:997–1010. doi: 10.1091/mbc.E04-10-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen EM, Fan S, Ma Y. BRCA1 regulation of transcription. Cancer Lett. 2006;236:175–185. doi: 10.1016/j.canlet.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 19.Fabbro M, Rodriguez JA, Baer R, Henderson BR. BARD1 induces BRCA1 intranuclear foci formation by increasing RING-dependent BRCA1 nuclear import and inhibiting BRCA1 nuclear export. J Biol Chem. 2002;277:21315–21324. doi: 10.1074/jbc.M200769200. [DOI] [PubMed] [Google Scholar]
- 20.Hashizume R, Fukuda M, Maeda I, Nishikawa H, Oyake D, et al. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J Biol Chem. 2001;276:14537–14540. doi: 10.1074/jbc.C000881200. [DOI] [PubMed] [Google Scholar]
- 21.Eakin CM, Maccoss MJ, Finney GL, Klevit RE. Estrogen receptor alpha is a putative substrate for the BRCA1 ubiquitin ligase. Proc Natl Acad Sci U S A. 2007;104:5794–5799. doi: 10.1073/pnas.0610887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reid LJ, Shakya R, Modi AP, Lokshin M, Cheng JT, et al. E3 ligase activity of BRCA1 is not essential for mammalian cell viability or homology-directed repair of double-strand DNA breaks. Proc Natl Acad Sci U S A. 2008;105:20876–20881. doi: 10.1073/pnas.0811203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y, Fan S, Hu C, Meng Q, Fuqua SA, et al. BRCA1 regulates acetylation and ubiquitination of estrogen receptor-alpha. Mol Endocrinol. 2010;24:76–90. doi: 10.1210/me.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Kim KI, Baek SH, Chung CH. Versatile protein tag, SUMO: its enzymology and biological function. J Cell Physiol. 2002;191:257–268. doi: 10.1002/jcp.10100. [DOI] [PubMed] [Google Scholar]
- 26.Seeler JS, Dejean A. Nuclear and unclear functions of SUMO. Nat Rev Mol Cell Biol. 2003;4:690–699. doi: 10.1038/nrm1200. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 28.Morris JR, Boutell C, Keppler M, Densham R, Weekes D, et al. The SUMO modification pathway is involved in the BRCA1 response to genotoxic stress. Nature. 2009;462:886–890. doi: 10.1038/nature08593. [DOI] [PubMed] [Google Scholar]
- 29.Park MA, Seok YJ, Jeong G, Lee JS. SUMO1 negatively regulates BRCA1-mediated transcription, via modulation of promoter occupancy. Nucleic Acids Res. 2008;36:263–283. doi: 10.1093/nar/gkm969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu J, Watkins T, Reddy A, Reddy ES, Rao VN. A novel mechanism whereby BRCA1/1a/1b fine tunes the dynamic complex interplay between SUMO-dependent/independent activities of Ubc9 on E2-induced ERalpha activation/repression and degradation in breast cancer cells. Int J Oncol. 2009;34:939–949. doi: 10.3892/ijo_00000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H, Leverson JD, Hunter T. Conserved function of RNF4 family proteins in eukaryotes: targeting a ubiquitin ligase to SUMOylated proteins. EMBO J. 2007;26:4102–4112. doi: 10.1038/sj.emboj.7601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uzunova K, Gottsche K, Miteva M, Weisshaar SR, Glanemann C, et al. Ubiquitin-dependent proteolytic control of SUMO conjugates. J Biol Chem. 2007;282:34167–34175. doi: 10.1074/jbc.M706505200. [DOI] [PubMed] [Google Scholar]
- 33.Qin Y, Xu J, Aysola K, Begum N, Reddy V, et al. Ubc9 Mediates Nuclear Localization and Growth Suppression of BRCA1 and BRCA1a Proteins. J Cell Physiol. 2011;226:3355–3367. doi: 10.1002/jcp.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qin Y, Xu J, Aysola K, Oprea G, Reddy A, et al. BRCA1 proteins regulate growth of ovarian cancer cells by tethering Ubc9. Am J Cancer Res. 2012;2:540–548. [PMC free article] [PubMed] [Google Scholar]
- 35.Ronen O, Malone JP, Kay P, Bivens C, Hall K, et al. Expression of a novel marker, Ubc9, in squamous cell carcinoma of the head and neck. Head Neck. 2009;31:845–855. doi: 10.1002/hed.21048. [DOI] [PubMed] [Google Scholar]
- 36.Dunnebier T, Bermejo JL, Haas S, Fischer HP, Pierl CB, et al. Common variants in the UBC9 gene encoding the SUMO-conjugating enzyme are associated with breast tumor grade. Int J Cancer. 2009;125:596–602. doi: 10.1002/ijc.24286. [DOI] [PubMed] [Google Scholar]
- 37.Driscoll JJ, Pelluru D, Lefkimmiatis K, Fulciniti M, Prabhala RH, et al. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood. 2010;115:2827–2834. doi: 10.1182/blood-2009-03-211045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mo YY, Yu Y, Ee PL, Beck WT. Overexpression of a dominant-negative mutant Ubc9 is associated with increased sensitivity to anticancer drugs. Cancer Res. 2004;64:2793–2798. doi: 10.1158/0008-5472.can-03-2410. [DOI] [PubMed] [Google Scholar]
- 39.Moschos SJ, Jukic DM, Athanassiou C, Bhargava R, Dacic S, et al. Expression analysis of Ubc9, the single small ubiquitin-like modifier (SUMO) E2 conjugating enzyme, in normal and malignant tissues. Hum Pathol. 2010;41:1286–1298. doi: 10.1016/j.humpath.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Lu J, Prochownik E. Dual Role for SUMO E2 Conjugase Ubc9 in Modulating the Transforming and Growth-promoting Properties of the HMGA1b Architectural Transcription Factor. J Biol Chem. 2007;18:13363–13371. doi: 10.1074/jbc.M610919200. [DOI] [PubMed] [Google Scholar]
- 41.Jin Z, Wang L, Cao Z, Cheng Y, Gao Y, et al. Temporal Evolution in Caveolin-1 Methylation Levels during Human Esophageal Carcinogenesis. BMC Cancer. 2014;14:345. doi: 10.1186/1471-2407-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastiani M, Parton RG. Caveolae at a Glance. J Cell Sci. 2010;123:3831–3836. doi: 10.1242/jcs.070102. [DOI] [PubMed] [Google Scholar]
- 43.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, et al. Caveolin-1 and Cancer Metabolism in the Tumor Microenvironment: Markers, Models, and Mechanisms. Annu Rev Pathol. 2012;7:423–467. doi: 10.1146/annurev-pathol-011811-120856. [DOI] [PubMed] [Google Scholar]
- 44.Razani B, Engelman JA, Wang XB, Schubert W, Zhang XL, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 45.Sloan EK, Stanley KL, Anderson RL. Caveolin-1 Inhibits Breast Cancer Growth and Metastasis. Oncogene. 2004;23:7893–7897. doi: 10.1038/sj.onc.1208062. [DOI] [PubMed] [Google Scholar]
- 46.Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, et al. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001;159:1635–1643. doi: 10.1016/S0002-9440(10)63010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Yu J, Zhan Q. BRCA1 regulates caveolin-1 expression and inhibits cell invasiveness. Biochem Biophys Res Commun. 2008;370:201–206. doi: 10.1016/j.bbrc.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 48.DelloRusso C, Welcsh PL, Wang W, Garcia RL, King MC, et al. Functional Characterization of a novel BRCA1-null ovarian cancer cell line in response to ionizing radiation. Mol Cancer Res. 2007;5:35–45. doi: 10.1158/1541-7786.MCR-06-0234. [DOI] [PubMed] [Google Scholar]
- 49.Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, Caveolae, and Endothelial Cell Function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 50.Asterholm IW, Mundy DI, Weng J, Anderson RG, Scherer PE. Altered mitochondrial function and metabolic inflexibility associated with loss of caveolin-1. Cell Metab. 2012;15:171–185. doi: 10.1016/j.cmet.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu H, Yue J, Pan Z, Wu H, Cheng Y, et al. Involvement of Caveolin-1 in repair of DNA damage through both homologous recombination and non-homologous end joining. PLoS One. 2010;5:e12055. doi: 10.1371/journal.pone.0012055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holt JT, Thompson ME, Szabo C, Robinson-Benion C, Arteaga CL, et al. Growth retardation and tumour inhibition by BRCA1. Nat Genet. 1996;12:298–302. doi: 10.1038/ng0396-298. [DOI] [PubMed] [Google Scholar]
- 53.Aprelikova ON, Fang BS, Meissner EG, Cotter S, Campbell M, et al. BRCA1-associated growth arrest is RB-dependent. Proc Natl Acad Sci U S A. 1999;96:11866–11871. doi: 10.1073/pnas.96.21.11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tait DL, Obermiller PS, Holt JT. Preclinical studies of a new generation retroviral vector for ovarian cancer BRCA1 gene therapy. Gynecol Oncol. 2000;79:471–476. doi: 10.1006/gyno.2000.5969. [DOI] [PubMed] [Google Scholar]
- 55.Randrianarison V, Marot D, Foray N, Cabannes J, Meret V, et al. BRCA1 carries tumor suppressor activity distinct from that of p53 and p21. Cancer Gene Ther. 2001;8:759–770. doi: 10.1038/sj.cgt.7700366. [DOI] [PubMed] [Google Scholar]
- 56.Marot D, Opolon P, Brailly-Tabard S, Elie N, Randrianarison V, et al. The tumor suppressor activity induced by adenovirus-mediated BRCA1 overexpression is not restricted to breast cancers. Gene Ther. 2006;13:235–244. doi: 10.1038/sj.gt.3302637. [DOI] [PubMed] [Google Scholar]