Abstract
Opioid systems mainly regulate physiological functions such as pain, emotional tone and reward circuitry in neural tissues (brain and spinal cord). These systems are also found in extraneural tissues (ganglia, gut, spleen, stomach, lung, pancreas, liver, heart, blood and blood vessels), and recent studies have elucidated their roles in various organs. The current review focuses on the roles of opioid systems in blood vessels, especially angiogenesis, during development and tumour malignancy. The balance between endogenous activators and inhibitors of angiogenesis delicately maintains a normally quiescent vasculature to sustain homeostasis. Disturbance of this balance causes pathogenic angiogenesis and, especially in tumours, several activators such as VEGF are highly expressed in the tumour microenvironment and strongly induce tumour angiogenesis, the so-called angiogenic switch. Recently, we demonstrated that κ opioid receptor agonists function as anti-angiogenic factors, which impede the angiogenic switch, in vascular development and tumour angiogenesis by inhibiting the expression of receptors for VEGF. In clinical medicine, angiogenesis inhibitors that target VEGF signalling such as bevacizumab are used as anti-cancer drugs. Although therapies that inhibit tumour angiogenesis have been highly successful for tumour therapy, most patients eventually develop resistance to this anti-angiogenic therapy. Thus, we must identify novel targets for anti-angiogenic agents to sustain inhibition of angiogenesis for tumour therapy. The regulation of responses to κ opioid receptor ligands could be useful for controlling vascular formation under physiological conditions and in cancers, and thus could offer therapeutic benefits beyond the relief of pain.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: opioid, angiogenesis, endothelial cells, embryonic stem cells, tumour, cancer therapy
Introduction
One of the earliest events in organogenesis is the development of the vascular system, which contributes to the formation of most organs in our bodies. The vascular system is first formed as a primitive vascular network by the differentiation and assembly of vascular progenitor cells derived from mesodermal cells. These progenitor cells undergo a complex remodelling process, in which growth, migration, sprouting and pruning lead to the development of a functional circulatory system. Earlier studies have suggested that many of the events in normal vascular formation during embryogenesis are recapitulated during de novo angiogenesis in adults such as tumour angiogenesis and neovascularization induced after tissue damage (Carmeliet, 2003). Furthermore, the disordered vascular function triggers the development of lifestyle-related diseases such as hypertension, diabetes and hyperlipidaemia. Thus, a better understanding of vascular biology may lead to novel strategies for the treatment of a variety of diseases.
It has been recognized for hundreds of years that the vascular network is closely associated with the neuronal network throughout development and in adulthood. Blood vessels deliver oxygen and nutrients throughout the body, and also provide some nerve-related growth factors to guide neurons to target organs. On the other hand, neural tissues also provide vascular-related growth factors such as VEGF and members of the Wnt signalling pathways, under physiological conditions, indicating that the vasculature could communicate with neurons and form complicated three-dimensional vascular networks. VEGF-A derived from neurons is important for blood vessel ingression, correct vascular density, fine-tuning of the blood vessel pattern and arterial differentiation (Kutcher et al., 2004; Mukouyama et al., 2005; James et al., 2009). Members of the Wnt subfamily derived from neurons promote the acquisition of characteristics of the blood–brain barrier (BBB) in intra-neural vessels (Stenman et al., 2008; Daneman et al., 2009). Furthermore, repulsive axon guidance molecules such as plexin/semaphorine/neuropilin (NRP) coordinate with VEGF-A signalling to determine the pattern of blood vessel ingression in the neural tube (Miao et al., 1999; Bates et al., 2003). Therefore, vascular–nerve networks play critical roles in vascular formation in both embryos and adults.
Although endogenous opioids were first characterized in the brain, these transmitters and their receptors (μ κ and δ; receptor nomenclature follows Alexander et al., 2013a) are found in both neural (brain and spinal cord) and extraneural tissues (ganglia, gut, spleen, stomach, lung, pancreas, liver, heart, blood and blood vessels). Opioids and opioid receptors are present in blood vessels from the later stages of the rat embryo [embryonic day (E)-16] through to adulthood (Zagon et al., 1996; Wu et al., 1998). Treatment with opioid peptides inhibited both angiogenesis in a chick chorioallantoic membrane model (Blebea et al., 2000) and DNA synthesis in rat vascular walls (Zagon et al., 1996). In adults, the endogenous opioid system has been shown to be active in hemodynamic and cardiovascular responses, such as haemorrhagic shock, sepsis and trauma (Molina, 2002). The κ opioid receptor agonist U-50,488H has beneficial effects on vascular injury after spinal cord trauma by improving vascular permeability and oedema (Qu et al., 1993). Moreover, morphine, an agonist at μ opioid receptors, suppresses tumour angiogenesis through the inhibition of hypoxia-inducible transcription factors (HIFs), which enhances the expression of VEGF-A and VEGF receptors (Koodie et al., 2010). These findings suggest that opioid systems play important roles in vascular functions, although their physiological roles and molecular mechanisms remain largely unknown.
Roles of opioid systems in vascular development
VEGF signalling in vascular development
Several factors affecting vascular formation, such as VEGF, NRP, angiopoietins, TGF-β, PDGF, fibroblast growth factor (FGF), ephrin and notch have been identified over the past few decades, mainly by the characterization of vascular-mutant phenotypes in mice. Among these factors, VEGF signalling is a key modulator of vascular development during embryogenesis and for neovascularization in the adult (Coultas et al., 2005). In mammals, five VEGF ligands, VEGF-A, -B, -C, -D and placenta growth factor, have been identified and have been shown to bind in an overlapping pattern to three receptor tyrosine kinases, known as VEGF receptor-1, -2, -3 (VEGFR1–3; receptor nomenclature follows Alexander et al., 2013b)), as well as to co-receptors such as heparin sulphate proteoglycans and NRPs. VEGF-A heterozygote knockout mice die early in gestation due to failure of the vascular formation (Carmeliet et al., 1996). On the other hand, the two- to threefold overexpression of VEGF-A from its endogenous locus results in abnormal heart formation and lethality at E12.5 to E14.0 (Miquerol et al., 2000), indicating that strictly balanced VEGF function is important in normal embryogenesis. Furthermore, the intensity of VEGF signalling is strictly regulated through ligand-receptor interaction. VEGFR2 (also known as Flk1 in mice or KDR in humans) is tyrosine-phosphorylated much more efficiently than VEGFR1 (also known as Flt1) upon VEGF binding (Millauer et al., 1993; Waltenberger et al., 1994; Shibuya, 2006). Although VEGFR1 tyrosine kinase-deficient homozygous mice developed normal vessels and survived (Hiratsuka et al., 1998), mice that were homozygous for point mutation at Tyr1173 of VEGFR2 (Tyr1175 in human VEGFR2) died at E8.5 to E9.5 without any organized blood vessels or yolk sac blood islands, and haematopoietic progenitors were severely reduced, as seen with Flk-1 null mice (Sakurai et al., 2005). Interestingly, VEGFR1-null mice die at midgestation with vascular overgrowth and disorganization (Fong et al., 1995). Taken together, these findings suggest that VEGFR2 is the major receptor in endothelial cells (ECs) for VEGF-induced responses, and VEGF signal intensity on VEGFR2 is regulated by the binding of VEGF to the higher affinity receptor, VEGFR1.
Another receptor for VEGF, NRP1, is expressed in ECs of blood vessels and endocardial cells of the heart (Kitsukawa et al., 1995; Kawakami et al., 1996; Soker et al., 1998). NRP1 is also expressed in particular classes of developing neurons and functions as a receptor for the class 3 semaphorins that mediate semaphorin-elicited inhibitory axon guidance signals to neurons (Kitsukawa et al., 1995; Kawakami et al., 1996; He and Tessier Lavigne, 1997). NRP1, together with VEGFR2, forms a specific receptor for VEGF165, an isoform of VEGF, and the VEGFR2–VEGF165–NRP1 complex potently enhances VEGFR2 signalling (Soker et al., 1998). NRP1-null mice die midway through gestation at E10.5 to E12.5 and exhibit defects in the heart, vasculature, and nervous system (Kawakami et al., 1996). Overexpression of NRP1 resulted in the excess production of blood vessels and malformed hearts (Kitsukawa et al., 1995). These findings indicated that NRP1 plays a critical function in the formation of blood vessels, along with VEGF.
Inhibitory effects of the κ opioid agonists in vascular formation
Many studies on vascular development have focused on gene knockout and gene inhibition using mice and zebra fish. Although these studies have led to the discovery of essential factors in vascular development, they could not identify the sufficient conditions required for vascular formation. To clarify the ‘constructive’ mechanisms that underlie vascular development, we have developed a novel embryonic stem (ES)/induced pluripotent stem (iPS) cell differentiation system that exhibits early vascular development using VEGFR2-positive cells as common progenitors for vascular cells (Figure 1A) (Yamashita et al., 2000; Narazaki et al., 2008). In the early embryo and in differentiating ES/iPS cells, VEGFR2 expression marks a common progenitor for both blood and endothelium. ES/iPS cell-derived VEGFR2+ cells can differentiate into both ECs and mural cells (vascular smooth muscle cells and pericytes) and form mature vascular-like structures in vitro. With the use of this system, we had proposed that vascular formation was accomplished via two mechanisms (Figure 1B) (Yamamizu et al., 2010; Yamamizu and Yamashita, 2011): first, a basal mechanism for common EC differentiation, where VEGF signalling plays a central role, and second, a vascular diversification mechanism that works on the basis of common EC differentiation. Vascular diversification such as artery and vein formation can only be achieved by the action of specific mechanisms in the presence of the basal EC machinery. We have shown that cAMP/PKA signalling contributes to common EC differentiation through the upregulation of VEGF-A receptors, VEGFR2 and NRP1 (Figure 1B) (Yamamizu et al., 2009).
Figure 1.
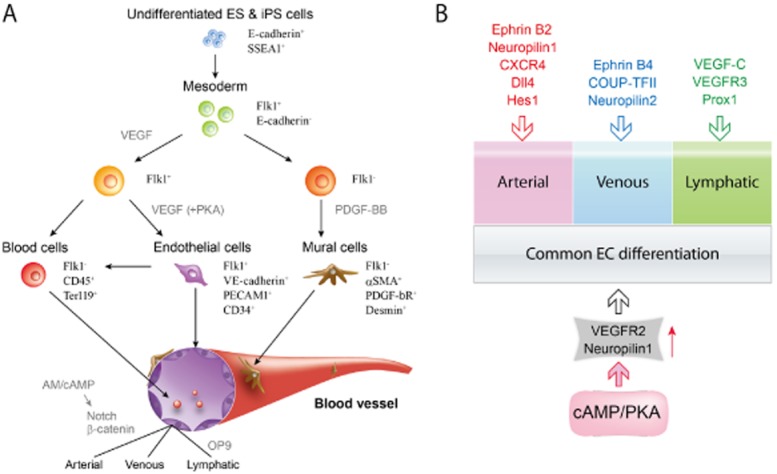
Vascular differentiation system using ES cells and iPS cells (A). Flk1 (VEGFR2) positive cells derived from ES/iPS cells are vascular progenitors that can differentiate into ECs, mural cells and blood cells. ECs have specific characters of arterial-venous-lymphatic ECs. (B) EC differentiation and vascular diversification in vascular development. Vascular formation in embryogenesis is considered to have two main mechanisms: (i) a basal mechanism for common EC differentiation, in which VEGF signalling plays a central role; and (ii) a vascular diversification mechanism working on the basis of common EC differentiation. Vascular diversification, such as artery and vein formation, can be achieved only by activating specific mechanisms in the presence of the basal EC machinery. cAMP/PKA signalling contributes to common EC differentiation through up-regulation of VEGF-A receptors, Flk1 and NRP1. Other protein critically involved in differentiation into arterial, venous or lymphatic vessels are: delta like ligand 4 (Dll 4); hairy and enhancer of split-1 (Hes 1); prospero homeobox 1 (Prox 1); COUP transcription factor 2 (COUP-TFII) (adapted from Yamamizu and Yamashita, 2011).
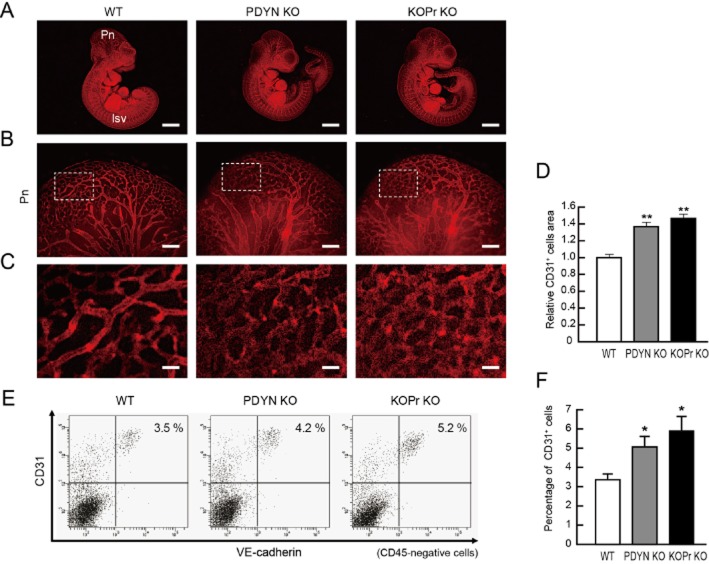
Opioid systems are mainly present in neural tissues and could be involved in neurogenesis during brain development (Zhu et al., 1998; Tripathi et al., 2008). The three opioid receptors, μ, δ and κ, mainly act as inhibitory G (Gi) protein-coupled receptors through which endogenous opioids (endorphins, enkephalins and dynorphins) regulate physiological functions (Kieffer and Gaveriaux Ruff, 2002). These receptors also activate other G protein-dependent signalling such as Gs or Gq, and G protein-independent signalling, for instance through β-arrestin (Galandrin et al., 2007). We investigated whether opioid systems (ligands and receptors) were involved in vascular formation with our vascular differentiation system using ES cells. Interestingly, κ opioid receptors, but not μ or δ opioid receptors, were highly expressed in vascular progenitors and ECs, and negatively regulated EC differentiation and in vitro vascular formation via the inhibition of cAMP/PKA signalling (Yamamizu et al., 2011). Activation of κ opioid receptors with U50,488H and TRK820 inhibited the expression of VEGFR2 and NRP1, but not other VEGF receptors. Mice lacking κ opioid receptors (κ opioid receptor-null) or dynorphin (an endogenous κ opioid receptor agonist) showed a significant increase in vascular formation in early embryos (Figure 2). Moreover, ectopic vascular invasion into somites of E10.5 embryos accompanied by decreased plexinD1 expression in ECs was observed in both strains of null mice (Yamamizu et al., 2011). Therefore, the κ opioid receptor system may be a dual inhibitory regulator of EC differentiation and of vascular angiogenesis.
Figure 2.
Representative results of WT, prodynorphin-null (PDYN KO) and κ opioid receptor-null (KOPr KO) mouse embryos at E10.5 (A). Whole-mount CD31 (red) staining. Left panels, WT mice. Pn; perineural vascular plexus, Isv; intersomitic vessels. Middle panels, PDYN KO mice. Right panels, KOPr KO mice. Scale bars: 2 mm. (B) High-magnification views of CD31-stained Pn region. Scale bars: 200 μm. (C) Higher magnification views corresponding to boxed regions in Figure 2B. Scale bars: 40 μm. (D) Quantitative evaluation of CD31+ area in Pn. CD31 staining of WT mice was set as 1.0. (n = 3, **P < 0.01 vs. WT). (E) Flow cytometry. X-axis: VE-cadherin, Y-axis: CD31. Percentages of CD31+/VE-cadherin+/CD45-ECs in the embryo are indicated. (F) Quantitative evaluation of CD31+/VE-cadherin+/CD45-ECs in the embryo. n = 3; *P < 0.05 vs. WT (adapted from Yamamizu et al., 2011).
Roles of the κ opioid receptor system in tumour angiogenesis
Angiogenic switch in tumours
Tumour angiogenesis is required for tumour progression, to provide nutrients and oxygen and to remove metabolic wastes and carbon dioxide (Folkman, 1971; Carmeliet and Baes, 2008). The balance between endogenous activation and inhibition of angiogenesis critically maintains a normally quiescent vasculature to sustain homeostasis. One characteristic feature of tumour blood vessels is that they have lost the appropriate balance between positive and negative controls and fail to become quiescent, leading to the constant progression of tumour angiogenesis (Bergers and Benjamin, 2003). Therefore, restoration of the balance between activation and inhibition of angiogenesis is a critical treatment strategy for tumours.
Growth factors and hypoxia are known to induce VEGF-A gene expression. The hypoxia-inducible transcription factor, HIF, is a strong inducer of VEGF and contributes to the formation of vascular tubes in embryogenesis as well as in adults. Many studies have shown that HIF is highly expressed in various types of tumours, thereby enhancing angiogenesis via VEGF and reproducing tumour cells. Mice lacking HIF1a-, HIF2a- and HIF-related genes exhibit vascular defects and death at E9.5–E10.5 (Dunwoodie, 2009), indicating that HIF-related VEGF production regulates vascular formation. Moreover, oncogene signalling molecules such as Ras and Myc in cancer cells, up-regulate VEGF expression, which would lead to the formation of vasculature and the proliferation of tumours (Carmeliet, 2005).
Angiogenesis inhibitors in cancer therapy
The concept of using angiogenesis inhibitors as anticancer drugs was received with considerable scepticism when first presented by Dr. Folkman in the early 1970s (Folkman, 1971). Solid tumours cannot grow beyond 2 to 3 mm in diameter without being able to recruit their own blood supply. Bevacizumab, a humanized monoclonal antibody that is specific for human VEGF-A, was the first anti-angiogenic agent approved by the Food and Drugs Administration (FDA) in 2004 for the treatment of colorectal cancer, renal cell cancer, non-small cell lung cancer, and glioblastoma (Ferrara et al., 2004). Furthermore, sunitinib and sorafenib were approved by the FDA in 2008 as multi-target tyrosine kinase inhibitors (TKIs) and have demonstrated efficacy against various solid tumours in clinical trials (Llovet et al., 2008; Ivy et al., 2009; Huang et al., 2010). TKIs can interact physically with a highly conserved kinase domain shared by VEGFR1–3, as well as PDGF receptors, FGF receptors, EGF receptor, Raf kinase and cKit. Although VEGF-targeted therapy for cancer has been highly successful for the prevention of tumour angiogenesis so far, most patients eventually acquire resistance to anti-angiogenic therapy and rapid vascular regrowth in tumours occurs after the discontinuation of anti-VEGF therapy. Furthermore, treatment with VEGF-targeted drugs has side effects, such as hypertension and proteinuria-related kidney dysfunction. Thus, there is a clear need to identify novel targets for anti-angiogenic therapeutic agents to achieve a continuous inhibition of angiogenesis for tumour therapy.
To date, approximately 30 endogenous inhibitors of angiogenesis have been identified (Nyberg et al., 2005). Many endogenous inhibitors including thrombospondin1 (TSP1), which was the first protein to be recognized as an endogenous angiogenesis inhibitor, are fragments of naturally occurring extracellular matrix and basement membrane proteins (Cao, 2001). The expression of TSP1 is inversely correlated with tumour progression in melanoma, lung and breast carcinoma (Zabrenetzky et al., 1994). Suppression of TSP1 augmented tumour angiogenesis through the production of matrix metalloprotease 9 and the enhancement of VEGFR2 signalling (Zabrenetzky et al., 1994). In contrast, TSP1 overexpression resulted in delayed tumour growth by the inhibition of tumour angiogenesis (Rodriguez Manzaneque et al., 2001). Although many studies on these endogenous angiogenesis inhibitors have shown that they significantly inhibit tumour angiogenesis and tumour growth, it is still difficult to accurately control their expression and to apply them in clinical practice.
Potential of κ opioid receptor agonists in cancer therapy
Opioid analgesics such as morphine have been broadly used to relieve pain from all types of cancer. The effect of morphine on tumour growth is still controversial. Independent studies have shown that morphine can either decrease or increase tumour growth in mice (Gupta et al., 2002; Tegeder et al., 2003; Koodie et al., 2010). A recent study showed that morphine suppressed tumour angiogenesis through the inhibition of HIF transcription, which enhances the expression of VEGF and VEGF receptors (Koodie et al., 2010). Moreover, morphine inhibited tumour cell proliferation through activation of p53 (Tegeder et al., 2003). On the other hand, morphine stimulated HUVEC proliferation and promoted tumour neovascularization in a human breast tumour, and further potentiated endothelial-pericyte interaction via PDGF-BB and PDGF receptor-β (PDGFR-β), thereby enhancing coverage of tumour vessels through pericyte recruitment (Gupta et al., 2002; Luk et al., 2012). Furthermore, methylnaltrexone, a peripherally restricted antagonist of μ, exerted a synergistic effect with 5-fluorouracil and bevacizumab on inhibition of VEGF-induced human pulmonary microvascular EC proliferation and migration (Singleton et al., 2008). However, naltrexone effectively induced new blood vessel growth in the chick chorioallantoic membrane assay (Blebea et al., 2000). In-depth investigations would be needed in purified ECs from tumours, but not tumours themselves, for elucidation of regulatory mechanisms of tumour angiogenesis by opioids.
Based on our earlier study of vascular development (Yamamizu et al., 2011), we investigated whether opioid agonists could act as anti-cancer drugs through the inhibition of VEGF signalling. In HUVEC and in ECs purified from adult mice, κ opioid receptors but not δ or μ opioid receptors, were highly expressed. Treatment with the κ opioid receptor agonists U50,488H and TRK820 significantly inhibited HUVEC migration and vascular tube formation by suppressing VEGFR2 expression (Yamamizu et al., 2013). Treatment with nor-BNI, a κ opioid receptor antagonist, blocked the effects of κ opioid receptor agonists on HUVEC migration. Interestingly, Lewis lung carcinoma (LLC) or B16 melanoma cells grafted in KOPr-knockout mice showed increased proliferation and markedly enhanced tumour angiogenesis, compared with those in wild-type mice. In contrast, repeated intraperitoneal injection of TRK820 significantly inhibited tumour growth by suppressing tumour angiogenesis (Figure 3) (Yamamizu et al., 2013). Nevertheless, we still do not know if the κ opioid receptor system in vivo acts as an anti-angiogenic mediator only in tumour vasculatures, or if it also is functional in other cell lineages such as pericytes, blood cells and the tumours themselves. Furthermore, because our studies showed that LLC or B16 melanoma grafted in prodynorphin-null mice showed increased proliferation of tumours compared with those in wild-type mice (unpublished data, Yamamizu et al., 2013), more careful studies using κ opioid receptor antagonists, which inhibit endogenous dynorphin will be needed.
Figure 3.
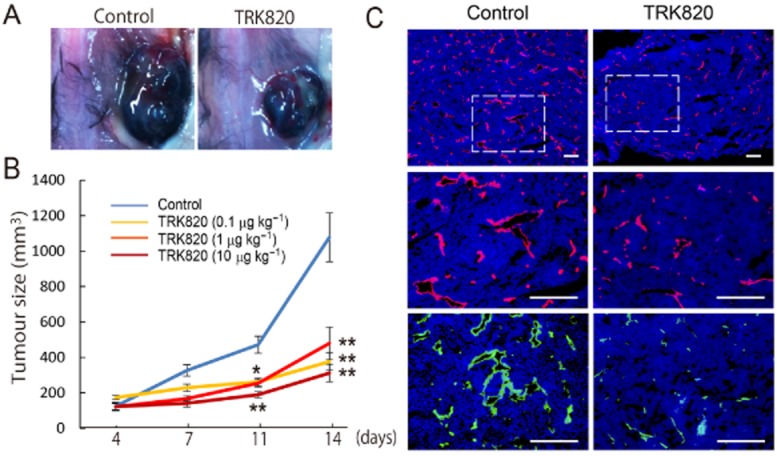
Effects of the κ opioid receptor agonist TRL-820 on melanoma. (A) Example of a mouse bearing B16 melanoma without treatment (control, left) or with TRK-820 treatment (right). (B) Quantitative analysis of tumour size among PBS-treated (n = 16) and TRK820 (0.1 μg·kg−1 (n = 9), 1 μg·kg−1 (n = 17), 10 μg·kg−1 (n = 9)-treated mice at 4, 7, 11, 14 days after tumour transplantation. **P < 0.01, *P < 0.05 vs. Control. (C) Fluorescent staining for CD31 (red) and VE-cadherin (green) at 14 days after tumour transplantation. Nuclei are stained with DAPI (blue). Left panel, PBS treated. Right panel, TRK820 (1 mg·kg−1)-treated. Scale bars: 200 μm (adapted from Yamamizu et al., 2013).
The κ opioid receptor agonist TRK820 (nalfurafine), which has been clinically approved in Japan for use in haemodialysis-related uremic pruritus, could be useful for tumour therapy by suppressing tumour angiogenesis, and thus could offer therapeutic benefits beyond the relief of cancer pain. Furthermore, because opioids have been used as analgesics for more than 2000 years, there is considerable experience of the clinical use of opioids. As opioid systems function through ligand–receptor interaction, it should be relatively easy to apply opioid agonists to cancer therapy. However, patients also develop tolerance to opioid receptor agonists, including TRK820, through repeated use (Suzuki et al., 2004). Our results showed that although a low dose (0.1–10 μg·kg−1, b.i.d.) of TRK820, which is effective for managing itching and pain in mice, inhibited tumour angiogenesis and tumour growth, constant treatment with a much higher dose (150 μg·kg−1) had no significant effect on tumour growth (unpublished data; Yamamizu et al., 2013). These results suggest that continuous treatment with high doses of κ opioid receptor agonists might lead to the development of tolerance to their anti-angiogenic effects on tumours or might induce biphasic effects for tumour angiogenesis or ligand off-target effects on tumour vasculatures. Therefore, more precise and careful observations are required to develop effective tumour therapies with κ opioid receptor agonists. Nevertheless, a better understanding of κ opioid receptor ligands as anti-angiogenic regulators and the ability to inhibit tumour angiogenesis by manipulating the opioid ligand-receptor system may lead to an feasible cancer therapy.
Summary and future direction
De novo angiogenesis is a critical process both in embryogenesis and in many cancers. The balance between angiogenic activators and inhibitors controls sprouting, elongation and stabilization of the blood vessels in most organs and in tumours. Although we have made significant progress in our understanding of opioid agonists as anti-angiogenic factors in vascular development and in tumours (Figure 4), much work remains to be done. We still do not fully understand the origins of opioids that act as angiogenesis inhibitors or the intensity and degree of the contribution of opioids in physiological angiogenesis in development and tumours. Furthermore, although many diseases of the vascular system can also affect the CNS, and vice versa, we do not fully understand whether the opioids from neural tissues contribute to vascular function and formation under pathological conditions. The multiple roles of VEGF signalling in endothelial development and function have made it the most popular target for therapeutic interventions in angiogenesis. A more refined understanding of the complex interaction between opioid systems and VEGF signalling, which controls all aspects of vascular formation and remodelling should provide novel and more specific targets for future therapeutic intervention.
Figure 4.
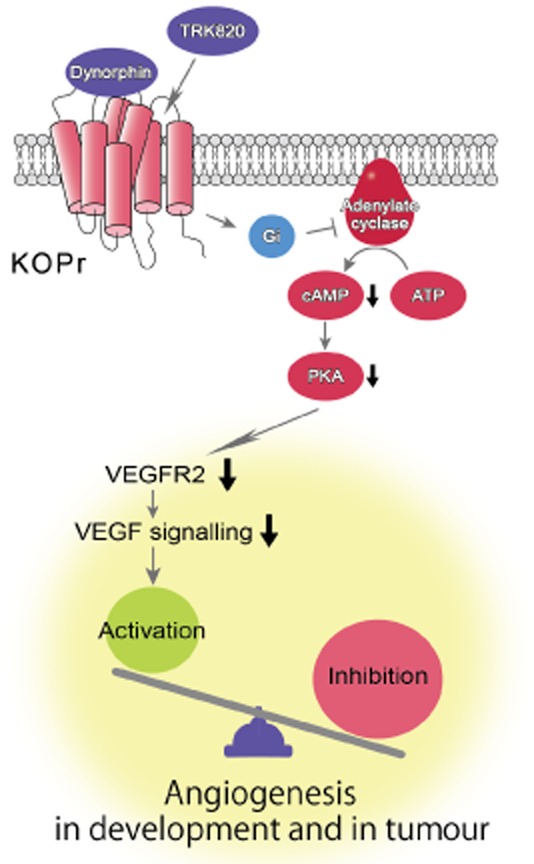
Signalling through κ opioid receptors (KOPr) induced by dynorphin or TRK820 regulates VEGF signalling, especially VEGFR2 expression, by activating the cAMP/PKA pathway in ECs. The balance between the expression of activators and inhibitors of angiogenesis controls angiogenesis in development and in tumours (adapted from Yamamizu et al., 2011).
Acknowledgments
We thank Dr JK Yamashita for supervising the studies on vascular formation, Dr H Nagase for giving us TRK820 and S Katayama for helping with the figures. This study was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan, the Ministry of Health, Labour and Welfare of Japan, the Project for Realization of Regenerative Medicine, the Japan Society for the Promotion of Science, and the Intramural Research Program of the NIH, National Institute on Aging.
Glossary
Abbreviations
- BBB
blood–brain barrier
- E
embryonic day
- ECs
endothelial cells
- ES
embryonic stem
- FGF
fibroblast growth factor
- Gi
inhibitory G protein
- HIF
hypoxia inducible factor
- iPS
induced pluripotent stem
- LLC
Lewis lung carcinoma
- NRP
neuropilin
- TKIs
tyrosine kinase inhibitors
- TSP1
thrombospondin1
Conflict of interest
The authors declared that they have no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013b;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Taylor GI, Minichiello J, Farlie P, Cichowitz A, Watson N, et al. Neurovascular congruence results from a shared patterning mechanism that utilizes semaphorin3A and neuropilin-1. Dev Biol. 2003;255:77–98. doi: 10.1016/s0012-1606(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Blebea J, Mazo JE, Kihara TK, Vu JH, McLaughlin PJ, Atnip RG, et al. Opioid growth factor modulates angiogenesis. J Vasc Surg. 2000;32:364–373. doi: 10.1067/mva.2000.107763b. [DOI] [PubMed] [Google Scholar]
- Cao Y. Endogenous angiogenesis inhibitors and their therapeutic implications. Int J Biochem Cell Biol. 2001;33:357–369. doi: 10.1016/s1357-2725(01)00023-1. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl. 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Baes M. Metabolism and therapeutic angiogenesis. N Engl J Med. 2008;358:2511–2512. doi: 10.1056/NEJMcibr0802500. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the mammalian embryo. Dev Cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- He Z, Tessier Lavigne M. Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Ding Y, Li Y, Luo WM, Zhang ZF, Snider J, et al. Sunitinib acts primarily on tumor endothelium rather than tumor cells to inhibit the growth of renal cell carcinoma. Cancer Res. 2010;70:1053–1062. doi: 10.1158/0008-5472.CAN-09-3722. [DOI] [PubMed] [Google Scholar]
- Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nat Rev Clin Oncol. 2009;6:569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami A, Kitsukawa T, Takagi S, Fujisawa H. Developmentally regulated expression of a cell surface protein, neuropilin, in the mouse nervous system. J Neurobiol. 1996;29:1–17. doi: 10.1002/(SICI)1097-4695(199601)29:1<1::AID-NEU1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- Koodie L, Ramakrishnan S, Roy S. Morphine suppresses tumor angiogenesis through a HIF-1alpha/p38MAPK pathway. Am J Pathol. 2010;177:984–997. doi: 10.2353/ajpath.2010.090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher ME, Klagsbrun M, Mamluk R. VEGF is required for the maintenance of dorsal root ganglia blood vessels but not neurons during development. FASEB J. 2004;18:1952–1954. doi: 10.1096/fj.04-2320fje. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Luk K, Boatman S, Johnson KN, Dudek OA, Ristau N, Vang D, et al. Influence of morphine on pericyte-endothelial interaction: implications for antiangiogenic therapy. J Oncol. 2012;2012:458385–458394. doi: 10.1155/2012/458385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, Wizigmann Voos S, Schnurch H, Martinez R, Moller NP, Risau W, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Miquerol L, Langille BL, Nagy A. Embryonic development is disrupted by modest increases in vascular endothelial growth factor gene expression. Development. 2000;127:3941–3946. doi: 10.1242/dev.127.18.3941. [DOI] [PubMed] [Google Scholar]
- Molina PE. Stress-specific opioid modulation of haemodynamic counter-regulation. Clin Exp Pharmacol Physiol. 2002;29:248–253. doi: 10.1046/j.1440-1681.2002.03638.x. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941–952. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- Nyberg P, Xie L, Kalluri R. Endogenous inhibitors of angiogenesis. Cancer Res. 2005;65:3967–3979. doi: 10.1158/0008-5472.CAN-04-2427. [DOI] [PubMed] [Google Scholar]
- Qu ZX, Xu J, Hogan EL, Hsu CY. Effect of U-50488h, a selective opioid kappa receptor agonist, on vascular injury after spinal cord trauma. Brain Res. 1993;626:45–49. doi: 10.1016/0006-8993(93)90561-z. [DOI] [PubMed] [Google Scholar]
- Rodriguez Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- Singleton PA, Garcia JG, Moss J. Synergistic effects of methylnaltrexone with 5-fluorouracil and bevacizumab on inhibition of vascular endothelial growth factor-induced angiogenesis. Mol Cancer Ther. 2008;7:1669–1679. doi: 10.1158/1535-7163.MCT-07-2217. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Izumimoto N, Takezawa Y, Fujimura M, Togashi Y, Nagase H, et al. Effect of repeated administration of TRK-820, a kappa-opioid receptor agonist, on tolerance to its antinociceptive and sedative actions. Brain Res. 2004;995:167–175. doi: 10.1016/j.brainres.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Tegeder I, Grosch S, Schmidtko A, Haussler A, Schmidt H, Niederberger E, et al. G protein-independent G1 cell cycle block and apoptosis with morphine in adenocarcinoma cells: involvement of p53 phosphorylation. Cancer Res. 2003;63:1846–1852. [PubMed] [Google Scholar]
- Tripathi A, Khurshid N, Kumar P, Iyengar S. Expression of delta- and mu-opioid receptors in the ventricular and subventricular zones of the developing human neocortex. Neurosci Res. 2008;61:257–270. doi: 10.1016/j.neures.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Waltenberger J, Claesson Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- Wu Y, McLaughlin PJ, Zagon IS. Ontogeny of the opioid growth factor, [Met5]-enkephalin, preproenkephalin gene expression, and the zeta opioid receptor in the developing and adult aorta of rat. Dev Dyn. 1998;211:327–337. doi: 10.1002/(SICI)1097-0177(199804)211:4<327::AID-AJA4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Yamashita JK. Roles of cyclic adenosine monophosphate signaling in endothelial cell differentiation and arterial-venous specification during vascular development. Circ J. 2011;75:253–260. doi: 10.1253/circj.cj-10-0915. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Kawasaki K, Katayama S, Watabe T, Yamashita JK. Enhancement of vascular progenitor potential by protein kinase A through dual induction of Flk-1 and neuropilin-1. Blood. 2009;114:3707–3716. doi: 10.1182/blood-2008-12-195750. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka Kanie M, et al. Convergence of Notch and beta-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189:325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamizu K, Furuta S, Katayama S, Narita M, Kuzumaki N, Imai S, et al. The kappa opioid system regulates endothelial cell differentiation and pathfinding in vascular development. Blood. 2011;118:775–785. doi: 10.1182/blood-2010-09-306001. [DOI] [PubMed] [Google Scholar]
- Yamamizu K, Furuta S, Hamada Y, Yamashita A, Kuzumaki N, Narita M, et al. κ Opioids inhibit tumor angiogenesis by suppressing VEGF signaling. Sci Rep. 2013;3:3213. doi: 10.1038/srep03213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, et al. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- Zabrenetzky V, Harris CC, Steeg PS, Roberts DD. Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int J Cancer. 1994;59:191–195. doi: 10.1002/ijc.2910590209. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Wu Y, McLaughlin PJ. Opioid growth factor-dependent DNA synthesis in the neonatal rat aorta. Am J Physiol. 1996;270:R22–R32. doi: 10.1152/ajpregu.1996.270.1.R22. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998;18:2538–2549. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]