Abstract
Addiction is a devastating disorder that affects 15.3 million people worldwide. While prevalent, few effective treatments exist. Orexin receptors have been proposed as a potential target for anti-craving medications. Orexins, also known as hypocretins, are neuropeptides produced in neurons of the lateral and dorsomedial hypothalamus and perifornical area, which project widely throughout the brain. The absence of orexins in rodents and humans leads to narcolepsy. However, orexins also have an established role in reward seeking. This review will discuss some of the original studies describing the roles of the orexins in reward seeking as well as specific works that were presented at the 2013 International Narcotics Research Conference. Orexin signalling can promote drug-induced plasticity of glutamatergic synapses onto dopamine neurons of the ventral tegmental area (VTA), a brain region implicated in motivated behaviour. Additional evidence suggests that orexin signalling can also promote drug seeking by initiating an endocannabinoid-mediated synaptic depression of GABAergic inputs to the VTA, and thereby disinhibiting dopaminergic neurons. Orexin neurons co-express the inhibitory opioid peptide dynorphin. It has been proposed that orexin in the VTA may not mediate reward per se, but rather occludes the ‘anti-reward’ effects of dynorphin. Finally, orexin signalling in the prefrontal cortex and the central amygdala is implicated in reinstatement of reward seeking. This review will highlight recent work describing the role of orexin signalling in cellular processes underlying addiction-related behaviours and propose novel hypotheses for the mechanisms by which orexin signalling may impart drug seeking.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: orexin, hypocretin, dynorphin, morphine, addiction, dopamine, VTA
Introduction
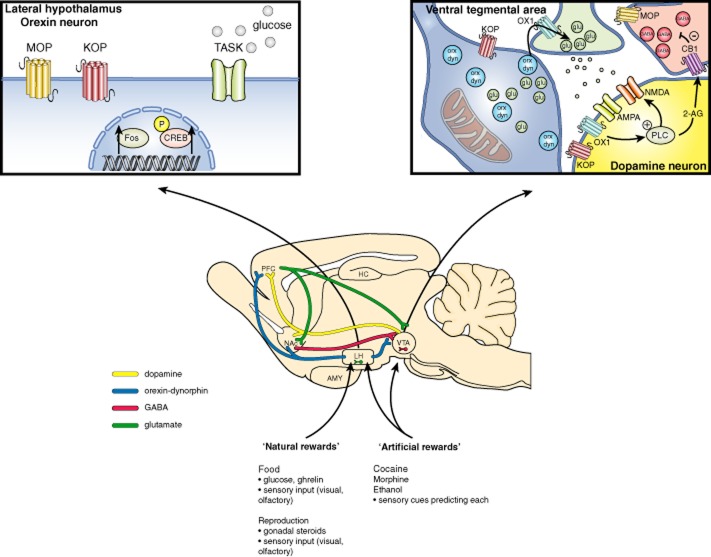
Neurons containing orexin, also known as hypocretin, are firmly established as regulators of reward seeking. Orexin-A and -B (hypocretin-1 and -2) are peptides produced in neurons residing in the lateral and dorsomedial hypothalamus and perifornical area (de Lecea et al., 1998; Sakurai et al., 1998) that have widespread projections throughout the brain (Peyron et al., 1998). Orexin containing neurons project from the lateral hypothalamus (LH) to many areas of the mesolimbic ‘reward pathway’ including the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (Di Chiara and Imperato, 1985; Koob and Bloom, 1988; Wise and Rompre, 1989) and these neurons are primarily implicated in reward-seeking behaviour. Within the VTA, 20% of neuronal inputs originating in the LH show immunolabelling for orexin (Balcita-Pedicino and Sesack, 2007). Moreover, orexin fibres co-distribute with dopamine fibres in both the medial prefrontal cortex (mPFC) and the medial shell of the NAc (Fadel and Deutch, 2002) suggesting that orexin and dopamine may interact at several levels in the reward system. Orexin innervation is present throughout the entirety of the VTA. Approximately 15% of orexin axons make appositional contacts in the VTA with 5% showing identifiable synaptic specializations onto both dopaminergic and GABAergic neurons (Balcita-Pedicino and Sesack, 2007). Because a function of many neuropeptides, including orexin, is the modulation of fast amino acid neurotransmitter signalling, and neuropeptide release is not limited to synaptic specializations, the low synaptic incidence in the VTA is likely to be not relevant to the ability of orexin to modulate the activity of VTA dopaminergic and GABAergic neurons. This is supported by the observation that orexin axons contain many dense core vesicles within the VTA (Balcita-Pedicino and Sesack, 2007), which are capable of exocytosis at extra-synaptic sites. From these sites, orexin can diffuse small distances to modulate nearby synapses. Orexin neurons co-express glutamate and the vesicular glutamate transporters VGlut1 and VGlut2 (Rosin et al., 2003; see Alexander et al., 2013a). Thus, the orexin-containing neurons that do synapse in the VTA likely represent a minor source of glutamate (Figure 1). Finally, orexin neurons also co-express the opioid dynorphin (Chou et al., 2001), and therefore may co-release dynorphin in the VTA or at other projection targets. This review aims to discuss (i) the involvement of hypothalamic orexin neurons in drug taking, seeking and withdrawal; (ii) the role of orexin receptor signalling in the VTA and ascending projection targets on drug seeking and reinstatement of drug seeking; and (iii) the possible role of co-released orexin and dynorphin on drug reward. Furthermore, specific work that was presented at the 2013 International Narcotics Research Conference (INRC) will be highlighted.
Figure 1.
Simplified diagram of pathways involved in orexin signalling. Neurohumoural information about natural rewards is integrated by orexin neurons of the LH. Drugs of abuse may act both locally in the LH and at orexin terminals in VTA. Exposure to rewards or reward cues increases Fos expression and phospho-CREB in orexin neurons (inset, upper left). In the VTA (inset, upper right) orexin can affect glutamate release onto dopaminergic DA neurons as well as stimulating them directly via postsynaptic OX1 receptors. Within DA neurons, OX1 activation can recruit synthetic enzymes (PLC) for endocannabinoids such as 2-AG, which can inhibit GABA release from interneurons in a retrograde fashion. Co-released dynorphin may also modulate the excitatory effects of its co-transmitter orexin. MOP, KOP; μ-opioid, κ−opioid receptors: TASK, K2P potassium channels.
Differential effects of OX1 and OX2 receptors on drug seeking
Once released, orexins elicit their effects via two GPCRs: the orexin-1 (OX1) receptor and orexin-2 (OX2) receptor (Sakurai et al., 1998; receptor nomenclature follows Alexander et al., 2013a). Both OX1 and OX2 receptors are somewhat promiscuous in their G-protein signalling with evidence linking both receptor subtypes to Gq, Gs and Gi/o interactions (Kukkonen and Leonard, 2014). Although the Gq pathway appears to have important functional roles, a lack of direct methods to measure G-protein activation has inhibited a definitive assessment of orexin receptor G-protein coupling (see Kukkonen and Leonard, 2014). In line with the extensive orexinergic projection fields throughout the neuraxis, OX1 and OX2 receptors are widely distributed within the brain (Trivedi et al., 1998). For example, OX1 receptors are more highly expressed in cortical regions, the bed nucleus of the stria terminalis and the locus coeruleus, whereas OX2 receptor density is enriched over OX1 receptors in the NAc and specific thalamic/hypothalamic regions. The VTA contains relatively similar expression of both receptors (Sakurai et al., 1998; Trivedi et al., 1998).
Recent evidence suggests a dichotomous role of OX1 and OX2 receptors in the brain. OX1 receptors are important for the neurobiological effects that drive drug seeking for morphine (Harris et al., 2005; 2007), cocaine (Harris et al., 2005; Borgland et al., 2006), nicotine (Pasumarthi et al., 2006; Hollander et al., 2008; Plaza-Zabala et al., 2010; 2012) and alcohol (Lawrence et al., 2006; Dayas et al., 2008; Richards et al., 2008; Moorman and Aston-Jones, 2009; Jupp et al., 2011b; Kim et al., 2012; Srinivasan et al., 2012). In contrast, OX2 receptors have been implicated more strongly in sleep/wake cycle regulation and arousal (Willie et al., 2003). While some studies suggest that OX2 receptors play a lesser role in drug seeking (Smith et al., 2009; Shoblock et al., 2011), the role of OX2 receptors in ethanol reinforcement and ethanol-seeking behaviour is less clear. To determine the role of OX2 receptors in ethanol-taking and ethanol-seeking behaviour, rats were trained to self-administer ethanol (10% w/v) or sucrose (0.7–1% w/v) in the presence of reward-associated cues. i.c.v. administration of the selective OX2 receptor antagonist TCS-OX2-29 reduced self-administration of ethanol, but not sucrose, and had no effect on cue-induced reinstatement of ethanol seeking (Brown et al., 2013). Furthermore, intra-NAc core, but not shell, infusions of TCS-OX2-29 decreased responding for ethanol (Brown et al., 2013). Thus, OX2 in addition to OX1 receptors may represent a potential therapeutic target for the treatment of alcohol use disorders. However, unlike OX1 receptors, no effect of OX2 receptor antagonism was observed on cue-induced reinstatement of ethanol seeking, suggesting a more prominent role for OX2 receptors in ethanol self-administration compared with cue-conditioned ethanol seeking. Importantly, however, these data are restricted to cue-driven ethanol seeking; it is quite possible that OX2 receptors may be implicated in other forms of reward seeking, such as stress-mediated or drug-primed reward seeking. Future studies are therefore required to further elucidate the varied roles of OX1 and OX2 receptors in cue, stress or drug-primed reward seeking, including identification of the anatomic loci and mechanisms underlying these effects. Furthermore, while morphine seeking and withdrawal are dependent on OX1 receptor signalling (Georgescu et al., 2003; Harris et al., 2005), no studies have addressed whether OX2 receptors are required for morphine seeking, reinstatement or withdrawal.
An fMRI study in rodents demonstrated that selective antagonists to either OX1 or OX2 receptors inhibited blood oxygen level-dependent (BOLD) responses to amphetamine. However, the selective OX1 receptor antagonist modulated functional responses in the striatum, whereas the OX2 receptor antagonist attenuated the amphetamine-induced response predominantly in the cortex (Gozzi et al., 2011). In a subsequent experiment, the same doses of the OX2 receptor antagonist were strongly sedating, while the OX1 receptor antagonist significantly reduced a cocaine-induced conditioned place preference (CPP) (Gozzi et al., 2011). Thus, the functional differences observed between OX1 and OX2 receptors on different aspects of drug seeking or arousal may be due to differential regional expression of these receptors in the brain.
Hypothalamic orexin neurons are activated by drugs, context, stress and withdrawal
The LH has been studied for its role in motivated behaviour for more than half a century (Hess, 1954). Early investigators found that non-contingent electrical stimulation of this tissue could evoke a wide variety of behavioural responses from simple increases in ambulation to more complex, goal-directed activities like feeding, drinking or copulation (Valenstein et al., 1970; Valenstein, 1973; Hoebel, 1976). These studies reached their apotheosis with the discovery that rodents (and indeed humans) would vigorously perform arbitrary operant responses for electrical stimulation to the LH (Olds and Milner, 1954; Heath, 1963). Since this time, the LH has been recognized as a critical node in a complex circuit that regulates reinforcement and reward (Kauer and Malenka, 2007). The LH appears to control responding not only for natural rewards like food or receptive mates, but also pharmacological rewards like drugs of abuse.
There is not only a close resemblance between the anatomical distribution of the orexin neuronal population and sites in the LH known to support rewarding self-stimulation (Valenstein et al., 1970; Peyron et al., 1998; Hollander et al., 2008; 2012), but also for the functional effects of orexin peptides when compared with LH stimulation. Indeed, central administration of orexin peptides evokes many of the same behaviours observed decades before with electrical stimulation of the LH (see Table 1). These observations prompted research on the role of orexin peptides in reward and motivated behaviour. This line of inquiry has been fruitful. A body of literature has emerged supporting the view that the orexin system fulfils a central role in integrating signals from the periphery conveying information about macronutrient balance (Burdakov et al., 2006; Karnani et al., 2011), and hormonal competence for reproductive behaviour (Muschamp et al., 2007; Di Sebastiano et al., 2011), with circadian information (Estabrooke et al., 2001; Mileykovskiy et al., 2005) in order to coordinate arousal levels appropriate for the acquisition of food or receptive mates (Adamantidis and de Lecea, 2008). These goal-directed behaviours are engaged, in part, via robust projections of the orexin neurons to reward-responsive dopamine neurons in the VTA (Fadel and Deutch, 2002; Balcita-Pedicino and Sesack, 2007). This natural reward circuitry can be co-opted by repeated exposure to drugs of abuse, resulting in increased drug-seeking behaviour and the emergence of a set of neural adaptations that are putative markers of drug dependence.
Table 1.
Electrical stimulation of lateral hypothalamus and central infusion of orexins (hypocretins) both activate a diverse array of behaviours in rats
| Behaviour | LH stimulation citation | Central orexin (hypocretin) citation | Injection site | Dose |
|---|---|---|---|---|
| Feeding | Hoebel, 1969; Hoebel et al., 1962; Margules et al., 1962 | Dube et al., 1999 Haynes et al., 1999 Sakurai et al.,1998 | LH, PVN, DMH i.c.v. i.c.v. | 1 nmol Hcrt-1/Orx A 23.4 nmol Hcrt-1/Orx A 3, 30 nmol Hcrt-1/Orx A 3, 30 nmol Hcrt-2/Orx B |
| Drinking | Mogenson and Stevenson, 1967 | Kunii et al.,1999 | i.c.v. | 10 nmol Hcrt-1/Orx A 10 nmol Hcrt-2/Orx B |
| Copulation | Caggiula et al., 1966; Herberg, 1963; Vaughan et al., 1962 | Gulia et al., 2003 | mPOA | 0.3 nmol Hcrt-1/Orx A |
| Locomotion | Rolls et al., 1972 | Kotz et al., 2002 | LH | 1 nmol Hcrt-1/Orx A |
| Gnawing of non-food objects | Valenstein, 1973; Valenstein et al., 1968 | España et al., 2002 | i.c.v. | 3 nmol Hcrt-1/Orx A |
mPOA, medial preoptic area; PVN, paraventricular nucleus of hypothalamus.
Drug-induced Fos activation in orexin neurons
The anatomical inputs to, and outputs from, orexin neurons make them an ideal candidate for mediating certain aspects of reward and motivation. Therefore, many studies have examined the role of orexin neuron activity in drug-seeking behaviours. Harris et al. (2005) first reported that orexin neurons in the LH, but not the dorsomedial nucleus of the hypothalamus (DMH) or the perifornical area (PFA), were activated by cues associated with both drug (cocaine and morphine) and food rewards. In a CPP task, whereby animals learn to associate distinct contextual cues with the subjective effects of experimenter-administered drugs, preference for the drug-paired environment positively correlates with the level of Fos protein, a surrogate marker of neural activation, in LH orexin neurons whereas animals showing no preference have Fos levels similar to controls (Harris et al., 2005). Interestingly, this effect seems to be distinct from novel object preference, which does not activate orexin neurons in the LH (Harris et al., 2005). Moreover, stimulation of the LH, or microinfusion of orexin-A into the VTA, can reinstate an extinguished CPP; with both effects being inhibited by the selective OX1 receptor antagonist, SB 334867 (Harris et al., 2005). Conversely, foot shock, a stressful stimulus that can also reinstate drug seeking, does not activate LH orexin neurons but rather activates those in the DMH and PFA (Harris et al., 2005). Together, these results led to the hypothesis that discrete populations of orexin neurons display a functional dichotomy in responses to pharmacological or environmental stimuli, and in turn, to organizing behaviour. It was suggested that orexin neurons situated in the DMH and PFA might contribute to behaviours linked with arousal and foot shock stress (Harris and Aston-Jones, 2006; Sharf et al., 2010) whereas those located in the LH might have a preferential role in reward seeking (Harris and Aston-Jones, 2006). If so, this dichotomy might arise from preferential targeting of orexin neurons within each subregion; such that orexin neurons in the DMH and PFA may mediate arousal by projecting to arousal-related regions such as the locus coeruleus; and LH orexin neurons may influence reward through projections to the brain reward circuitry including, but not limited to, the VTA (Harris and Aston-Jones, 2006). However, this idea has been contradicted by a recent report that orexin neurons projecting both to arousal and reward-related areas, the locus coeruleus and VTA, respectively, are not segregated to the DMH/PFA and LH but rather are spread throughout the entire orexin field (González et al., 2012). In fact, VTA-projecting orexin neurons outnumber locus coeruleus-projecting neurons in the medial portions of the orexin field (González et al., 2012). In addition, amphetamine-sensitized rats show c-Fos+orexin double labelling in all three subregions of the orexin field (McPherson et al., 2007) and restraint stress was found to reinstate extinguished cocaine seeking via activation of LH, but not PFA orexin neurons (Tung and Chiou, 2012). Therefore, an alternative hypothesis has been proposed whereby differential activation of orexin neurons may be a result of distinct upstream activation of afferent projections to orexin neurons. In line with this hypothesis is the finding that orexin neurons in the medial regions are preferentially innervated by hypothalamic neurons while those in the LH receive preferential afferent input from the brain stem and reward-related regions (Yoshida et al., 2006).
In this light, Sartor and Aston-Jones (2012) characterized which afferents to LH orexin neurons are activated by reward-related stimuli, and if these inputs could drive activity in orexin neurons to promote drug place preference. They focused on the lateral septum, a brain region that sends afferents to the LH (Yoshida et al., 2006), and is activated by drugs and environmental information associated with reward (Shoji et al., 1997; 1998). Here, they demonstrated that neurons projecting from the rostral, but not the caudal, lateral septum to the LH, but not to the DMH or PFA, were Fos activated following cocaine CPP and that the percentage of Fos activation correlated with preference scores (Sartor and Aston-Jones, 2012). Importantly, this circuit may be a small part of a more complex circuit in which the lateral septum integrates contextual information with motivational processes (Sartor and Aston-Jones, 2012). Therefore, it will be interesting to see if these data hold true for other rewards or drugs of other pharmacological classes, like morphine.
Withdrawal-induced Fos activation in hypothalamic orexin neurons
Morphine withdrawal induced by naloxone administration increases Fos activation of LH orexin neurons in mice (Georgescu et al., 2003) and rats (Laorden et al., 2012). In contrast to Fos expression induced by morphine CPP (Harris et al., 2005), Fos expression induced by morphine withdrawal was localized to DMH and PFA, but was absent in LH orexin neurons of mice (Georgescu et al., 2003). However, this was not the case in rats where withdrawal also induced Fos expression in the LH (Laorden et al., 2012). Interestingly, morphine withdrawal increased both orexin and μ-opioid receptor mRNA selectively in the LH (Zhou et al., 2006). Increased Fos induction by morphine withdrawal was attenuated in orexin peptide knockout mice (Sharf et al., 2008) or in wild-type mice (Georgescu et al., 2003) or rats (Laorden et al., 2012) treated with an OX1 receptor antagonist. Finally, systemic SB 334867 inhibited morphine withdrawal-induced Fos expression in the hypothalamus, NAc shell, bed nucleus of the stria terminalis and central amygdala, but not the VTA or the locus coeruleus (Sharf et al., 2008; Laorden et al., 2012). Surprisingly, direct infusion of SB 334867 into the locus coeruleus attenuated somatic signs of morphine withdrawal in rats (Azizi et al., 2010). These results suggest that, in addition to conditioned drug seeking, hypothalamic orexin neurons are also activated during morphine withdrawal.
Drug-induced plasticity at orexin neurons
In addition to drug-induced Fos activation of orexin neurons, drugs have been reported to alter synaptic transmission within the LH, including that onto orexin neurons. Acute application of Met-enkephalin or morphine can depress the firing rate and the frequency of miniature EPSCs (mEPSCs) onto orexin neurons (Li and van den Pol, 2008). Furthermore, 1 h pre-exposure to morphine reduced morphine-induced inhibition of firing 30 min later (Li and van den Pol, 2008). So far, it has not been determined how in vivo morphine exposure can modify synaptic transmission or plasticity of orexin neurons.
Drug-induced synaptic plasticity in reward-associated areas including the VTA and the NAc is essential to both the development and the expression of drug-related behaviours (reviewed in Lüscher and Malenka, 2011). LH hypothalamic neurons also appear to undergo drug-induced plasticity. Recent reports from Rao et al. (2013) and Yeoh et al. (2012) described cocaine-induced plasticity of glutamatergic inputs to orexin neurons. In mice that received repeated cocaine in a CPP paradigm, there was a long-lasting increase in the AMPA/NMDA receptor ratio, a measure of synaptic strength, as well as the amplitude of AMPA receptor mEPSCs, suggesting an increase in postsynaptic AMPA receptor expression (Rao et al., 2013; receptor nomenclature follows Alexander et al., 2013b). In contrast, Yeoh et al. (2012) observed enhanced excitatory inputs to PFA/LH neurons including orexin neurons, but no postsynaptic changes, after 7 days of cocaine self-administration in rats. The difference in these responses may be due to the duration or method of cocaine delivery (3 days vs. 7 days and experimenter-administered vs. self-administered cocaine). This plasticity at synapses onto orexin neurons was not dependent on OX1 receptor signalling as cocaine-induced strengthening of glutamatergic synapses onto orexin neurons was not blocked by systemic SB 334867 (Rao et al., 2013). This finding differentiates drug-induced plasticity in the LH from that at dopamine neurons in the VTA, which is dependent on OX1 receptor signalling (Borgland et al., 2006), as discussed below.
Effects of orexin receptor activation in the VTA
Although there is clear evidence of drug-induced modulation of orexin neurons, it is the downstream release of orexins in target projection regions that likely represents a critical step in the initiation and maintenance of drug-seeking behaviour. Highlighting this is the finding that cocaine CPP is inhibited by SB 334867 despite the persistence of cocaine-induced plasticity of orexin neurons (Rao et al., 2013). Therefore, plasticity at the level of orexin neurons is likely to represent a mechanism to increase the excitability of orexin neurons in order to enhance the probability of downstream release. At these downstream sites, orexin can modulate synaptic transmission and influence motivated behaviour.
Acute effects of orexin in the VTA
In whole cell patch-clamp electrophysiology experiments using midbrain slices, direct application of orexin-A or orexin-B increased the firing rate of VTA dopamine neurons (Korotkova et al., 2003). Furthermore, orexin-A or -B concentration dependently increased NMDA receptor EPSCs via a PKC/PLC-dependent signalling pathway (Figure 1; Borgland et al., 2006; 2008). Although acute application of orexin-A had no effect on AMPA receptor EPSCs when measured 15 min later, both the amplitude of AMPA receptor mEPSCs and the AMPA/NMDA receptor ratio were increased 3–4 h later, suggesting that acute potentiation of NMDA receptor currents led to a late phase potentiation of AMPA receptors. Accordingly, an NMDA receptor antagonist blocked the increase in AMPA receptor mEPSC amplitude (Borgland et al., 2006). Thus, orexin-A potentiation of NMDA receptor EPSCs may serve to facilitate the induction of synaptic plasticity at VTA dopaminergic neurons.
Chiou et al. (2013) proposed another cellular mechanism involving endocannabinoids to explain how orexin increases VTA dopaminergic activity. Endocannabinoids, especially 2-arachidonoylglycerol (2-AG), are synthesized and then released locally in an activity-dependent and receptor-regulated manner (Di Marzo et al., 1994). Endocannabinoids can be generated when certain GPCRs coupled to Gq/11, including OX1 receptors, are activated (Kano et al., 2009; Ho et al., 2011), resulting in retrograde inhibition of neurotransmitter release via activation of presynaptic cannabinoid CB1 receptors (Kano et al., 2009). In a slice electrophysiology study, Chiou et al. (2013) demonstrated that activation of OX1 receptors on VTA dopaminergic neurons could initiate the synthesis of 2-AG by postsynaptic DAG lipase (DAGL) and subsequent retrograde signalling onto CB1 receptor-expressing GABAergic terminals (Matyas et al., 2008). Orexin-A depressed inhibitory postsynaptic currents (IPSCs) via a presynaptic mechanism. This effect was (i) inhibited by OX1 and CB1, but not OX2, receptor antagonists; (ii) prevented by inhibitors of G-protein signalling, PLC or DAGL; and (iii) potentiated and prolonged by inhibiting the major 2-AG degrading enzyme, monoglycerol lipase (Ludanyi et al., 2011). These results suggest that in VTA dopaminergic neurons, orexin-A initiates a Gq/11PCR-coupled PLC-DAGL enzymic pathway to generate 2-AG, which then travels retrogradely across the synapse to inhibit GABA release through presynaptic CB1 receptors (unpublished observations presented at the 2013 INRC meeting; Chiou et al., 2013; Figure 1). This is likely to result in disinhibition of VTA dopamine neurons.
Acute application of orexin-A or -B increases the firing rate of GABAergic neurons (Korotkova et al., 2003), which presumably decreases VTA dopaminergic activity. Indeed, in a preliminary study, Tung and Chiou (2012) found that orexin-A depressed IPSCs in VTA dopaminergic neurons in a bell-shaped concentration–response curve. In the presence of a CB1 receptor antagonist, higher concentrations of orexin-A potentiated IPSCs whereas lower concentrations depressed IPSCs. This suggests that higher concentrations of orexin-A are needed to directly activate GABAergic neurons, as compared with the concentrations that initiate 2-AG-mediated retrograde disinhibition. Interestingly, orexin-A and -B were equally effective at increasing the firing rate of GABAergic neurons in the VTA (Korotkova et al., 2003). Given that OX1 receptors display higher affinity for orexin-A than orexin-B, whereas OX2 receptors have equal affinity for orexin-A and -B (Sakurai et al., 1998), it remains to be elucidated if the direct neuronal excitatory effect of orexin is mainly mediated by OX2 receptors while OX1 receptors may mediate the generation of 2-AG and its subsequent signalling cascade.
Drug-induced modulation of synaptic transmission in the VTA is thought to promote a switch from tonic to burst firing, a phenomenon that increases the efficiency of dopamine release in target regions (Overton and Clark, 1991; Suaud-Chagny et al., 1992; Tong et al., 1996). Indeed, orexin-A or orexin-B application to VTA midbrain slices increased dopamine neuron firing and in some cases was capable of inducing burst firing activity (Korotkova et al., 2003). However, dopaminergic neurons were not uniformly excited by orexin application as a substantial number of dopaminergic neurons were unaffected by orexin application (Korotkova et al., 2003). With the emerging notion of heterogeneity of dopaminergic neurons in the VTA both in terms of cellular properties and circuit level connections (Lammel et al., 2008; 2011; 2012; Margolis et al., 2008), such a varied response to orexin application within the VTA could suggest that orexin functions within specific circuits in the VTA to modulate different aspects of motivated behaviour. Accordingly, orexin-A preferentially activates VTA dopaminergic neurons that project to the mPFC and the NAc shell (Vittoz et al., 2008). Regardless of differential activation of dopamine neurons in the VTA by orexin, intra-VTA application of orexin-A is sufficient to increase dopamine release in the mPFC (Vittoz and Berridge, 2006; Vittoz et al., 2008) and the NAc (Narita et al., 2006; 2007; España et al., 2011).
Effect of orexin signalling on drug-induced plasticity in the VTA
The ability of orexin to induce synaptic plasticity in the VTA may represent a mechanism by which environmental drug-related stimuli, known to activate orexin neurons (Harris et al., 2005; 2007), modulate excitatory transmission in the VTA. Morphine, cocaine and other drugs of abuse strengthen AMPA-mediated synaptic transmission onto dopamine neurons when tested 24 h after drug exposure (Ungless et al., 2001; Saal et al., 2003; Borgland et al., 2004), an effect that is dependent on NMDA receptor activation (Ungless et al., 2001; Argilli et al., 2008). Interestingly, systemic administration of the OX1 antagonist SB 334867 blocks cocaine-induced synaptic plasticity in the VTA (Borgland et al., 2006). Preliminary evidence suggests that SB 334867 administered systemically or within the VTA also blocks morphine-induced plasticity at VTA dopamine neuronal synapses (unpublished observations presented at the 2013 INRC meeting). These results are interesting as acute application of morphine depresses firing and glutamatergic activity onto orexin neurons, implicating reduced orexin release (Li and van den Pol, 2008). However, further studies are required to determine how in vivo exposure to morphine subsequently modifies activity of orexin neurons. Nevertheless, it appears that drug-induced plasticity of VTA dopaminergic neurons requires orexin signalling, regardless of the mechanism of action of the drug. Interestingly, both systemic and intra-VTA SB 334867 administration attenuates cocaine-induced increases in dopamine concentrations in the NAc core (España et al., 2010), while intra-VTA orexin-A infusions increase dopamine concentrations and the efficacy of cocaine-mediated dopamine reuptake inhibition (España et al., 2011). Taken together, blockade of orexin-A signalling in the VTA can inhibit cocaine-mediated strengthening of synapses and output of VTA dopaminergic neurons (España et al., 2010). Current studies are underway to determine if systemic or intra-VTA SB 334867 can prevent morphine-induced increases in terminal dopamine release.
Drug-induced plasticity in the VTA is thought to underlie some of the core behavioural features of addiction. For example, locomotor sensitization, which manifests as a progressive and persistent increase in locomotor activity with repeated administration of a drug (Robinson and Berridge, 1993), is dependent on NMDA receptor activation in the VTA (Kalivas and Alesdatter, 1993) and results in increased dopaminergic transmission. Consistent with the effects of orexin on NMDA receptors in the VTA, the development of morphine-, cocaine- and amphetamine-induced behavioural sensitization is also inhibited by the systemic administration of an OX1 receptor antagonist (Borgland et al., 2006; Narita et al., 2006; Quarta et al., 2009).
While acute application of orexin has prominent effects on synaptic transmission in the VTA, long-term drug exposure further alters these effects. Rats that self-administered cocaine or high-fat food had potentiated orexin-induced increases in NMDA receptor currents compared with those that received regular food (Borgland et al., 2009). This was observed along with an increase in AMPA receptor mEPSC frequency in these animals, suggesting that prolonged exposure to a positive reinforcer induces a presynaptic increase in glutamate release that is absent in naïve animals or those with a history of self-administering a less-salient reinforcer (Borgland et al., 2009). Further studies are required to assess if opioid self-administration, another salient reinforcer, also promotes an orexin-mediated increase in excitatory synaptic transmission at dopaminergic synapses.
Effects of orexin receptor activation in the VTA on drug seeking and reinstatement
Several lines of evidence suggest that endogenous orexins acting via OX1 receptors in the VTA play an important role in the reinstatement of extinguished reward seeking. First, activation of LH orexin neurons, which send substantial projections to the VTA (Fadel and Deutch, 2002), is strongly associated with cue-reinstated drug and food-seeking behaviours (Harris et al., 2005). Second, intra-VTA injection of orexin-A induced reward-seeking behaviours in extinguished rodents in an OX1 receptor-dependent manner (Mahler et al., 2012). Third, systemic or intra-VTA injection of an antagonist of OX1, but not OX2, receptors significantly reduced the reinstatement of extinguished seeking behaviours for cocaine, alcohol or morphine elicited by drug-predicting cues or yohimbine (Mahler et al., 2012), but not foot shock stress (Wang et al., 2009). Finally, OX1 receptor activation and AMPA transmission in VTA are simultaneously necessary for cues, but not cocaine, to trigger drug seeking (Mahler et al., 2013). It remains to be elucidated by in vivo studies if endogenous orexins released during the reinstatement induced by stress will increase VTA dopaminergic activity through a glutamate-receptor-mediated mechanism.
Chiou and colleagues further demonstrated that endocannabinoid-mediated disinhibition of dopaminergic neurons contributes to acute restraint stress-induced reinstatement of extinguished cocaine-seeking behaviour in mice (unpublished observations presented at the 2013 INRC meeting; Chiou et al., 2013). A 30 min restraint stress, which increased orexin-A levels in the VTA and Fos activation in LH orexin neurons, significantly reinstated extinguished cocaine CPP in mice. This acute stress-induced cocaine reinstatement was absent in CB1 receptor-knockout mice, and was prevented by intra-VTA injection of antagonists for OX1, CB1 or a DAGL inhibitor. Several previous studies have suggested that endocannabinoids are involved in the reinstatement of extinguished seeking behaviours for drugs of abuse. Indeed, cue- or swim-stress-induced seeking behaviours for cocaine (De Vries et al., 2001), heroin (Fattore et al., 2003), ethanol (Cippitelli et al., 2005) or nicotine (De Vries et al., 2005) were blocked by a CB1 receptor antagonist. Therefore, one could hypothesize that acute restraint stress activates hypothalamic orexin neurons, likely leading to downstream release of orexin in the VTA and the activation of OX1 receptors on dopaminergic neurons that causes an endocannabinoid-mediated retrograde inhibition of GABA release, resulting in disinhibition of VTA dopaminergic neurons and the initiation of cocaine-seeking behaviour.
Orexin receptor signalling at ascending projection targets on ethanol self-administration, ethanol seeking and stress-induced relapse
Alcohol dependence constitutes a major global public health problem and there remains a critical need for the development of medications for the treatment of alcohol use disorders. There is an extensive literature demonstrating that activation of orexin-containing neurons and orexin receptors modulate alcohol consumption and cue-induced relapse (see Kim et al. 2012). While their mechanism of action remains unclear, these receptors may be promising targets for the treatment of alcohol use disorders.
The Lawrence laboratory was the first to demonstrate a role for orexins in both ethanol consumption and cue-induced reinstatement of ethanol seeking in rats (Lawrence et al., 2006). These effects were specific, with a differential effect of OX1 receptor antagonism on the motivational strength of ethanol compared with sucrose (Jupp et al., 2011a). A feature of human addiction is an enduring propensity to relapse, which can be modelled in rodents for potential translational relevance. After long-term ethanol self-administration, rats were subjected to extinction training and then tested for cue-induced reinstatement of ethanol seeking either immediately after extinction or when extinction was followed by protracted abstinence (5 months). Representation of cues previously paired with ethanol availability was sufficient to precipitate reinstatement of ethanol seeking at both time points. Pretreatment with the OX1 receptor antagonist, SB 334867, effectively prevented cue-induced reinstatement of ethanol seeking both immediately after extinction and also after protracted abstinence (Jupp et al., 2011b). These data therefore provide evidence that cue-conditioned ethanol seeking involves the release of endogenous orexins acting upon OX1 receptors. Indeed, these findings are consistent with a role for orexins in the integration of cue salience, even after long-term withdrawal. While the VTA is heavily implicated as a site for orexin-mediated modulation of reward seeking (Harris et al., 2005; Borgland et al., 2006), Lawrence and colleagues sought to examine the potential for other regions to contribute to this behaviour. The prefrontal cortex (prelimbic and orbitofrontal) was identified by Fos expression as a potential locus where ascending orexinergic input could modulate cue-conditioned ethanol seeking. Thus, OX1 receptor antagonism attenuated reinstatement-induced Fos expression in prelimbic and orbitofrontal cortices (Jupp et al., 2011b). Therefore, in addition to acting within the VTA, ascending orexin projections may also be implicated in cue-driven ethanol seeking (Brown and Lawrence, 2013).
Orexins in the amygdala and stress-induced reinstatement of ethanol seeking and relapse
The orexin system plays a role in response to stress and orexin neurons are activated in response to arousal, which is a key component of the stress response system (Winsky-Sommerer et al., 2004; Boutrel et al., 2005; Richards et al., 2008). Orexins have been implicated in the post-stress anxiogenic behaviour in rats exposed to foot shock treatment, and antagonism of orexin receptors decreases not only anxiety (Chen et al., 2013b) but also blood pressure in spontaneously hypertensive rats (Li et al., 2013). The extended amygdala plays a critical role in the reinstatement of drug seeking, as inactivation of the central nucleus of the amygdala (CeA) prevents foot shock-induced reinstatement of cocaine seeking (McFarland et al., 2004). Projections from orexin neurons densely innervate the CeA (Peyron et al., 1998; Schmitt et al., 2012), a brain region implicated in emotional behaviours such as anxiety, fear and stress (Lin et al., 1999). A previous study had shown that in acute rat brain slices, orexins enhance firing of neurons of the central medial nucleus of the CeA (Bisetti et al., 2006). The central and medial amygdaloid projections regulate the hypothalamic-pituitary-adrenal axis and innervate orexin-containing neurons in the LH (Gray et al., 1989; Dayas et al., 1999; Nakamura et al., 2009). Furthermore, hypothalamic orexin neurons have reciprocal projections to the amygdala and play a role in vigilance and stress-related responses (Nishino, 2011). The OX1 receptor antagonist, GSK1059865, can inhibit the yohimbine (pharmacological stressor) activated extra-hypothalamic stress neurocircuits in the extended amygdala (Gozzi et al., 2013). Furthermore, the dual OX1 and OX2 receptor antagonist, almorexant, decreases fear-potentiated startle responses in rats, which is a model of conditioned fear involving the CeA (Steiner et al., 2012). Additionally, orexin receptor antagonists reduce panic responses induced by anxiogenic drugs (Johnson et al., 2012). Together, this suggests that orexins in the amygdala play a role in stress responses, including those underlying drug relapse.
Orexins in the mPFC: role in ethanol seeking and stress-induced relapse
The mPFC has been known to regulate seeking behaviour for most drugs of abuse including cocaine (Pietro et al., 2008; Pentkowski et al., 2010; Chen et al., 2013a; Cisler et al., 2013) and ethanol (George et al., 2001; Samson and Chappell, 2003). The mPFC, VTA and the NAc core have been proposed to interact in a dopamine-dependent manner to influence the behavioural aspects of ethanol seeking (Groenewegen et al., 1990; Cador, 1992; Yang et al., 1999). Most importantly, ambient environmental cues coupled with endogenous stimuli (level of stress, anxiety, dysphoria) are processed at the level of mPFC (Kalivas and Klitenick, 1993) to trigger alcohol-seeking behaviour. Depending on the rewarding salience of the stimuli, the mPFC causes an increase in the dopamine levels in the NAc core and VTA through glutamatergic innervation (Sesack et al., 2003; Geisler et al., 2007) in conjunction to the direct excitatory effects of ethanol on VTA dopaminergic neurons (Brodie et al., 1999). An ethanol-induced increase in dopamine affects the NAc core and mPFC so as to sustain the rewarding salience of the initial stimuli (i.e. self-administration) with a concomitant decrease in excitatory inputs from the mPFC (Seamans et al., 2001).
The mPFC-induced modulation of VTA dopaminergic neurons through glutamate transmission is enhanced by orexins particularly during the active/wake period (Moorman and Aston-Jones, 2010). The rewarding salience of the external stimuli and direct orexinergic innervation of VTA dopaminergic neurons are thought to strengthen the mPFC-VTA dopamine connection (Moorman and Aston-Jones, 2010) that drives bias towards reward-seeking and goal-directed responses. This role of orexin aligns perfectly with its ability to potentiate glutamate transmission in VTA dopaminergic neurons in cocaine-induced synaptic plasticity (Borgland et al., 2006). Orexin-A has also been shown to excite pyramidal cells in the mPFC (layers II–VI) (Yan et al., 2012) to strengthen intracortical connections and facilitate information processing between mPFC and other parts of the prefrontal cortex to achieve appropriate cognitive and reward-related responses. Stressful stimuli and stress-induced adaptations disrupt normal signalling in the PFC (Liu and Aghajanian, 2008; Caffino et al., 2013). Exposure to stress in cocaine-habituated rats causes dysregulation in glutamate neurotransmission in the mPFC at synaptic clefts leading to reduced glutamate reuptake and sensitization of glutamate neurons to stress (Caffino et al., 2013). These changes may be subjectively experienced as stressful or dysphoric and presumably manifest in a ‘depression-like’ state that may lead to stress-induced drug reinstatement. Orexins have been previously implicated in stress-induced alcohol reinstatement and the activity of excitatory orexin currents in the mPFC has been shown to be partially affected under stressful conditions (Liu and Aghajanian, 2008). This suggests that orexin transmission in the mPFC could exacerbate a stress-induced negative emotional state by decreasing arousal with a concomitant urge to mitigate the negative affective state by using drugs. As orexin receptor antagonists are optimized and reach the market for other medical indications, their use as part of a multifaceted approach in the treatment of addiction is indeed promising.
Effects of co-release of orexin and dynorphin on reward and drug seeking
While expression of orexin peptides is confined to several thousand cells in the LH, dynorphin and its cognate, κ-opioid, receptor are found more widely across the neuraxis (Chavkin et al., 1982; Mansour et al., 1994). Although orexin does appear to play an important role in the heightened arousal that accompanies stress (Winsky-Sommerer et al., 2004), the balance of evidence suggests that dynorphin release and κ-opioid receptor activation are responsible for the aversive components of stress (Van't Veer and Carlezon, 2013). For example, dynorphin and κ-opioid receptor expression are altered predictably in the NAc, amygdala and several other brain structures in animals subjected to stress (Shirayama et al., 2004; Knoll et al., 2011); central or peripheral administration of κ-opioid receptor agonists causes conditioned place aversions in rodents (Bals-Kubik et al., 1993), and produces anxiety and dysphoria in humans (Pfeiffer et al., 1986; Walsh et al., 2001); and the aversive effects of ‘stress peptides’ like corticotropin-releasing factor (CRF) exert their dysphoric effects via dynorphin and κ-opioid receptor activation (Land et al., 2008). Indeed, dynorphin- κ-opioid receptor signalling has been conceptualized to work in concert with CRF as part of a ‘brain anti-reward system’ that serves to offset the function of natural reward circuitry of which the orexin system may be a part (Koob and Le Moal, 2008).
Counter-intuitively, almost all (∼95%) orexin neurons in rats and mice express dynorphin (Chou et al., 2001). This pattern of expression is conserved in humans (Crocker et al., 2005), and in vitro data support co-release of orexin and dynorphin (Li and van den Pol, 2006), suggesting functional relevance. Interestingly, mRNA for both prepro-orexin and preprodynorphin increases after acute cocaine abstinence in rats exposed to chronic escalating doses of cocaine (Zhou et al., 2008), suggesting that orexin and dynorphin release may be sensitive to cocaine exposure and/or acute withdrawal. In contrast, withdrawal from morphine does not alter preprodynorphin mRNA (Zhou et al., 2006).
At this time, potential functions of this pattern of gene expression can only be speculated, but the presence of an excitatory (orexin) and inhibitory neuropeptide (dynorphin) in the same neuron suggests a number of possibilities. The first is a simple negative feedback mechanism whereby dynorphin can hyperpolarize orexin-dynorphin containing terminals and reduce the probability of transmitter release under conditions of prolonged excitation. Because many orexin-containing neurons are glutamatergic (Rosin et al., 2003), this motif may be important for modulating a potent source of excitatory output onto target neurons, thus preventing hyperexcitability in both target neurons and the orexin-dynorphin neurons themselves. Another possibility permits robust gain control of target neurons given differential expression of OX1 and κ-opioid receptors in different brain structures or nuclei. Feedforward disinhibition of target neurons by the expression of κ-opioid receptors on GABAergic interneurons may occur in parallel with direct excitation of OX1 receptor-expressing target neurons. This type of synaptic arrangement would facilitate rapid increases in firing rate and an accompanying transient spike in transmitter released by target neurons. This mechanism could, for instance, induce the type of burst firing and forebrain phasic dopamine release known to be important for reward learning (Schultz, 2002; Tsai et al., 2009; Steinberg et al., 2013).
These ideas remain untested; however, Muschamp and colleagues have used a combination of behavioural, cellular and molecular approaches to demonstrate the importance of orexin-dynorphin co-expression both on functioning of VTA dopaminergic neurons and some of the reward-associated behaviours they control. Preliminary data presented at the 2013 INRC meeting suggest that acute actions of orexin do not mediate reward per se. Rather, orexin appears to facilitate reward by occluding the ‘anti-reward’ effects of its co-transmitter dynorphin (Muschamp et al., 2012).
Summary
Dopaminergic neurons in the VTA are a key target of addictive drugs and neuroplasticity in this region may underlie some of the core features of addiction. Orexin neurons are activated by abused drugs, stress and cues predicting drugs or stress. Abused drugs can also induce synaptic plasticity of orexin neurons. However, this alone is not sufficient to drive drug-seeking behaviour. Indeed, orexin action in the VTA exerts modulatory effects on a variety of behaviours produced by drugs of abuse. Mechanisms underlying orexin-mediated effects in the VTA on drug-seeking behaviour have been proposed. Acute application of orexin potentiates excitatory synaptic transmission in the VTA, and inhibition of OX1 receptor signalling blocks both cocaine- or morphine-induced plasticity. Additionally, it has been proposed that orexins can induce an endocannabinoid-mediated retrograde inhibition of GABA release, resulting in a disinhibition of dopamine neurons. Orexins may also promote alcohol seeking as well as cue- or stress-induced relapse at sites outside the VTA. OX1 receptor antagonists blocked cue-induced ethanol seeking as well as Fos expression in the medial and orbitoprefrontal cortices. Furthermore, OX1 receptor signalling in the CeA as well as the mPFC has also been implicated in stress-induced relapse to ethanol seeking. These studies suggest that the ascending orexin projections may also play a critical role in alcohol-seeking behaviours. It will be important to determine if these sites are also involved in addiction-related behaviour for other drugs of abuse, including morphine. Finally, excitatory orexins are co-expressed and potentially co-released with the aversion-inducing neuropeptide, dynorphin. Opposing effects of these peptides have been proposed to facilitate reward by orexin-mediated occlusion of the ‘anti-reward’ effects of dynorphin. Taken together, the development of novel OX1 receptor antagonists could have excellent utility in the treatment of drug craving and relapse.
Acknowledgments
A. J. L. is a NHMRC Principal Research Fellow and supported by project grants from the NHMRC and ARC, the Pratt & Besen Family Trusts, and the Victorian Government's Operational Infrastructure Support Program. This mini-review discusses primarily the findings from past and present Lawrence laboratory members, all of whom are much appreciated. L. C. C. is supported by grants from the National Science Council, Taiwan (NSC 101–2325-B002–048 and NSC 102–2321-B002–066) and the National Health Research Institutes, Taiwan (NHRI-EX102-10251NI). S. E. B. is supported by funding from the Australian Research Council Future Fellowship and NHMRC. J. W. M. is supported by USPHS Grants, NIH F32-DA026250 and K99-DA031767. C. B. is supported by a Brain Canada Predoctoral Fellowship, S. L. B is supported by a CIHR New Investigator Award and an NSERC 372517.
Glossary
Abbreviations
- BOLD
blood oxygen level-dependent
- CeA
central nucleus of the amygdala
- CPP
conditioned place preference
- CRF
corticotropin-releasing factor
- DAGL
DAG lipase
- DMH
dorsomedial nucleus of the hypothalamus
- LH
lateral hypothalamus
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- PFA
perifornical area
- VTA
ventral tegmental area
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Transporters. Br J Pharmacol. 2013a;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013b;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2008;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi H, Mirnajafi-Zadeh J, Rohampour K, Semnanian S. Antagonism of orexin type 1 receptors in the locus coeruleus attenuates signs of naloxone-precipitated morphine withdrawal in rats. Neurosci Lett. 2010;482:255–259. doi: 10.1016/j.neulet.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Sesack SR. Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol. 2007;503:668–684. doi: 10.1002/cne.21420. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, et al. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Storm E, Bonci A. Orexin B/hypocretin 2 increases glutamatergic transmission to ventral tegmental area neurons. Eur J Neurosci. 2008;28:1545–1556. doi: 10.1111/j.1460-9568.2008.06397.x. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 2005;102(52):19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brown RM, Lawrence AJ. Ascending orexinergic pathways and alcohol-seeking. Curr Opin Neurobiol. 2013;23:467–472. doi: 10.1016/j.conb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Brown RM, Khoo SYS, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring (iP) rats. Int J Neuropsychopharmacol. 2013;16:2067–2079. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Burdakov D, Jensen LT, Alexopoulos H, Williams RH, Fearon IM, O'Kelly I, et al. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Cador M. Limbic-striatal interactions in reward related processes: modulation by the dopaminergic system. Clin Neuropharmacol. 1992;15(Suppl. 1 Pt A):548A–549A. doi: 10.1097/00002826-199201001-00285. [DOI] [PubMed] [Google Scholar]
- Caffino L, Calabrese F, Giannotti G, Barbon A, Verheij MM, Racagni G, et al. Stress rapidly dysregulates the glutamatergic synapse in the prefrontal cortex of cocaine-withdrawn adolescent rats. Addict Biol. 2013 doi: 10.1111/adb.12089. doi: 10.1111/adb.12089. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Hoebel BG. ‘Copulation-reward site’ in the posterior hypothalamus. Science. 1966;153(741):1284–1285. doi: 10.1126/science.153.3741.1284. [DOI] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013a;496(7445):359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Chen X, Wang H, Lin Z, Li S, Li Y, Bergen HT, et al. Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct Funct. 2013b doi: 10.1007/s00429-013-0626-3. doi: 10.1007/s00429-013-0626-3. [DOI] [PubMed] [Google Scholar]
- Chiou L-C, Tung L-W, Lee Y-S, Lu G-L, Lee H-J, Chagne L-Y, et al. 2013. Orexin-endocannabinoid signaling in stress-induced cocaine relapse, International Narcotic Research Conference, Cairns, Australia.
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Bilbao A, Hansson AC, del Arco I, Sommer W, Heilig M, et al. Cannabinoid CB1 receptor antagonism reduces conditioned reinstatement of ethanol-seeking behavior in rats. Eur J Neurosci. 2005;21:2243–2251. doi: 10.1111/j.1460-9568.2005.04056.x. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, et al. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, España RA, Papadopoulou M, Saper CB, Faraco J, Sakurai T, et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–1154. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–168. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano AR, Wilson-Perez HE, Lehman MN, Coolen LM. Lesions of orexin neurons block conditioned place preference for sexual behavior in male rats. Horm Behav. 2011;59:1–8. doi: 10.1016/j.yhbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–477. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- España RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fattore L, Spano MS, Cossu G, Deiana S, Fratta W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci. 2003;17:1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González JA, Jensen LT, Fugger L, Burdakov D. Convergent inputs from electrically and topographically distinct orexin cells to locus coeruleus and ventral tegmental area. Eur J Neurosci. 2012;35:1426–1432. doi: 10.1111/j.1460-9568.2012.08057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. Functional magentic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS ONE. 2011;6:e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Lepore S, Vicentini E, Merlo-Pich E, Bifone A. Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor yohimbine. Neuropsychopharmacology. 2013;38:2120–2130. doi: 10.1038/npp.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TS, Carney ME, Magnuson DJ. Direct projections from the central amygdaloid nucleus to the hypothalamic paraventricular nucleus: possible role in stress-induced adrenocorticotropin release. Neuroendocrinology. 1989;50:433–446. doi: 10.1159/000125260. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–118. [DOI] [PubMed] [Google Scholar]
- Gulia KK, Mallick HN, Kumar VM. Orexin A (hypocretin-1) application at the medial preoptic area potentiates male sexual behavior in rats. Neuroscience. 2003;116:921–923. doi: 10.1016/s0306-4522(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, Tadayyon M, et al. Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides. 1999;20:1099–1105. doi: 10.1016/s0196-9781(99)00105-9. [DOI] [PubMed] [Google Scholar]
- Heath RG. Electrical self-stimulation of the brain in man. Am J Psychiatry. 1963;120:571–577. doi: 10.1176/ajp.120.6.571. [DOI] [PubMed] [Google Scholar]
- Herberg LJ. A hypothalamic mechanism causing seminal ejaculation. Nature. 1963;198:219–220. doi: 10.1038/198219b0. [DOI] [PubMed] [Google Scholar]
- Hess WR. Diencephalon: Autonomic and Extrapyramidal Functions. 1954. Grune and Stratton: New York.
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF, et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci. 2011;31:14600–14610. doi: 10.1523/JNEUROSCI.2671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG. Feeding and self-stimulation. Ann N Y Acad Sci. 1969;157:758–778. doi: 10.1111/j.1749-6632.1969.tb12919.x. [DOI] [PubMed] [Google Scholar]
- Hoebel BG. Brain-stimulation reward and aversion in relation to behavior. In: Wauquier A, Rolls ET, editors. New York: Elsevier; 1976. pp. 335–372. Brain Stimulation Reward. [Google Scholar]
- Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science. 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011a;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, Lawrence AJ. Discrete cue-conditioned ethanol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol. 2011b;162:880–889. doi: 10.1111/j.1476-5381.2010.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, Klitenick MA. The circuitry mediating the motivational stimuli into adaptive motor responses. In: Kalivas PW, Barnes CD, editors. Boca Raton, FL: CRC Press; 1993. pp. 237–288. Limbic Motor Circuits and Neuropsychiatry. [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Karnani MM, Apergis-Schoute J, Adamantidis A, Jensen LT, de Lecea L, Fugger L, et al. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron. 2011;72:616–629. doi: 10.1016/j.neuron.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim AK, Brown RM, Lawrence AJ. The role of orexins/hypocretins in alcohol use and abuse: an appetitive-reward relationship. Front Behav Neurosci. 2012;6:78. doi: 10.3389/fnbeh.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, et al. Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Leonard CS. Orexin/hypocretin receptor signalling cascades. Br J Pharmacol. 2014;171:314–331. doi: 10.1111/bph.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunii K, Yamanaka A, Nambu T, Matsuzaki I, Goto K, Sakurai T. Orexins/hypocretins regulate drinking behaviour. Brain Res. 1999;842:256–261. doi: 10.1016/s0006-8993(99)01884-3. [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laorden ML, Ferenczi S, Pintér-Kübler B, González-Martín LL, Lasheras MC, Kovács KJ, et al. Hypothalamic orexin – a neurons are involved in the response of the brain stress system to morphine withdrawal. PLoS ONE. 2012;7:e36871. doi: 10.1371/journal.pone.0036871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Hindmarch CCT, Nattie EE, Paton JFR. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiolo. 2013;591:4237–4248. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26:13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, van den Pol AN. Mu-opioid receptor-mediated depression of the hypothalamic hypcrerin/orexin arousal system. J Neurosci. 2008;28:2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin XY, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludanyi A, Hu SS, Yamazaki M, Tanimura A, Piomelli D, Watanabe M, et al. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience. 2011;174:50–63. doi: 10.1016/j.neuroscience.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margules DL, Olds J. Identical ‘feeding’ and ‘rewarding’ systems in the lateral hypothalamus of rats. Science. 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, Freund TF, et al. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson CS, Featherby T, Krstew E, Lawrence AJ. Quantification of phosphorylated cAMP-response element-binding protein expression throughout the brain of amphetamine-sensitized rats: activation of hypothalamic orexin A-containing neurons. J Pharmacol Exp Ther. 2007;2323:805–812. doi: 10.1124/jpet.107.125732. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Stevenson IAF. Drinking and self-stimulation with electrical stimulation of the lateral hypothalamus. Physiol Behav. 1967;1:251–254. [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol-preferring Sprague-Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27:2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Onvani S, Hassinger LC, Kamenecka TM, et al. 2012. Opposing actions of hypocretin (orexin) and dynorphin co-transmission on motivated behavior. Program No. 498.20.2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012. Online. [DOI] [PMC free article] [PubMed]
- Nakamura S, Tsumori T, Yokota S, Oka T, Yasui Y. Amygdaloid axons innervate melanin-concentrating hormone- and orexin-containing neurons in the mouse lateral hypothalamus. Brain Res. 2009;1278:66–74. doi: 10.1016/j.brainres.2009.04.049. [DOI] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Miyatake M, Ikegami D, Kurahashi K, Suzuki T. Implication of protein kinase C in the orexin-induced elevation of extracellular dopamine levels and its rewarding effect. Eur J Neurosci. 2007;25:1537–1545. doi: 10.1111/j.1460-9568.2007.05403.x. [DOI] [PubMed] [Google Scholar]
- Nishino S. Hypothalamus, hypocretins/orexin, and vigilance control. Handb Clin Neurol. 2011;99:765–782. doi: 10.1016/B978-0-444-52007-4.00006-0. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of the septal area and other regions of the rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Overton P, Clark D. N-methyl-D-aspartate increases the excitability of nigrostriatal dopamine terminals. Eur J Pharmacol. 1991;201:117–120. doi: 10.1016/0014-2999(91)90332-k. [DOI] [PubMed] [Google Scholar]
- Pasumarthi RK, Reznikov LR, Fadel J. Activation of orexin neurons by acute nicotine. Eur J Pharmacol. 2006;535:172–176. doi: 10.1016/j.ejphar.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, et al. Stimulation of medial prefrontal cortex serotonin 2C (5-HT2C) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pietro N, Mashhoon Y, Heaney C, Yager L, Kantak K. Role of dopamine D1 receptors in the prefrontal dorsal agranular insular cortex in mediating cocaine self-administration in rats. Psychopharmacology (Berl) 2008;200:81–91. doi: 10.1007/s00213-008-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry. 2012;71:214–223. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2009;56:11–15. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Rao Y, Mineur YS, Gan G, Wang AH, Liu ZW, Wu X, et al. Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin -producing neurons in the lateral hypothalamus in mice. J Physiolo. 2013;591:1951–1966. doi: 10.1113/jphysiol.2012.246983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, et al. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kelly PH. Neural basis of stimulus-bound locomotor activity in the rat. J Comp Physiol Psychol. 1972;81:173–182. doi: 10.1037/h0033527. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Weston MC, Sevigny CP, Stornetta RL, Guyenet PG. Hypothalamic orexin (hypocretin) neurons express vesicular glutamate transporters VGLUT1 or VGLUT2. J Comp Neurol. 2003;465:593–603. doi: 10.1002/cne.10860. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci. 2012;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, et al. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, et al. Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 2011;215:191–203. doi: 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- Shoji S, Simms D, McDaniel WC, Gallagher JP. Chronic cocaine enhances gamma-aminobutyric acid and glutamate release by altering presynaptic and not postsynaptic gamma-aminobutyric acid B receptors within the rat dorsolateral septal nucleus. J Pharmacol Exp Ther. 1997;280:129–137. [PubMed] [Google Scholar]
- Shoji S, Simms D, Yamada K, Gallagher JP. Cocaine administered in vitro to brain slices from rats treated with cocaine chronically in vivo results in a gamma-aminobutyric acid receptor-mediated hyperpolarization recorded from the dorsolateral septum. J Pharmacol Exp Ther. 1998;286:509–518. [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, et al. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS ONE. 2012;7:e44726. doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, Jenck F. The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology (Berl) 2012;223:465–475. doi: 10.1007/s00213-012-2736-7. [DOI] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Chergui K, Chouvet G, Gonon F. Relationship between dopamine release in the rat nucleus accumbens and the discharge activity of dopaminergic neurons during local in vivo application of amino acids in the ventral tegmental area. Neuroscience. 1992;49:63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Antagonism of NMDA receptors but not AMPA/kainate receptors blocks bursting in dopaminergic neurons induced by electrical stimulation of the prefrontal cortex. J Neural Transm. 1996;103:889–904. doi: 10.1007/BF01291780. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung LW, Chiou LC. 2012. Orexin A increases dopaminergic activity via OX1 receptor-mediated endocannabinoid retrograde disinhibition in the ventral tegmental area- A novel addiction mechanism. Neuroscience 2012, Prog. No. 73.10, New Orleans, LA, USA.
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Valenstein ES. History of brain stimulation: investigations into the physiology of motivation. In: Valenstein ES, editor. Glenview, IL Scott: Foresman and Company; 1973. pp. 1–44. Brain Stimulation and Motivation. [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Modification of motivated behavior elicited by electrical stimulation of the hypothalamus. Science. 1968;159(819):1119–1121. doi: 10.1126/science.159.3819.1119. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Reexamination of the role of the hypothalamus in motivation. Psychol Rev. 1970;77:16–31. doi: 10.1037/h0028581. [DOI] [PubMed] [Google Scholar]
- Van't Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan E, Fisher AE. Male sexual behavior induced by intracranial electrical stimulation. Science. 1962;137:758–760. doi: 10.1126/science.137.3532.758-a. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Vittoz NM, Schmeichel B, Berridge CW. Hypocretin /orexin preferentially activates caudomedial ventral tegmental area dopamine neurons. Eur J Neurosci. 2008;28:1629–1640. doi: 10.1111/j.1460-9568.2008.06453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Yan J, He C, Xia JX, Zhang D, Hu ZA. Orexin-A excites pyramidal neurons in layer 2/3 of the rat prefrontal cortex. Neurosci Lett. 2012;520:92–97. doi: 10.1016/j.neulet.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK, Gorelova N. Developing a neuronal model for the pathophysiology of schizophrenia based on the nature of electrophysiological actions of dopamine in the prefrontal cortex. Neuropsychopharmacology. 1999;21:161–194. doi: 10.1016/S0893-133X(98)00112-2. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV. Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol. 2012;590:3677–3689. doi: 10.1113/jphysiol.2012.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, et al. Effects of cocaine place conditioning, chronic escalating-dose ‘binge’ pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]