Abstract
Several studies suggest that heteromerization between μ (MOP) and δ (DOP) opioid receptors modulates the signalling properties of the individual receptors. For example, whereas activation of MOP receptors by an agonist induces G protein-mediated signalling, the same agonist induces β-arrestin-mediated signalling in the context of the MOP-DOP receptor heteromer. Moreover, heteromer-mediated signalling is allosterically modulated by a combination of MOP and DOP receptor ligands. This has implications in analgesia given that morphine-induced antinociception can be potentiated by DOP receptor ligands. Recently reagents selectively targeting the MOP-DOP receptor heteromer such as bivalent ligands, antibodies or membrane permeable peptides have been generated; these reagents are enabling studies to elucidate the contribution of endogenously expressed heteromers to analgesia as well as to the development of side-effects associated with chronic opioid use. Recent advances in drug screening technology have led to the identification of a MOP-DOP receptor heteromer-biased agonist that activates both G protein-mediated and β-arrestin-mediated signalling. Moreover, this heteromer-biased agonist exhibits potent antinociceptive activity but with reduced side-effects, suggesting that ligands targeting the MOP-DOP receptor heteromer form a basis for the development of novel therapeutics for the treatment of pain. In this review, we summarize findings regarding the biological and functional characteristics of the MOP-DOP receptor heteromer and the in vitro and in vivo properties of heteromer-selective ligands.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: opioid, heteromer, trafficking, signalling, antinociception, tolerance, dependence, high-throughput screening
Introduction
G protein-coupled receptors (GPCR) comprise a large and most diverse family of seven transmembrane proteins that are encoded by more than 800 genes in the human genome (Fredriksson et al., 2003). Based on the guidelines of the International Union of Basic Clinical Pharmacology (IUPHAR), there are four main classes of GPCRs: class A rhodopsin-like receptors, class B secretin-like receptors, class C metabotropic glutamate/pheromone receptors and frizzled receptors (Venkatakrishnan et al., 2013).
Opioid receptors are members of the class A GPCR family. Three types of opioid receptors have been identified: μ, κ and δ opioid (MOP, KOP and DOP) receptors respectively (Kieffer, 1995; receptor nomenclature follows Alexander et al., 2013a). Opioid receptors are coupled to Gαi/o proteins, and their activation leads to inhibition of adenylyl cyclase activity and voltage-gated Ca2+ channels and to increases in MAPK phosphorylation and in the activity of inwardly rectifying K+ channels (Kir channels; nomenclature follows Alexander et al., 2013b) and phospholipase Cβ (Waldhoer et al., 2004). The signalling cascades initiated by the activation of opioid receptors induce the transcription of genes that regulate cellular differentiation, proliferation and survival (Chen et al., 2008). At the systems level, opioid receptor activation leads to a number of physiological responses including analgesia, feelings of euphoria and anxiety, respiratory depression, constipation, immunosuppression and changes in feeding and locomotor activity (Kieffer, 1995).
Over the last decade, several studies showed that GPCRs, including opioid receptors, can form homomers or heteromers (Satake and Sakai, 2008; Milligan, 2009; van Rijn et al., 2010; Smith and Milligan, 2010; Al-Hasani and Bruchas, 2011; Gonzalez-Maeso, 2011; Gomes et al., 2013a) and that these receptor–receptor interactions modulate ligand binding, receptor signalling and trafficking properties (Satake and Sakai, 2008; Gonzalez-Maeso, 2011; Gomes et al., 2013a; Petko et al., 2013). Heteromers involving opioid receptor types, particularly between KOP and DOP receptors and between MOP and DOP receptors, were reported in the early 2000s (Jordan and Devi, 1999; George et al., 2000; Gomes et al., 2000). Since then, heteromerization between MOP and DOP receptors has been extensively studied (Milligan, 2009; van Rijn et al., 2010; Al-Hasani and Bruchas, 2011; Costantino et al., 2012; Stockton and Devi, 2012) and found to expand the cellular responses of the MOP receptor protomer, resulting in an enhancement of morphine-induced antinociception (Gomes et al., 2004). MOP-DOP receptor heteromer has also been shown to play a role in the adverse side effects of opioids such as development of analgesic tolerance (Gupta et al., 2010; He et al., 2011). Ligands targeting the MOP-DOP receptor heteromer, such as bivalent ligands (Daniels et al., 2005; Lenard et al., 2007; Harvey et al., 2012) and a MOP-DOP receptor-biased agonist, have been reported (Gomes et al., 2013b); these are likely to help in the elucidation of the physiological effects of MOP-DOP receptor heteromers. This review will summarize what is presently known about MOP-DOP receptor heteromers and their selective ligands.
Evidence for the formation of MOP–DOP receptor heteromers
Computational analysis and crystal structure
Computational studies have been used to predict the transmembrane (TM) regions involved in the formation of opioid receptor heteromers (Filizola and Devi, 2012). An early study used a combination of three-dimensional (3D) homology modelling, based on the crystal structure of rhodopsin, and a subtractive correlated-mutation method to predict the involvement of TM1 of MOP receptors and TM4, TM5 and TM6 of DOP receptors in the heteromer interface (Filizola et al., 2002). Another study used a combination of 3D homology modelling, molecular dynamics simulations and analysis of protein–protein docking, cluster, shape complementarity and interaction energy to identify the possible MOP-DOP receptor heteromer interface (Liu et al., 2009). This analysis revealed that the most likely interface involved TM1 of MOP receptors and TM4 of DOP receptors and that the next likely interface involved TM6 of MOP receptors and TM4 of DOP receptors (Liu et al., 2009). Although both studies predict the involvement of TM1 of MOP receptors and TM4 of DOP receptors in the MOP-DOP receptor heteromeric interface, differences in the predictions for other likely interfaces could be due to the methodologies used. More recently, a study used energy transfer techniques and homology modelling methods to generate putative configurations for MOP-DOP receptor tetramers and predicted symmetric interactions of TM4, TM5 or TM1 at the MOP-DOP receptor heterodimeric interface (Golebiewska et al., 2011).
Recently, the crystal structures of the opioid receptor types revealed dimers and/or higher-order oligomers; for example, the MOP receptor crystal lattice comprises tightly packed parallel receptor dimers with an interface involving TM1, TM2 and helix 8 or TM5 and TM6 (Manglik et al., 2012). This would suggest that physiologically MOP receptors could be present as dimers or higher-order oligomers (Manglik et al., 2012). DOP receptors were shown to crystallize as an antiparallel arrangement of receptor proteins, suggesting the absence of physiologically relevant oligomeric contacts (Granier et al., 2012). However, examination of the amino acids involved in the MOP receptor dimer interface shows that they exhibit a high degree of homology with the corresponding amino acids in DOP receptors, suggesting that MOP-DOP receptors could share the same interface (Manglik et al., 2012). Taken together, both computational analysis and crystallization studies are consistent with the idea that opioid receptors could form dimeric, heteromeric and higher-order oligomeric complexes.
Anatomical evidence for MOP and DOP receptor heteromerization
Direct interactions between MOP and DOP receptors would require that both receptors be present not only in the same cell but also in the same subcellular compartment. Early electrophysiological studies examining the cell firing profile of single neurons using either MOP or DOP receptor agonists supported the presence of both receptors in the same neuron that was being investigated (Fields et al., 1980; Egan and North, 1981; Zieglgansberger et al., 1982). In addition, radioligand binding assays with commercially available neuroblastoma cell lines detected the presence of endogenous MOP and DOP receptors in these cells (Yu et al., 1986; Kazmi and Mishra, 1987; Baumhaker et al., 1993; Palazzi et al., 1996). In order to ascertain whether MOP-DOP receptor colocalization could be detected in brain and spinal cord, immunohistochemical studies using antibodies to endogenous receptors have been carried out. These studies detected MOP and DOP receptor colocalization to the same axonal terminals of the superficial dorsal horn (Arvidsson et al., 1995). Moreover, ultrastructural analysis carried out using a combination of electron microscopy and dual immunocytochemical labelling of MOP and DOP receptors indicated that the two receptors colocalized in the plasmalemma of dorsal horn neurons (Cheng et al., 1997) and in the dendrites or spines of the striatum (Wang and Pickel, 2001). These data showing colocalization of MOP and DOP receptors in brain and spinal cord were challenged by a report that examined DOP receptor localization in mice expressing enhanced green fluorescent protein-tagged receptor (eGFP–DOP receptor knockin mice). This study showed that in dorsal root ganglion neurons, MOP and DOP receptors were segregated from each other. MOP receptors were expressed on small, peptidergic C fibres, while DOP receptors were preferentially localized to medium-sized, non-peptidergic primary afferents and on large myelinated neurons (Scherrer et al., 2009). A limited colocalization of MOP with DOP receptors was found in <5% of the neurons, leading the authors to suggest that previous reports of colocalization between these two receptors were inaccurate due to the quality of the DOP receptor antibodies used in the studies (Scherrer et al., 2009). Several factors could account for the discrepancies in the colocalization of these two receptors. Firstly, the intensity of the staining obtained with the GFP antibody is much stronger than that obtained with the MOP receptor antibody (Scherrer et al., 2009). This could be due to an increase in Oprd1 transcription in eGFP–DOP receptor knock-in mice leading to higher levels of DOP receptors and/or to differences in the binding affinities/avidity of the anti-GFP antibody compared to the anti-MOP receptor antibody. Either of these conditions would lead to an overestimation of DOP receptor and underestimation of MOP receptor abundance and therefore extremely low colocalization between these two receptors. Secondly, the eGFP tag at the C-terminus has been known to increase the cell surface localization of DOP receptors (Wang et al., 2010), leading to different localization of endogenous DOP receptors and eGFP–DOP receptors (Wang et al., 2010; Zhang and Bao, 2012). Thirdly, molecular and pharmacological chaperones have been shown to help target DOP receptors to the cell surface by stabilizing receptor precursors and facilitating their release from the stringent quality control of the endoplasmic reticulum (Leskela et al., 2007; Decaillot et al., 2008). In addition, in vivo interactions with other proteins facilitate surface expression of DOP receptors (see Cahill et al., 2007) and decrease the expression of MOP receptors (Decaillot et al., 2008). Fourthly, it is possible that under physiological conditions MOP-DOP receptor heteromer levels are low and that they increase under pathological conditions such as pain or development of tolerance to morphine (see Cahill et al., 2007). Thus, although the eGFP-DOP receptor knock-in mouse is a good model for understanding the physiological role of DOP receptors, care has to be taken in the interpretation of the data, as the eGFP tag could disrupt interactions normally seen involving endogenous DOP receptors and other intracellular proteins.
A number of studies have tried to address the conflicting reports on the colocalization of MOP and DOP receptors in DRG neurons. These included studies that (i) used single-cell PCR in subsets of DRG neurons and reported the presence of MOP and DOP receptors in peptidergic small DRG neurons (Wang et al., 2010); (ii) used in situ hybridization to demonstrate the presence of MOP receptor and DOP receptor mRNA in DRG neurons that also expressed the mRNA for preprotachykinin A (a marker for peptidergic small neurons) (Wang et al., 2010); (iii) demonstrated the presence of DOP receptor immunoreactivity in peptidergic small DRG neurons using anti-DOP receptor antibodies that gave a signal with DRG neurons from wild-type but not from Oprd1 exon 1-deleted mice – reporting, interestingly, that high antibody dilutions (1:30 000 to 1:60 000) were needed to prevent non-specific binding (Wang et al., 2010); (iv) expressed myc-tagged DOP receptors and eGFP-tagged DOP receptors in small DRG neurons and, using antibodies to the epitope tags, showed that myc-DOP receptors localize to CGRP containing large dense-core vesicles, while eGFP–DOP receptors localize at the cell surface (Zhang and Bao, 2012); (v) used receptor-selective agonists to show that MOP and DOP receptors expressed by peptidergic nociceptors inhibited the release of substance P following formalin or capsaicin treatment and this could be blocked by receptor-selective antagonists (Beaudry et al., 2011); and (vi) used MOP-DOP receptor heteromer-selective antibodies to demonstrate the presence of heteromers in DRG neurons as well as in neurons in the pain pathway (Gupta et al., 2010). Taken together, these studies strongly support the presence of MOP and DOP receptors in peptidergic DRG neurons.
Behavioural evidence for MOP and DOP receptor heteromerization
Early behavioural studies provided indirect evidence for interactions between MOP and DOP receptors. One set of studies examined the effect of DOP receptor agonists on morphine-mediated antinociception and on the development of tolerance to this clinically used opioid. These studies showed that endogenous or synthetic DOP receptor agonists enhanced morphine-mediated analgesia as well as the development of tolerance to morphine (Vaught and Takemori, 1979a; b; Porreca et al., 1987). Another set of studies found that potent and selective DOP receptor antagonists could block morphine-mediated antinociception as well as development of tolerance following chronic morphine administration (Abdelhamid et al., 1991; Abul-Husn et al., 2007; Ballesta et al., 2012). More recently, a study examined the effect of DOP receptor ligands on antinociception mediated by [D-Ala2-N-Me-Phe4,Gly-ol5]-enkephalin (DAMGO) in naïve and morphine-tolerant mice; this study reported that the development of tolerance to morphine also led to tolerance to DAMGO, a MOP receptor-selective agonist (Szentirmay et al., 2013). Moreover, in morphine-tolerant mice, treatment with selective DOP receptor antagonists restored the antinociceptive effects of DAMGO to the levels observed with naïve animals (Szentirmay et al., 2013). Taken together, these studies indicated that DOP receptor could modulate MOP receptor-mediated antinociception and play a role in the development of tolerance following chronic administration of MOP receptor agonists.
The involvement of DOP receptors in MOP receptor-mediated antinociception has been examined using animal models with reduced DOP receptor levels or animals lacking individual receptors. For example, a study found that repeated i.c.v. injections with antisense oligonucleotides selective for DOP receptors reduced receptor levels in the brain and this, in turn, led to attenuation in the development of dependence on morphine (Sanchez-Blazquez et al., 1997). Also, studies with mice lacking MOP receptors showed that they exhibited reduced antinociception to DOP receptor-selective agonists compared with wild-type controls (Matthes et al., 1996; 1998). Examination of DOP receptor function in these mice showed that it was comparable to that of wild-type controls, thereby suggesting that optimal DOP receptor-mediated analgesia requires the presence of MOP receptors (Matthes et al., 1998). In addition, studies with mice lacking DOP receptors demonstrated that they exhibited normal morphine-mediated antinociceptive responses, although they did not develop antinociceptive tolerance to morphine (Zhu et al., 1999). Taken together, these studies support the notion that the interactions between MOP and DOP receptors could play a role in the development of tolerance to morphine.
Biochemical evidence for MOP and DOP receptor heteromerization
Biochemical evidence for the formation of MOP and DOP receptor complexes was first provided by studies in recombinant systems using co-immunoprecipitation methods and epitope-tagged (Flag or myc) receptors (George et al., 2000; Gomes et al., 2000). For example, cells transfected with either myc-DOP receptors, Flag-MOP receptors or a combination of both receptors were subjected to immunoprecipitation with antibodies to the myc epitope tag (to enable immunoprecipitation of DOP receptors). The immunoprecipitates containing DOP receptors were subjected to Western blot analysis with antibodies to the Flag epitope to detect the presence of MOP receptors. This strategy led to the detection of a distinct signal only in immunoprecipitates from cells co-expressing MOP and DOP receptors (George et al., 2000; Gomes et al., 2000). Interestingly, the level of interacting MOP-DOP receptor complexes decreased when using cells co-expressing myc-tagged MOP receptors along with a Flag-tagged C-terminally truncated DOP receptor (Fan et al., 2005), suggesting that the C-terminal region might be involved in MOP-DOP receptor heteromerization. Co-immunoprecipitation studies carried out with spinal cord membranes from wild-type and DOP receptor knockout mice detected interacting complexes only in membranes from wild-type animals (Gomes et al., 2004). While these co-immunoprecipitation studies imply that MOP and DOP receptors are in interacting complexes, they do not demonstrate direct interaction between the two receptors. Biophysical assays, such as proximity-based energy transfer assays, have been used to explore the possibility of interaction (i.e. if the two receptors are in close enough proximity to directly interact) in live cells. Studies using the bioluminescence resonance energy transfer (BRET) assay, where one of the receptors is C-terminally tagged with luciferase (Luc) and the partner receptor is C-terminally tagged with either yellow fluorescent protein or GFP, showed that MOP receptors and DOP receptors are within <10 nm in live cells and therefore are in close enough proximity to associate with each other (Gomes et al., 2004; Wang et al., 2005; Hasbi et al., 2007). This was supported by another study involving fractionation of cells co-expressing MOP receptor-Luc and DOP receptor-GFP followed by BRET assays that showed that MOP and DOP receptors were in sufficiently close proximity to directly interact in the plasma membrane (Hasbi et al., 2007). In addition, this study showed that MOP receptor-Luc interacted preferentially with Gαz-GFP in the presence of DOP receptors and with Gαi-GFP in its absence (Hasbi et al., 2007). Taken together, these results show that in live cells MOP and DOP receptors are in sufficiently close proximity to interact and that this leads to a change in receptor associated G protein from Gαi to Gαz.
The use of heteromer-selective antibodies (Gupta et al., 2010) or agents that disrupt heteromer formation (He et al., 2011; Kabli et al., 2013) has provided further support for the presence of MOP-DOP receptor heteromers in interacting complexes. MOP-DOP receptor heteromer-selective antibodies (that do not recognize individual receptors) were instrumental in demonstrating the presence of native MOP-DOP receptor heteromers in brain regions involved in pain processing (Gupta et al., 2010). Additional evidence for MOP-DOP receptor heteromeric interactions comes from the use of agents that disrupt these interactions, such as membrane-permeable TAT (YGRKKRRQRRR) peptides fused to either the peptide representing TM1 of MOP receptors or to the peptide representing the distal portion of the C-tail of DOP receptors (He et al., 2011; Kabli et al., 2013). The TAT peptide fused to TM1 of MOP receptors was found to disrupt MOP-DOP receptor heteromers both in vitro and in vivo (He et al., 2011). Moreover, in vivo disruption of the MOP-DOP receptor heteromer was found to lead to an increase in morphine-mediated antinociception (He et al., 2011). Disruption of this heteromer was also observed following substitutions of the G-G-G sequence in the carboxyl terminal tail of DOP receptors or of the S-V-R sequence in the third intracellular loop of MOP and DOP receptors (O'Dowd et al., 2012) or by using a TAT peptide fused to the peptide corresponding to the distal C-tail of DOP receptors (Kabli et al., 2013). While these studies suggest that disruption of the MOP-DOP receptor might be achieved by targeting different domains (TM1, C-terminal tail), collectively they provide direct evidence in support of the presence of MOP-DOP receptor heteromers in interacting complexes.
Modulation of MOP and DOP receptor properties by heteromerization
In the following sections we describe how the binding, signalling and trafficking properties of cells expressing MOP-DOP receptors differ from those expressing individual receptors. Although these differences could be due to receptor cross-talk, the data from these studies, taken together with co-immunoprecipitation and proximity-based assay studies, provide further support for MOP-DOP receptor heteromerization.
Pharmacological and signalling properties of MOP-DOP receptor heteromers
A number of studies have examined the changes in the binding properties of selective ligands in cells and/or tissues expressing MOP-DOP receptor heteromers. In a study comparing the binding of selective synthetic agonists and of endogenous opioid peptides at MOP-DOP receptor heteromers with that at individual receptor homomers, the authors noted that cells expressing such heteromers exhibited ∼10-fold decrease in affinity for selective synthetic agonists and a 2–3-fold increase in affinity of endogenous opioid peptides compared with cells expressing individual receptors (George et al., 2000). Another study examined the effect of low non-signalling doses of ligands to one receptor protomer on radiolabelled agonist binding to the partner protomer, and reported that selective ligands (binding to one of the protomers) could allosterically increase the radiolabelled agonist binding to the partner protomer by affecting the rate of dissociation of the radiolabelled ligand only in cells co-expressing MOP and DOP receptors (Gomes et al., 2000; 2004; 2011). These changes in the pharmacological properties of MOP-DOP receptor heteromers compared with MOP or DOP receptor homomers suggested possible differences in signalling properties between heteromers and homomers.
When the intracellular signalling between MOP-DOP receptor heteromers and MOP or DOP receptor homomers was examined, interesting properties were revealed. The occupancy of one of the protomers (by low non-signalling doses of an agonist, antagonist or inverse agonist) in the MOP-DOP receptor heteromer was found to lead to an enhancement in the signalling activity of the partner protomer (Gomes et al., 2000; 2004; 2011). A study examining the G proteins associated with the MOP-DOP receptor heteromer by carrying out [35S]GTPγS binding found that the receptors were associated with Pertussis toxin-insensitive Gαz in cells co-expressing both receptors and with Pertussis toxin-sensitive Gαi in cells expressing individual receptors (George et al., 2000; Fan et al., 2005; Hasbi et al., 2007). However, another study found that DAMGO inhibited Ca2+-mediated signalling in cells expressing MOP receptors while increasing Pertussis toxin-dependent Ca2+ signalling in cells co-expressing MOP and DOP receptors (Charles et al., 2003); this suggested an involvement of Pertussis toxin-sensitive G proteins in the heteromer-mediated effects. These differences could be due to differences in experimental conditions and the type of cells used in these studies. Together, these studies suggest that MOP-DOP receptor heteromerization leads to a switch in the G protein or signalling pathway associated with the receptor. Interestingly, a study examining the localization of β-arrestin in cells expressing the MOP-DOP receptor heteromer found that the latter was associated with and signalled via a β-arrestin-2-mediated pathway (Rozenfeld and Devi, 2007). Furthermore, the heteromer-mediated signalling was shown to lead to changes in the spatiotemporal dynamics of ERK1/2 phosphorylation, including cytosolic retention of ERK1/2 leading to phosphorylation of its cytosolic substrates and resulting in differential activation of transcription factors (Rozenfeld and Devi, 2007). Taken together, these studies suggest that MOP-DOP receptor heteromerization leads to a switch in signalling and activation of different signal transduction pathways, thereby increasing the repertoire of signalling of MOP and DOP receptors (Table 1).
Table 1.
Ligands modulating MOP-DOP receptor heteromer signalling or trafficking
| Ligand | Ligand binding and signalling | Trafficking | References |
|---|---|---|---|
| DAMGO | Induces prolonged ERK1/2 activation that is blocked by β-arrestin-2 siRNA1 Activates Ca2+-mediated signalling2 Binding is increased in the presence of a DOP receptor antagonist3 | Internalizes the MOP-DOP receptor heteromer and this is blocked by DOP receptor antagonists4 | 1Rozenfeld et al., 2007 2Charles et al., 2003 3Gomes et al., 2000 4Milan-Lobo and Whistler, 2011 |
| Methadone | Not reported | Induces internalization and degradation of MOP-DOP receptor heteromer, and this is blocked by DOP receptor antagonists | Milan-Lobo and Whistler, 2011 |
| Deltorphin II | Induces β-arrestin recruitment, and this is blocked by MOP-DOP receptor heteromer-selective antibody5 | Induces internalization of MOP-DOP receptor heteromers6 | 5Gomes et al., 2013b 6Hasbi et al., 2007 |
| DPDPE, DSLET | Not reported | Does not internalize MOP-DOP receptor heteromers | Hasbi et al., 2007 |
| Bivalent ligands (oxymorphone + ENIT, naltrexone+DM-SNC80) | These ligands show low affinity at DOP receptors and enhanced affinity at MOP-DOP receptor heteromers | Not reported | Harvey et al., 2012 |
| Bivalent ligand (MDAN21) | Not reported | Does not internalize MOP-DOP receptor heteromers, and this is reversed by naltrindole. | Yekkirala et al., 2013 |
| Monovalent ligands (MA19, DN20) | Not reported | Combination of these ligands facilitates internalization of MOP-DOP receptor heteromer. | Yekkirala et al., 2013 |
| Biased agonist (CYM51010) | CYM51010 induces both β-arrestin- and G protein-mediated signalling. | Not reported | Gomes et al., 2013b |
Trafficking properties of MOP-DOP receptor heteromers
A number of studies have examined the trafficking properties of MOP-DOP receptor heteromers. Relative to the studies examining trafficking of the heteromers from the cell surface to the intracellular compartment, fewer studies have explored the trafficking of the heteromers from an intracellular compartment to the cell surface. In the latter case there are conflicting reports about whether MOP-DOP receptor heteromers are present only at the cell surface or if they are pre-assembled in the endoplasmic reticulum prior to trafficking to the cell surface. One study used cells where MOP receptors were constitutively expressed and where DOP receptor expression could be induced and reported that MOP and DOP receptors heteromerized only at the cell surface and this required interactions with G proteins (Law et al., 2005). Another study used BRET in combination with cell fractionation to show that MOP-DOP receptor heteromerization could be detected in the endoplasmic reticulum, where the heteromers were associated with Gαz protein (Hasbi et al., 2007). These differences in detection of the site of heteromerization between MOP and DOP receptors could be due to the differences in the experimental conditions as the first study used a staggered receptor expression (inducing DOP receptors expression in cells already expressing MOP receptors) while the other study used co-expression of MOP receptor-luciferase and DOP receptor-GFP. Finally, a study examining the biosynthesis and maturation of MOP-DOP receptor heteromers reported the requirement for chaperone proteins for efficient cell surface expression of this heteromer. Thus, in cells co-expressing MOP and DOP receptors, a significant portion of the heteromer was found to localize to the Golgi apparatus; heteromer expression at the cell surface required the presence of receptor transport protein 4 (Decaillot et al., 2008). This chaperone was found to protect the heteromer from ubiquitination and proteasomal degradation during folding and maturation (Decaillot et al., 2008). It is not clear if this chaperone contributes to the unique binding and signalling properties of the MOP-DOP receptor heteromer.
A number of studies have examined the trafficking of MOP-DOP receptor heteromers from the cell surface to an intracellular compartment (endocytosis). One study found that the agonist to one receptor protomer promoted endocytosis of that protomer and not of the MOP-DOP receptor heteromer (Law et al., 2005). Other studies found that heteromer endocytosis is probe-selective, that is, some agonists (DAMGO, deltorphin II, SNC80, methadone) but not others (D-penicillamine(2,5)-enkephalin, DPDPE; [D-Ser2, Leu5, Thr6]-enkephalin, DSLET) induce endocytosis of MOP-DOP receptor heteromers (Law et al., 2005; Hasbi et al., 2007; Kabli et al., 2010; Milan-Lobo and Whistler, 2011). Moreover, endocytosis induced by select MOP receptor agonists was found to be blocked by DOP receptor-selective antagonists (Milan-Lobo and Whistler, 2011). Additionally, endocytosed MOP-DOP receptor heteromers were targeted for degradation, in contrast to MOP receptor homomers, which were found to be recycled back to the cell surface (Milan-Lobo and Whistler, 2011). These studies suggest that heteromerization leads to changes in receptor trafficking properties. Studies using MDAN21, a bivalent MOP-DOP receptor heteromer-selective ligand, found that it did not induce endocytosis of the heteromer, while a combination of individual monovalent pharmacophores (DN20 and MA19) did (Yekkirala et al., 2013). This led to the suggestion that the spacer arm in MDAN21 that joins the DN20 and MA19 pharmacophores helps in effective bridging of both protomers in the MOP-DOP receptor heteromer, thereby immobilizing it and preventing its internalization (Table 1). Additional studies are needed to characterize the underlying molecular mechanisms involved in the differential trafficking of MOP-DOP receptor heteromers.
Heteromer-biased ligands
Over the last several decades a number of ligands have been identified as being selective for one opioid receptor type versus the others. It is likely that some of these ligands exhibit differential selectivity towards the heteromer as compared to individual receptors. Studies evaluating the selectivity of some classical MOP receptor and DOP receptor ligands in the context of the MOP-DOP receptor heteromer as well as signalling pathways activated by these ligands have been initiated. In addition, compounds selective for MOP-DOP receptor heteromers have also been synthesized/identified. These are described below along with their in vivo antinociceptive effects and possible side-effects.
Classical MOP receptor agonists
Assays to measure intracellular calcium release via chimeric G proteins or GTPγS binding via the native G proteins have been used to examine the signalling properties of classical as well as of clinically used MOP receptor agonists (DAMGO, morphine, fentanyl and methadone) in cells stably expressing either homomeric or heteromeric opioid receptors (Yekkirala et al., 2010; 2012). These signalling assays showed that the potency of DAMGO, morphine, fentanyl and methadone was ∼7–12 fold greater at MOP-DOP receptor heteromers than at homomeric MOP receptors while showing no activity with homomeric DOP receptors (Yekkirala et al., 2010; 2012). These results suggested that these MOP receptor agonists are more potent at inducing signalling at MOP-DOP receptor heteromers compared to MOP receptor homomers. Moreover, the DOP receptor-selective antagonist naltrindole antagonized the signalling mediated by morphine, fentanyl and methadone only in cells expressing MOP-DOP receptor heteromers, and it also antagonized the antinociception mediated by these drugs in monkeys (Yekkirala et al., 2012). These findings led the authors to suggest that MOP-DOP receptor heteromers are the primary targets for the antinociceptive effects of morphine, fentanyl and methadone as well as in the development of tolerance and dependence to these drugs.
Studies also show that selective ligands activate distinct signalling pathways in cells expressing MOP-DOP receptor heteromers compared with those expressing MOP receptor homomers. For example, in cells expressing MOP receptors alone, treatment with DAMGO activates Gαi/o-mediated signalling, whereas in cells expressing MOP-DOP receptor heteromers it activates β-arrestin-mediated signalling (Rozenfeld and Devi, 2007) (see ‘Pharmacological and signalling properties of MOP-DOP receptor heteromers’ above). Taken with the report suggesting the involvement of β-arrestin-mediated signalling in the development of tolerance, this would suggest that the MOP-DOP receptor heteromer plays a role in this process (Bohn et al., 1999). Indeed, in vivo studies have suggested the involvement of such heteromers in the modulation of morphine-mediated antinociception; these include studies showing that the antinociceptive effect of morphine (acting primarily through μ opioid receptors) is enhanced by Leu-enkephalin, an endogenous DOP receptor agonist; by FK33824, a synthetic analogue of enkephalin; and by TIPPΨ (H-Tyr-Tic[CH2NH]-Phe-Phe-OH), a DOP receptor antagonist (Lee et al., 1980; Gomes et al., 2004). Other studies have supported the involvement of DOP receptors in the development of tolerance to morphine; these include the demonstration that naltrindole, a DOP receptor antagonist, can block the development of antinociceptive tolerance to morphine (Abdelhamid et al., 1991) and that DOP receptor knockout mice do not develop antinociceptive tolerance to morphine (Zhu et al., 1999; Nitsche et al., 2002). More recently, in a study using heteromer-selective antibodies, an increase in MOP-DOP receptor heteromer levels in the brain and spinal cord after chronic morphine treatment has been reported (Gupta et al., 2010). Finally, a study with the TAT peptide targeting TM1 of MOP receptors (which disrupts heteromer formation) showed that pretreatment with the peptide prevents the development of antinociceptive tolerance to morphine (He et al., 2011). Taken together, these findings are consistent with the idea that the MOP-DOP receptor heteromeric complex plays a role in the development of antinociceptive tolerance to morphine (Table 2).
Table 2.
Evidence for physiological effect of ligands targeting MOP-DOP receptor heteromers
| Ligand | Experimental model | Outcome | References |
|---|---|---|---|
| Morphine | Tail-immersion test (acute treatment) | Antinociception (i.m.) Morphine antinociception (ED50 of 2.67 mg/kg) is lowered by pretreatment with 3.2 mg/kg of naltrindole (ED50 of 15.48 mg/kg). | Yekkirala et al., 2012 |
| Fentanyl | Tail-immersion test (acute treatment) | Antinociception (i.m.) Fentanyl antinociception (ED50 of 0.011 mg/kg) is lowered by pretreatment with 3.2 mg/kg of naltrindole (ED50 of 0.048 mg/kg). | Yekkirala et al., 2012 |
| Methadone | Tail-immersion test (acute treatment) | Antinociception (i.m.) Methadone antinociception (ED50 of 1.79 mg/kg) is lowered by pretreatment with 3.2 mg/kg of naltrindole (ED50 of 4.35 mg/kg). | Yekkirala et al., 2012 |
| SNC80 | Tail-flick test (acute treatment) | Antinociception (i.t.) SNC80 antinociception (ED50 of ∼50 nmol) is lowered in the MOP receptor and DOP receptor knockout mice (ED50 of 131 nmol and 327 nmol, respectively). | Metcalf et al., 2012 |
| Bivalent ligand (MDAN) | Tail-flick test (acute treatment) | Antinociception (s.c., i.c.v., and i.t.) MDAN21 exhibits 100 times more potent antinociception (i.c.v., ED50 of 0.04 nmol) than morphine (i.c.v., ED50 of 4.1 nmol) without development of tolerance or dependence. | Daniels et al., 2005 |
| Biased agonist (CYM51010) | Tail-flick test (acute treatment) | CYM51010 (s.c.) exhibits equipotent antinociception to morphine with lesser antinociceptive tolerance. | Gomes et al., 2013b |
| TAT-fused peptide (MOP receptor TM1–TAT) | Tail-flick test (chronic morphine treatment) | Disruption of MOP-DOP receptor interaction by TAT-fused peptide increases morphine antinociception and decreases the development of antinociceptive tolerance. | He et al., 2011 |
| TAT-fused peptide (DOP receptor carboxyl tail–TAT) | Forced swim test Novelty-induced hypophagia Elevated plus maze | Disruption of MOP-DOP receptor interaction by TAT-fused peptide inhibits UFP-512 antidepressant-like and anxiolytic-like effects. | Kabli et al., 2013 |
| MOP-DOP receptor heteromer selective antibody | IHC, ELISA (chronic morphine treatment) | Increase of MOP-DOP receptor heteromers in various brain regions after chronic morphine treatment. | Gupta et al., 2010 |
i.m, intramuscularly; i.t, intrathecal; s.c., subcutaneously; i.c.v, intracerebroventricularly; IHC, immunohistochemistry.
Classical DOP receptor agonists
The involvement of MOP-DOP receptor heteromers in the antinociceptive effects of SNC80, a DOP receptor-selective agonist, has also been investigated. Studies using cells co-expressing a chimeric G protein with either opioid receptor heteromers or individual receptor homomers found that SNC80 elicited a robust release in intracellular calcium only in cells expressing MOP-DOP receptor heteromers (Metcalf et al., 2012). In addition, behavioural studies showed that the antinociceptive effect of SNC80 was abolished in animals lacking MOP receptors (Sora et al., 1999; Metcalf et al., 2012). Furthermore, the antinociceptive efficacy of SNC80 was right-shifted by approximately threefold and sixfold in MOP or DOP receptor knockout mice, respectively (Metcalf et al., 2012). Taken together, these results indicate that the presence of both MOP and DOP receptors are necessary for the antinociceptive activity of SNC80 (Table 2). One study used a combination of highly selective MOP receptor agonists with DOP receptor antagonists (and vice versa) to explore MOP-DOP receptor heteromer-mediated signalling. For example, the occupancy of the DOP receptor (by selective antagonist) was found to reverse MOP receptor-mediated signalling in cells co-expressing MOP-DOP receptor heteromers from β-arrestin-mediated to Gαi/o-mediated; this occupancy of DOP receptors was also found to lead to an enhancement of morphine-mediated antinociception (Gomes et al., 2004; Rozenfeld and Devi, 2007). These results indicate that DOP receptor ligands could function as allosteric modulators of MOP receptor activity (within the MOP-DOP heteromer). Finally, using a TAT peptide fused to the peptide corresponding to the distal C-tail of DOP receptors to disrupt MOP-DOP receptor heteromers, Kabli and colleagues (Kabli et al., 2013) reported that this led to a loss of the antidepressant and anxiolytic effects of the DOP receptor agonist UFP-512, suggesting a potential role for this heteromer in anxiety and depression.
Bivalent ligands
Attempts to synthesize heteromer-selective ligands have led to the generation of bivalent ligands such as MDAN21. This compound consists of a DOP receptor antagonist pharmacophore, DN20, separated by a 21-atom spacer from the MOP receptor agonist pharmacophore MA19 (Daniels et al., 2005). MDAN21 was found to exhibit 100 times higher potency as compared with morphine without significant development of tolerance or dependence (Daniels et al., 2005). Moreover, MDAN21 did not induce receptor internalization in cells expressing MOP-DOP receptor heteromers, probably accomplishing this by bridging both protomers and effectively immobilizing the heteromer at the cell surface (Yekkirala et al., 2013). Other bivalent ligands consisting of a high-affinity mu agonist (oxymorphone) linked by a spacer arm to a low-affinity delta antagonist (ENTI) or a high-affinity mu antagonist (naltrexone) joined by a spacer arm to a low-affinity delta agonist (DM-SNC80) have also been generated (Harvey et al., 2012). However, the antinociceptive effects of these ligands and their side-effects have not been adequately evaluated. Taken together, these studies demonstrate that bivalent ligands provide critical tools to explore in vitro and in vivo properties of MOP-DOP receptor heteromers.
Screening for ligands targeting the MOP-DOP receptor heteromer and their pharmacology in pain regulation
From studies described above, the unique pharmacological and signalling properties of MOP-DOP receptor heteromers make them potential targets for the development of new therapeutics to treat pain with reduced side-effects. This would require high-throughput screening (HTS) of large libraries of drug-like compounds, which could lead to the identification of MOP-DOP receptor heteromer-selective/biased ligands. This would require suitable screening assays. Below we describe a few of the HTS assays suitable for the screening of heteromer-selective ligands.
High-throughput screening using calcium signalling
Several assays that measure G protein-mediated signalling, such as adenylyl cyclase/cAMP, phospholipase C/Ca2+ and Rho assays, can be used for HTS. Of these, HTS assays that measure the release of intracellular Ca2+ are commonly used to screen for ligands to receptors that are normally coupled to Gαq. Moreover, measurement of intracellular Ca2+ release has also been used to screen for ligands for receptors that couple to Gαi or Gαs, due to the development of promiscuous chimeric G proteins such as Gαqs or Gαqi, which provide a calcium readout for these receptors that do not normally signal via the Gαq pathway (Harvey et al., 2013). Thus, the activation of opioid receptors that are co-expressed with chimeric Gαqi protein can be detected by monitoring the release of intracellular Ca2+.
Recently, a screening assay for the detection of heteromer-mediated signalling has been described that makes use of carboxyl-terminally truncated GPCRs fused to chimeric Gαqi proteins. These receptors, upon agonist binding, do not induce intracellular Ca2+ release; Ca2+ release is observed only when these chimeric receptors are co-expressed with the wild-type receptor (van Rijn et al., 2013). This method allowing for the selective detection of Ca2+ release by MOP-DOP receptor heteromers was developed using wild-type MOP receptors and Gαqi-fused DOP receptors (van Rijn et al., 2013). An important advantage of this method is that in cells expressing receptor heteromers, it detects only heteromer-mediated signalling. Using this assay, ADL5859 was found to be poor at eliciting a signal from MOP-DOP receptor heteromers, compared with DOP receptor homomers (van Rijn et al., 2013). Thus, this Ca2+ signalling-based assay could be useful in the identification of heteromer-selective compounds.
High-throughput screening using β-arrestin signalling
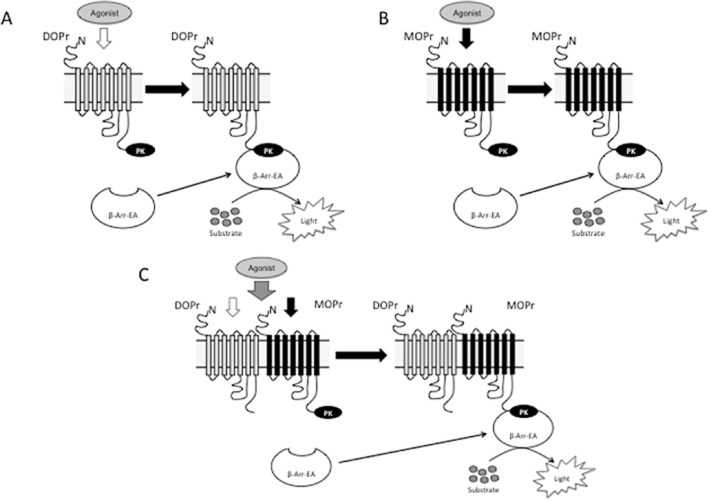
This assay makes use of receptor activation-mediated arrestin recruitment coupled to an enzyme-fragment complementation assay leading to reconstitution of β-galactosidase activity (Figure 1). For MOP-DOP receptor heteromer screening, a cell line expressing MOP receptors C-terminally tagged with a small fragment of β-galactosidase and β-arrestin tagged to a complementary β-galactosidase fragment and untagged DOP receptors was generated (MOPβgal-DOP cells; DiscoveRx, Fremont, CA, USA). The basic premise of this assay is that activation of DOP receptors in MOPβgal-DOP receptor cells will induce the recruitment of β-arrestin to MOP receptors and reconstitution of active β-galactosidase activity. Hence, deltorphin II, a DOP receptor agonist, would bind to DOP receptors within the MOP-DOP receptor heteromer, leading to an increase in recruitment of β-arrestin to MOP receptors. The extent to which the increase in recruitment represents activation of the heteromer is then tested using the MOP-DOP receptor heteromer-selective antibody (which selectively blocks the heteromer). Such an assay was developed and showed that a significant portion (∼60%) of DOP receptor agonist-mediated recruitment could be blocked by the MOP-DOP receptor heteromer-selective antibody (Gomes et al., 2013b). As in this assay, activation of the MOP receptor will also lead to an increase in recruitment of β-arrestin to the receptor, all positive ‘hits’ need to be confirmed using counter-screens with cells expressing only MOP receptors (MOPβgal receptor; DiscoveRx). A recent study used this assay to identify CYM51010 as a MOP-DOP receptor heteromer-biased agonist (Gomes et al., 2013b).
Figure 1.
High-throughput screening assay for heteromer-selective ligands using cells expressing DOPβgal receptor, MOPβgal receptor or MOPβgal-DOP receptor. (A & B) Schematics of monomer or homomer-mediated β-arrestin recruitment. Treatment of cells expressing either DOP receptor (DOPr) (A) or MOP receptor (MOPr) (B) tagged with ProLink/β-galactosidase donor (PK) (DOPβgal receptor and MOPβgal receptor, respectively) and β-arrestin (β−Arr-EA) tagged with a β-galactosidase activator (EA) with receptor-selective agonists leads to recruitment of β-arrestin to the receptor and reconstitution of a functionally active β-galactosidase whose activity can be measured by addition of an enzyme-specific substrate. (C) Schematic of heteromer-mediated β-arrestin recruitment. Treatment of cells expressing untagged DOP receptor and MOP receptor tagged with ProLink/β-galactosidase donor (PK) (MOPβgal-DOP receptor) and β-arrestin tagged with a β-galactosidase activator (EA) with a DOP receptor-selective agonist leads to recruitment of β-arrestin to MOP receptor and reconstitution of a functionally active β-galactosidase whose activity can be measured by addition of an enzyme-specific substrate, only if both receptors form interacting complexes. Modified from Gomes et al. (2013b).
The strategy used to identify CYM51010 as a MOP-DOP receptor heteromer-selective agonist involved the primary screening of a low MW library (∼335 461 compounds) at a single concentration using either MOPβgal receptor-DOP receptor or 5HT5Aβgal cells (Pinello et al., 2010; Gomes et al., 2013b). Comparison of the hits between the two cell lines identified 885 hits as unique to MOPβgal-DOP receptor cells; these were subjected to a secondary screen (single concentration in triplicate). This led to the identification of 346 hits as unique for MOPβgal-DOP receptor cells, which were then subjected to a tertiary screen (10-point dilution series in triplicate) using cells expressing either MOPβgal-DOP receptors, MOPβgal receptors, DOPβgal receptors or 5HT5Aβgal receptors (Pinello et al., 2010; Gomes et al., 2013b). Comparison of the dose–response curves led to the identification of 94 compounds as potential MOPβgal-DOP receptor-biased agonists; these were selected based on the criteria that they exhibited an EC50 of ≤10 μM with MOPβgal-DOP receptor cells and a fivefold difference in EC50 between MOPβgal-DOP receptor and either MOPβgal receptor or DOPβgal receptor cells (Gomes et al., 2013b). For validation of the identified hits, 14 compounds selected based on their potency, efficacy and uniqueness of structure compared with known opioid ligands were repurchased and tested in signalling assays. One of the tested compounds, CYM51010, exhibited a high intrinsic efficacy (1197 ± 31% over basal) for β-arrestin recruitment and for G protein-mediated signalling (via GTPγS binding assay; 168 ± 3% over basal) in MOPβgal-DOP receptor cells compared with MOPβgal receptor or DOPβgal receptor cells (Gomes et al., 2013b). Moreover, antinociception assays (tail-flick test) showed that CYM51010 exhibited antinociceptive activity similar to that of morphine and that chronic administration of this small molecule agonist resulted in less antinociceptive tolerance compared to morphine (Gomes et al., 2013b). Also, the MOP-DOP receptor heteromer-selective antibody significantly, albeit partly, blocked CYM51010-induced β-arrestin recruitment, GTPγS binding and intrathecal antinociception (Gomes et al., 2013b), indicating that CYM51010 exerted the majority of its effect via MOP-DOP receptor heteromer activation. The unique signalling properties of CYM51010 and its potent antinociceptive effects make it a novel candidate or a good lead compound for the development of new analgesics with lower antinociceptive tolerance compared with morphine.
Development of therapeutics: from the lab bench to clinical use
Drugs targeting MOP-DOP receptor heteromers could be novel therapeutics for the treatment of chronic pain and of mood disorders such as anxiety or depression. However, the considerable efforts to identify such novel therapeutics targeting heteromers raise the question as to when they will be clinically available. In general, drug development is a multi-year, multi-million-dollar proposition where the majority of promising compounds fail to reach the clinic. It has been reported that the average time from target identification to approval by the U.S. Food and Drug Administration (FDA) is 13.5 years (Paul et al., 2010).
There are several stages in drug development, including (i) identification of a potential therapeutic drug; (ii) examination of its potential side-effects in animal models; (iii) determination of the absorption, distribution, metabolism and elimination (ADME) properties of the compound in animals; (iv) if data from (ii) and (iii) are unfavourable, examination of whether chemical modifications in the structure of the potential drug will lead to a compound with fewer side-effects and better ADME parameters; and (v) human clinical trials, which are generally carried out in three phases. Phase I trials involve testing of the drug in a small group of people to evaluate the safety and dosage range and to identify side-effects in humans. Phase II trials involve testing the drug in a larger group of people in order to test its effectiveness to treat a disease and further evaluate its safety. Phase III trials are generally conducted in large groups of people in different countries to confirm the effectiveness of the drug, monitor side-effects, compare its therapeutic effects with those of commonly used treatments and collect information that will allow the drug to be safely used. After the drug passes the clinical trials it needs to be approved by the FDA for clinical use.
The National Institute of Health RoadMap programme created the Molecular Libraries Initiative and the Molecular Libraries Screening Center Network to facilitate the establishment of translational and chemical screening programs at academic screening centers (http://www.slas.org/screeningFacilities/facilityList.cfm) (Macarron et al., 2011). The use of these libraries in combination with HTS carried out using in vitro cell culture systems provides researchers with a rapid means to narrow down the search for potential hits. However, HTS-derived hits need to be optimized, characterized for their side-effect profile, and subjected to clinical trials and FDA approval. In general, the number of new drugs approved by the FDA is around 20 per year, and around 10 can be developed by large pharmaceutical companies, of which 20–30% would be considered ‘first-class’ medicines (Paul et al., 2010).
In the case of drugs targeting MOP-DOP receptor heteromers, none are currently under clinical trials. A potential therapeutic for the treatment of diarrhea-predominant irritable bowel syndrome (IBS-D) was developed by Furiex Pharmaceuticals (Morrisville, NC, USA) (Breslin et al., 2012; Wade et al., 2012; Dove et al., 2013) and is currently undergoing phase III clinical studies. This compound, named eluxadoline, is a locally active mixed MOP receptor agonist/DOP receptor antagonist with low oral bioavailability. However, the mechanism of action of this drug and whether it exerts its effect by activation of MOP receptors or of MOP-DOP receptor heteromers is not known. Further studies are needed to evaluate this. In animal models of altered gastrointestinal function, eluxadoline is able to normalize faecal output without completely blocking gastrointestinal transit, unlike the pure MOP receptor agonist loperamide (Wade et al., 2012). This suggests that eluxadoline will exhibit weaker side-effects compared to loperamide (Wade et al., 2012). The phase II clinical trials revealed that patients suffering from IBS-D, when given eluxadoline, showed an improvement of their symptoms based on decrease in abdominal pain and normal stool consistency (Dove et al., 2013). These promising results suggest that further studies are needed to evaluate eluxadoline for the treatment of IBS-D.
Summary and perspective
Over the last decade, the heteromerization between MOP and DOP receptors has been extensively studied. These studies are helping us understand how heteromerization between these two receptor types modulates individual receptor pharmacology, signalling and trafficking properties. Most of these studies have used heterologous cells. Research using the recent generation of antibodies that selectively recognize endogenous MOP-DOP receptor heteromers and TAT peptides that disrupt MOP-DOP receptor heteromers is beginning to identify the physiological roles of these heteromers. Heteromer-selective antibodies have begun to detect the presence of MOP-DOP receptor heteromers in endogenous tissue as well as changes in the levels of these heteromers following drug administration or in a pathological condition such as development of antinociceptive tolerance, while TAT peptides are helping to elucidate the contribution of MOP-DOP receptor heteromers to morphine-mediated antinociception and development of tolerance to the drug. Moreover, efforts are being made towards the identification of MOP-DOP receptor heteromer-selective ligands. In this context, studies reveal that classic drugs such as morphine, fentanyl or methadone that were once thought to be conventional MOP receptor agonists in fact exhibit greater signalling potency in cells co-expressing MOP and DOP receptors, suggesting that they could be targeting heteromers. Thus studies are needed to rigorously evaluate classical MOP receptor- or DOP receptor-selective ligands with regard to their binding and signalling to receptor homomers and heteromers. It is interesting to note that binding of morphine to MOP-DOP receptor heteromers leads to β-arrestin-mediated signalling, and the latter has been implicated in the development of tolerance to morphine. In this context, the heteromer-selective ligands described in this review, both bivalent ligands and CYM51010, exhibited similar or greater antinociceptive activity compared with morphine but with lesser development of antinociceptive tolerance. Given that CYM51010 can activate both β-arrestin- and G protein-mediated signalling, this suggests that this compound is likely to be a good candidate for the development of drugs for treatment of pain and with reduced abuse liability.
Glossary
Abbreviations
- BRET
bioluminescence resonance energy transfer
- DAMGO
[D-Ala2-N-Me-Phe4,Gly-ol5]-enkephalin
- DPDPE
D-penicillamine(2,5)-enkephalin
- DSLET
[D-Ser2, Leu5, Thr6]-enkephalin
- GFP
green fluorescent protein
- HTS
high-throughput screening
- IBS-D
diarrhoea-predominant irritable bowel syndrome
- i.t
intrathecal
- TIPPΨ
H-Tyr-Tic[CH2NH]-Phe-Phe-OH
- TM
transmembrane domain
Conflict of interest
None.
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J Pharmacol Exp Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- Abul-Husn NS, Sutak M, Milne B, Jhamandas K. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol. 2007;151:877–887. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, et al. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesta JJ, Cremades J, Rodriguez-Munoz M, Garzon J, Faura CC. Sensitivity to mu-opioid receptor-mediated anti-nociception is determined by cross-regulation between mu- and delta-opioid receptors at supraspinal level. Br J Pharmacol. 2012;166:309–326. doi: 10.1111/j.1476-5381.2011.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumhaker Y, Wollman Y, Goldstein MN, Sarne Y. Evidence for mu-, delta-, and kappa-opioid receptors in a human neuroblastoma cell line. Life Sci. 1993;52:PL205–PL210. doi: 10.1016/0024-3205(93)90061-7. [DOI] [PubMed] [Google Scholar]
- Beaudry H, Dubois D, Gendron L. Activation of spinal mu- and delta-opioid receptors potently inhibits substance P release induced by peripheral noxious stimuli. J Neurosci. 2011;31:13068–13077. doi: 10.1523/JNEUROSCI.1817-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Breslin HJ, Diamond CJ, Kavash RW, Cai C, Dyatkin AB, Miskowski TA, et al. Identification of a dual delta OR antagonist/mu OR agonist as a potential therapeutic for diarrhea-predominant irritable bowel syndrome (IBS-d) Bioorg Med Chem Lett. 2012;22:4869–4872. doi: 10.1016/j.bmcl.2012.05.042. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Charles AC, Mostovskaya N, Asas K, Evans CJ, Dankovich ML, Hales TG. Coexpression of delta-opioid receptors with micro receptors in GH3 cells changes the functional response to micro agonists from inhibitory to excitatory. Mol Pharmacol. 2003;63:89–95. doi: 10.1124/mol.63.1.89. [DOI] [PubMed] [Google Scholar]
- Chen YL, Law PY, Loh HH. The other side of the opioid story: modulation of cell growth and survival signaling. Curr Med Chem. 2008;15:772–778. doi: 10.2174/092986708783955518. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- Costantino CM, Gomes I, Stockton SD, Lim MP, Devi LA. Opioid receptor heteromers in analgesia. Expert Rev Mol Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci U S A. 2005;102:19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove LS, Lembo A, Randall CW, Fogel R, Andrae D, Davenport JM, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329–338 e321. doi: 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Egan TM, North RA. Both mu and delta opiate receptors exist on the same neuron. Science. 1981;214:923–924. doi: 10.1126/science.6272393. [DOI] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J Biol Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- Fields HL, Emson PC, Leigh BK, Gilbert RF, Iversen LL. Multiple opiate receptor sites on primary afferent fibres. Nature. 1980;284:351–353. doi: 10.1038/284351a0. [DOI] [PubMed] [Google Scholar]
- Filizola M, Devi LA. Grand opening of structure-guided design for novel opioids. Trends Pharmacol Sci. 2012;34:6–12. doi: 10.1016/j.tips.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filizola M, Olmea O, Weinstein H. Prediction of heterodimerization interfaces of G-protein coupled receptors with a new subtractive correlated mutation method. Protein Eng. 2002;15:881–885. doi: 10.1093/protein/15.11.881. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Golebiewska U, Johnston JM, Devi L, Filizola M, Scarlata S. Differential response to morphine of the oligomeric state of mu-opioid in the presence of delta-opioid receptors. Biochemistry. 2011;50:2829–2837. doi: 10.1021/bi101701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA. G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol. 2011;79:1044–1052. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Chandrakala MV, Devi LA. Disease-specific heteromerization of G-protein-coupled receptors that target drugs of abuse. Prog Mol Biol Transl Sci. 2013a;117:207–265. doi: 10.1016/B978-0-12-386931-9.00009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A, Saldanha AS, Negri A, Pinello CE, et al. Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci U S A. 2013b;110:12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J. GPCR oligomers in pharmacology and signaling. Mol Brain. 2011;4:20. doi: 10.1186/1756-6606-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, et al. Structure of the delta-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JH, Long DH, England PM, Whistler JL. Tuned-affinity bivalent ligands for the characterization of opioid receptor heteromers. ACS Med Chem Lett. 2012;3:640–644. doi: 10.1021/ml300083p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey JH, van Rijn RM, Whistler JL. A FLIPR assay for evaluating agonists and antagonists of GPCR heterodimers. Methods Mol Biol. 2013;995:43–54. doi: 10.1007/978-1-62703-345-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, et al. Agonists at the delta-opioid receptor modify the binding of micro-receptor agonists to the micro-delta receptor hetero-oligomer. Br J Pharmacol. 2010;161:1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Nguyen T, Balboni G, O'Dowd BF, George SR. Antidepressant-like and anxiolytic-like effects following activation of the mu-delta opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.115. doi: 10.1038/mp.2013.115; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kazmi SM, Mishra RK. Comparative pharmacological properties and functional coupling of mu and delta opioid receptor sites in human neuroblastoma SH-SY5Y cells. Mol Pharmacol. 1987;32:109–118. [PubMed] [Google Scholar]
- Kieffer BL. Recent advances in molecular recognition and signal transduction of active peptides: receptors for opioid peptides. Cell Mol Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor–G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Lee NM, Leybin L, Chang JK, Loh HH. Opiate and peptide interaction: effect of enkephalins on morphine analgesia. Eur J Pharmacol. 1980;68:181–185. doi: 10.1016/0014-2999(80)90319-2. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Leskela TT, Markkanen PM, Pietila EM, Tuusa JT, Petaja-Repo UE. Opioid receptor pharmacological chaperones act by binding and stabilizing newly synthesized receptors in the endoplasmic reticulum. J Biol Chem. 2007;282:23171–23183. doi: 10.1074/jbc.M610896200. [DOI] [PubMed] [Google Scholar]
- Liu X, Kai M, Jin L, Wang R. Computational study of the heterodimerization between mu and delta receptors. J Comput Aided Mol Des. 2009;23:321–332. doi: 10.1007/s10822-009-9262-7. [DOI] [PubMed] [Google Scholar]
- Macarron R, Banks MN, Bojanic D, Burns DJ, Cirovic DA, Garyantes T, et al. Impact of high-throughput screening in biomedical research. Nat Rev Drug Discov. 2011;10:188–195. doi: 10.1038/nrd3368. [DOI] [PubMed] [Google Scholar]
- Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, et al. Crystal structure of the micro-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf MD, Yekkirala AS, Powers MD, Kitto KF, Fairbanks CA, Wilcox GL, et al. The delta opioid receptor agonist SNC80 selectively activates heteromeric mu–delta opioid receptors. ACS Chem Neurosci. 2012;3:505–509. doi: 10.1021/cn3000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan-Lobo L, Whistler JL. Heteromerization of the mu- and delta-opioid receptors produces ligand-biased antagonism and alters mu-receptor trafficking. J Pharmacol Exp Ther. 2011;337:868–875. doi: 10.1124/jpet.111.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br J Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd BF, Ji X, O'Dowd PB, Nguyen T, George SR. Disruption of the mu-delta opioid receptor heteromer. Biochem Biophys Res Commun. 2012;422:556–560. doi: 10.1016/j.bbrc.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Palazzi E, Ceppi E, Guglielmetti F, Catozzi L, Amoroso D, Groppetti A. Biochemical evidence of functional interaction between mu- and delta-opioid receptors in SK-N-BE neuroblastoma cell line. J Neurochem. 1996;67:138–144. doi: 10.1046/j.1471-4159.1996.67010138.x. [DOI] [PubMed] [Google Scholar]
- Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, et al. How to improve R&D productivity: the pharmaceutical industry's grand challenge. Nat Rev Drug Discov. 2010;9:203–214. doi: 10.1038/nrd3078. [DOI] [PubMed] [Google Scholar]
- Petko J, Justice-Bitner S, Jin J, Wong V, Kittanakom S, Ferraro TN, et al. MOR is not enough: identification of novel mu-opioid receptor interacting proteins using traditional and modified membrane yeast two-hybrid screens. PLoS ONE. 2013;8:e67608. doi: 10.1371/journal.pone.0067608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinello C, Guerrero M, Eberhart C, Volmar CH, Saldanha SA, Cayanan C, et al. Characterization of an agonist probe for opioid receptor mu 1 (OPRM1)-opioid receptor delta 1 (OPRD1) heterodimerization. 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK148496 (accessed 5/4/2013) [PubMed]
- Porreca F, Heyman JS, Mosberg HI, Omnaas JR, Vaught JL. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Ther. 1987;241:393–400. [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Harvey JH, Brissett DI, DeFriel JN, Whistler JL. Novel screening assay for the selective detection of G-protein-coupled receptor heteromer signaling. J Pharmacol Exp Ther. 2013;344:179–188. doi: 10.1124/jpet.112.198655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Garcia-Espana A, Garzon J. Antisense oligodeoxynucleotides to opioid mu and delta receptors reduced morphine dependence in mice: role of delta-2 opioid receptors. J Pharmacol Exp Ther. 1997;280:1423–1431. [PubMed] [Google Scholar]
- Satake H, Sakai T. Recent advances and perceptions in studies of heterodimerization between G protein-coupled receptors. Protein Pept Lett. 2008;15:300–308. doi: 10.2174/092986608783744207. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NJ, Milligan G. Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes. Pharmacol Rev. 2010;62:701–725. doi: 10.1124/pr.110.002667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li XF, Funada M, Kinsey S, Uhl GR. Visceral chemical nociception in mice lacking mu-opioid receptors: effects of morphine, SNC80 and U-50488. Eur J Pharmacol. 1999;366:R3–R5. doi: 10.1016/s0014-2999(98)00933-9. [DOI] [PubMed] [Google Scholar]
- Stockton SD, Jr, Devi LA. Functional relevance of mu-delta opioid receptor heteromerization: a role in novel signaling and implications for the treatment of addiction disorders: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:167–172. doi: 10.1016/j.drugalcdep.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmay AK, Kiraly KP, Lenkey N, Lacko E, Al-Khrasani M, Friedmann T, et al. Spinal interaction between the highly selective mu agonist DAMGO and several delta opioid receptor ligands in naive and morphine-tolerant mice. Brain Res Bull. 2013;90:66–71. doi: 10.1016/j.brainresbull.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Vaught JL, Takemori AE. Differential effects of leucine and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J Pharmacol Exp Ther. 1979a;208:86–90. [PubMed] [Google Scholar]
- Vaught JL, Takemori AE. A further characterization of the differential effects of leucine enkephalin, methionine enkephalin and their analogs on morphine-induced analgesia. J Pharmacol Exp Ther. 1979b;211:280–283. [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA, et al. Modulation of gastrointestinal function by MuDelta, a mixed micro-opioid receptor agonist/micro-opioid receptor antagonist. Br J Pharmacol. 2012;167:1111–1125. doi: 10.1111/j.1476-5381.2012.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- Wang D, Sun X, Bohn LM, Sadee W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: comparison with plasmalemmal mu-opioid receptors. J Neurosci. 2001;21:3242–3250. doi: 10.1523/JNEUROSCI.21-09-03242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Kalyuzhny AE, Portoghese PS. Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [d-Ala(2)-MePhe(4)-Glyol(5)]enkephalin as selective mu-delta agonists. ACS Chem Neurosci. 2010;1:146–154. doi: 10.1021/cn9000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Banks ML, Lunzer MM, Negus SS, Rice KC, Portoghese PS. Clinically employed opioid analgesics produce antinociception via mu-delta opioid receptor heteromers in Rhesus monkeys. ACS Chem Neurosci. 2012;3:720–727. doi: 10.1021/cn300049m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid protomers by bivalent ligands. ACS Chem Biol. 2013;8:1412–1416. doi: 10.1021/cb400113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VC, Richards ML, Sadee W. A human neuroblastoma cell line expresses mu and delta opioid receptor sites. J Biol Chem. 1986;261:1065–1070. [PubMed] [Google Scholar]
- Zhang X, Bao L. Interaction and regulatory functions of mu- and delta-opioid receptors in nociceptive afferent neurons. Neurosci Bull. 2012;28:121–130. doi: 10.1007/s12264-012-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- Zieglgansberger W, French ED, Mercuri N, Pelayo F, Williams JT. Multiple opiate receptors on neurons of the mammalian central nervous system. In vivo and in vitro studies. Life Sci. 1982;31:2343–2346. doi: 10.1016/0024-3205(82)90152-7. [DOI] [PubMed] [Google Scholar]