Abstract
Within the opioid family of receptors, δ (DOPrs) and μ opioid receptors (MOPrs) are typical GPCRs that activate canonical second-messenger signalling cascades to influence diverse cellular functions in neuronal and non-neuronal cell types. These receptors activate well-known pathways to influence ion channel function and pathways such as the map kinase cascade, AC and PI3K. In addition new information regarding opioid receptor-interacting proteins, downstream signalling pathways and resultant functional effects has recently come to light. In this review, we will examine these novel findings focusing on the DOPr and, in doing so, will contrast and compare DOPrs with MOPrs in terms of differences and similarities in function, signalling pathways, distribution and interactions. We will also discuss and clarify issues that have recently surfaced regarding the expression and function of DOPrs in different cell types and analgesia.
LINKED ARTICLES
This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2
Keywords: β-arrestin, δ opioid receptor, μ opioid receptor, pain, primary afferent neurons, receptor trafficking
Introduction
Of the opioid family of receptors, the μ opioid receptor (MOPr) is the most well known. In binding with morphine and other semi-synthetic opioids, MOPrs are a well-studied clinical target. Unfortunately, MOPr agonists also induce a number of unwanted effects such as constipation, respiratory depression, analgesic tolerance, dependence and euphoria, which limit medical use and may lead to non-medical abuse.
Another member of the opioid receptor family, the δ opioid receptor (DOPr), has high sequence similarity to the MOPr, yet has different physiological and pharmacological properties and is not selectively targeted by an approved pharmaceutical product. Our knowledge of how this receptor functions in different cell types and under different pathological conditions is rapidly evolving. We will present recent evidence of the roles that this receptor may play under different conditions and in different cell types, and discuss how trafficking of this receptor influences DOPr function.
The concept that the location of a GPCR such as the DOPr, either intracellular or in different cell types, plays an important role in how the receptor functions is not novel. However, the location, and hence the function, of the DOPr has recently been the subject of some debate. This has resulted in some confusion as to the role of the DOPr under normal or physiological conditions. We will discuss these issues and describe recent findings of where DOPrs are localized and how this receptor functions. Novel interactions, pathways and physiological effects of DOPr activation will also be described suggestive of possible clinical roles of this receptor.
Part I. An overview of DOPr localization, trafficking and function
In the following section, we will first explore the anatomical and cellular localization of DOPrs. This will be followed by an assessment of our current knowledge of the intracellular localization and trafficking of DOPrs. We will then examine recent insights into how DOPrs regulate physiological and pathological states. An underlying theme of how DOPr localization, whether at the regional, cellular or intracellular levels, influences DOPr function will be developed throughout. Where possible and where relevant, we will also compare and contrast DOPrs with MOPrs so as to further our understanding and functional relevance of these GPCRs as distinct receptors or MOPr–DOPr heteromers.
Anatomical localization of DOPrs in the mammalian nervous system
In the CNS, MOPr and DOPr differ in their anatomical location. Although MOPrs are distributed throughout the CNS with highest densities in the thalamus, striatum, interpeduncular complex, medial habenular nucleus, cortex, superior and inferior colliculi, and in the superficial layers of the spinal cord (Mansour et al., 1994b; Le Merrer et al., 2009), DOPrs are discretely expressed in specific regions of the brain with high densities of the receptor found in the olfactory bulb, cortex, striatum and amygdala. Along the pain pathways, DOPrs are also expressed in several structures involved in the perception (peripheral nerve endings), transmission (dorsal root ganglia neurons and grey matter of the spinal cord) and integration of painful stimuli (parabrachial nucleus, amygdala, hypothalamus, thalamus, cerebral cortex, periaqueductal grey area and rostroventral medulla) as well as in areas involved in the regulation of mood (Mansour et al., 1994a; 1995; Cahill et al., 2001a; Mennicken et al., 2003). More recently, DOPrs were also shown to be expressed in peripheral NF200-positive axons surrounding hair follicles and other mechanosensory organs so likely regulates cutaneous mechanical hypersensitivity (Bardoni et al., 2014).
Significant differences in DOPr expression exist across species. A good example of this is the progressive specialization of DOPr localization within the nociceptive pathway across the phylogenetic tree. In rodent dorsal root ganglia neurons, DOPr expression is dispersed across different cell types whereas in primates, DOPr mRNA is primarily detected in small- and medium-sized dorsal root ganglion cells and DOPr-binding sites are concentrated in laminae I–II of the spinal cord (Mennicken et al., 2003). Furthermore, pharmacological (Pasquini et al., 1992) and immunogold labelling of DOPr has revealed that this receptor is mainly localized in the cytoplasm of cells (Cheng et al., 1995; Elde et al., 1995; Zhang et al., 1998; Cahill et al., 2001a; Gendron et al., 2006), suggesting that DOPrs are one of the few GPCRs that are sorted to the cell surface via the regulated secretory pathway (Guan et al., 2005; Cahill et al., 2007; Zhang et al., 2010; Zhao et al., 2011). Consistent with the high level of MOPrs on the cell membrane in nervous tissues, like most other GPCRs, MOPrs are delivered to the cell surface by the constitutive secretory pathway (Hamel and Beaudet, 1984; Van Bockstaele et al., 1996). Furthermore, as MOPrs may be recycled (Yu et al., 2010; Roman-Vendrell et al., 2012), it is possible that those present on the cell membrane may be from either newly synthesized or recycled receptor pools.
DOPr trafficking and function
Both MOPrs and DOPrs are Gi/o-coupled receptors, agonists of which activate canonical GPCR signalling cascades to reduce nociception, enhance euphoria or reduce anxiety, among other effects and recently described in several reviews (Al-Hasani and Bruchas, 2011; Williams et al., 2013; Charbogne et al., 2014). Novel trafficking and protein interactions, particularly of the DOPr, have recently come to light that may influence receptor signalling and are presented here.
Pre-assembled signalling complexes
GPCRs are often portrayed as single molecules present on the cell membrane. Upon binding to an agonist, these receptors recruit proteins to different regions of the receptor to activate downstream effector cascades. However, GPCRs have also been found as pre-assembled, receptor-specific protein complexes that are activated once on the cell membrane. For example, DOPrs may exist as a pre-assembled signalosome containing STAT5B, cSrc, Gα and Gβγ, so allowing enhanced STAT5 transcription in a cSrc and G-protein-dependent manner (Georganta et al., 2010). Both DOPrs and MOPrs may also be constitutively associated with spinophilin, an actin-associated and dendritic spine-enriched protein (Fourla et al., 2012). In a recombinant cell line setting, spinophilin is central to an agonist-specific complex consisting of a regulator of G-protein signalling (RGS) molecule, different Gα subunits and Gβγ subunits. This specificity could explain the ability of spinophilin to reduce DOPr, but not necessarily MOPr, induced inhibition of AC and ERK phosphorylation, but enhance receptor internalization (Fourla et al., 2012; Stratinaki et al., 2013). The role of members of the RGS family, RGS4, 9 and 10, in altering opioid receptor function in rodent models of opioid tolerance, analgesia and dependence is currently under examination (Leontiadis et al., 2009; Psifogeorgou et al., 2011; Georgoussi et al., 2012; Lamberts et al., 2013; Stratinaki et al., 2013). Furthermore, the reduced expression of RGS4 or 10 in the prefrontal cortex of opiate addicts suggests that these proteins may be involved in the human condition of opiate abuse (Rivero et al., 2012).
Protein interactions that influence DOPr and MOPr biosynthetic pathways
The export of DOPrs to the cell membrane appears to be a critical step in regulating DOPr function. In transfected cells, DOPrs undergo extensive post-translational sorting in the endoplasmic reticulum (ER) where up to 50% of the immature receptor may be degraded (Petaja-Repo et al., 2000; 2001). The remaining receptor forms a ternary complex with calnexin and a Ca2+ sensing ATPase to regulate receptor maturation in a Ca2+ and receptor-dependent manner (Petaja-Repo et al., 2002; Leskela et al., 2007; Tuusa et al., 2010).
In contrast to DOPrs, much less is known of proteins that influence MOPr biosynthesis, possibly a result of the constitutive release of MOPrs to the cell membrane in a comparatively unregulated manner. Some insight into this process has recently been provided by Law and colleagues who, in using a targeted proteomic approach, identified a role for ribophorin I as a chaperone for MOPrs to the cell membrane (Ge et al., 2009). Ribophorin I is one of two subunits of oligosaccharide transferase. This membrane protein complex is found in the rough ER and forms part of a quality control mechanism targeting misfolded proteins to a degradative fate. An interesting finding with respect to MOPr–DOPr interactions is that DOPrs and MOPrs may dimerize within the biosynthetic pathway (Hasbi et al., 2007; Decaillot et al., 2008), and that this is required to achieve full MOPr inhibitory coupling of voltage-gated ion channels in dorsal root ganglia neurons (Walwyn et al., 2009).
Agonist-induced receptor trafficking alters receptor function
Similar to many GPCRs, ligand-activated DOPrs and MOPrs are phosphorylated by kinases such as G-protein receptor kinase (GRK) 2, 3 or 5, to recruit β-arrestin 1 or 2 and initiate internalization. After activation by an agonist, GRK-mediated phosphorylation of the carboxy-terminal tail (Thr358, Thr361 and Ser363 residues) of DOPr is rapidly observed (Pei et al., 1995; Kramer et al., 2000; Law et al., 2000; Lowe et al., 2002; Navratilova et al., 2005; Zhang et al., 2005). This leads to the recruitment of β-arrestin 1 and 2 (Kovoor et al., 1999; Cen et al., 2001a; b; Whistler et al., 2001; Navratilova et al., 2005; Zhang et al., 2005), which in turn results in receptor desensitization and internalization of the ligand–receptor complex in clathrin-coated vesicles via a dynamin-dependent mechanism (Keith et al., 1996; Chu et al., 1997; Gaudriault et al., 1997; Ko et al., 1999; Law et al., 1999; Hasbi et al., 2000).
Removing a GPCR from the cell membrane has traditionally been equated with receptor desensitization and subsequent resensitization or degradation and down-regulation (Pippig et al., 1993; 1995). However, recent studies of MOPr function suggest that this may not always be the case. Several investigators have shown that inhibition of receptor phosphorylation, β-arrestin 2 recruitment or internalization enhances receptor resensitization (Arttamangkul et al., 2006; Dang et al., 2011; Doll et al., 2011; Quillinan et al., 2011; and reviewed by Dang and Christie, 2012; Williams et al., 2013). These findings suggest that MOPr internalization slows receptor resensitization, possibly by increasing the relative proportion of desensitized receptors on the cell membrane. An interesting interpretation of this finding is that morphine tolerance may not be equated with the relatively poor efficacy of morphine to induce receptor internalization.
Ligand-induced trafficking of endogenous and overexpressed DOPrs has also been shown to regulate receptor function. Mice expressing DOPr–eGFP at the DOPr locus were used to demonstrate that the efficacy of 4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide (SNC80), a selective DOPr agonist, to induce hyperlocomotion was reduced if more receptors were internalized (Pradhan et al., 2009). In transfected cells, Audet and colleagues used BRET to assess the inter-relationship between agonist, arrestin recruitment, internalization, recycling and signalling. They showed that the binding of peptidergic agonists such as [D-Pen2,5]enkephalin, [D-Pen2,D-Pen5]enkephalin (DPDPE) to DOPrs moved the carboxy (C)-terminal tail away from Gβγ to resulting in transient β-arrestin 2 recruitment. This led to receptor recycling and sustained analgesia. In contrast, SNC80, a non-peptidergic agonist, was found to alter the C-terminal folding to bring it closer to the amino terminal domain of Gγ2, allowing for sustained β-arrestin 2 recruitment, prolonged Gβγ association and ultimately prolonged receptor desensitization with minimal recycling (Audet et al., 2012). This results in acute analgesic tolerance to repeated SNC80 but not DPDPE (Audet et al., 2012). GPCR-associated sorting protein-1-bound DOPrs targeted for degradation are then actively transferred into lysosomes in an ubiquitin-dependent process (Whistler et al., 2002; Henry et al., 2010). Together these in vitro and in vivo data suggest that DOPrs, in contrast to MOPrs, may fit the traditional model of GPCR desensitization and trafficking, whereby internalization leads to enhanced receptor resensitization in an agonist-specific manner.
The functional effects of DOPr agonists
In the following section we will examine the ability of DOPrs to alter diverse physiological and pathological states.
Analgesia
The role of MOPr and DOPr in the control of pain has been thoroughly described (for reviews, see Gaveriaux-Ruff and Kieffer, 2011; Bodnar, 2013). Although these receptors share common roles in nociceptive pathways, at the spinal level MOPr and DOPr agonists were recently shown to inhibit distinct types of pain (Scherrer et al., 2009). Indeed, it was found that MOPr agonists specifically alleviate thermal pain while DOPr agonists inhibit mechanical pain. These findings opposed numerous studies in which the spinal MOPr agonist DAMGO was shown to efficiently alleviate both heat (Porreca et al., 1984; Malmberg and Yaksh, 1992; Nagasaka and Yaksh, 1995; Kondo et al., 2005; Scherrer et al., 2009; van Rijn et al., 2012; Normandin et al., 2013) and mechanically induced nociception (Nichols et al., 1995; Sluka et al., 2002; Kondo et al., 2005; Chen and Pan, 2006; Joseph and Levine, 2010; van Rijn et al., 2012; Normandin et al., 2013). Similarly, the activation of spinal DOPr by selective agonists was shown to equally relieve heat (Stewart and Hammond, 1994; Tseng et al., 1997; Qiu et al., 2000; Cahill et al., 2001b; 2003; Morinville et al., 2003; Gendron et al., 2007a; b; Beaudry et al., 2009; Dubois and Gendron, 2010; Normandin et al., 2013) and mechanical hyperalgesia (Miaskowski et al., 1990; 1991; Sutters et al., 1990; Holdridge and Cahill, 2007; Scherrer et al., 2009; Joseph and Levine, 2010; Otis et al., 2011; Normandin et al., 2013). More recently, using an in vivo electrophysiological approach to measure the activation of the diffuse nociceptive inhibitory controls, we demonstrated that spinal MOPr- and DOPr-selective agonists equally attenuate thermal and mechanically induced nociception (Normandin et al., 2013). In addition, the conditional deletion of either MOPr or DOPr in NaV1.8-positive primary afferent neurons respectively reduced MOPr- and DOPr-mediated peripheral analgesia (Gaveriaux-Ruff et al., 2011; Weibel et al., 2013). The latter studies not only support a similar role for MOPr and DOPr in pain control but also challenge the recent views that the distinction between pain modalities occurs at the level of primary afferents (Abrahamsen et al., 2008; Cavanaugh et al., 2009; Scherrer et al., 2009) rather than at the spinal and/or supraspinal levels (Perl, 2007).
Anxiety, stress and depression
DOPr activation can also reduce depression, possibly as a result of the ability of DOPrs to relieve stress or anxiety, as recently reviewed in Le Merrer et al. (2009) and Pradhan et al. (2011). This has been shown by a reduction in the immobility induced by the forced swim test (Jutkiewicz et al., 2003; 2005b) or of the conditioned suppression of locomotor activity following foot-shock (Saitoh et al., 2004; Nieto et al., 2005) in rodents. High levels of DOPr expression in the central nucleus of the amygdala may play an important role in this effect (Randall-Thompson et al., 2010). Based on these preclinical data, a phase II clinical trial was initiated to examine the effects of a DOPr agonist, AZD2327, on major depressive disorders. This small trial of 22 participants, 14 of which received AZD2327, failed, but some symptoms of depression were reduced in patients with co-morbid anxiety, reflective of preclinical findings in rodents (http://clinicaltrials.gov/show/NCT00759395).
Addiction
DOPr expression in different limbic and corticolimbic regions suggests that this receptor could alter euphoric states. In contrast with MOPrs, there has been little evidence that DOPr agonists result in overt drug-seeking behaviours. There is, however, evidence that DOPrs may influence drug-seeking behaviours induced by psychostimulants such as cocaine or amphetamine (Dikshtein et al., 2013; Bosse et al., 2014). In examining the persistence of cocaine seeking in self-administering rats, β-endorphin reduced cocaine reinstatement after forced abstinence by activating DOPrs in the nucleus accumbens (NAcc) (Dikshtein et al., 2013). This contrasts with the findings of Simmons and Self (2009) who showed that β-endorphin, acting on MOPrs, but not DOPrs, reinstates previously extinguished cocaine-seeking behaviours. Interestingly, these differences could have resulted from the fact that forced abstinence (Dikshtein et al., 2013) or extinction (Simmons and Self, 2009) could induce different cellular responses. In addition, DOPr activation by deltorphin II-based peptides has also been shown to enhance the locomotor sensitization to cocaine in a dose-dependent manner (Kotlinska et al., 2010). This role of DOPrs could be linked to a particular aspect of addiction-related behaviours: the cognitive control of decision making (Laurent et al., 2012; Bertran-Gonzalez et al., 2013). Epidemiological evidence of the association of a single nucleotide polymorphism in OPRD1 with cocaine addiction in some human populations (Crist et al., 2013) complements these preclinical studies in rodents. DOPrs may also play a role in the profile of morphine-induced addiction; DOPr inhibition or a lack of functional DOPs in rodents reduces the rewarding properties of morphine (Chefer and Shippenberg, 2009; Shippenberg et al., 2009; Billa et al., 2010; Le Merrer et al., 2011), possibly mediated by DOPr regulation of spatial and contextual cues (Le Merrer et al., 2012). There has also been evidence of DOPrs playing a role in the addiction profile induced by alcohol where behavioural responding to ethanol increases DOPr function in several regions. This suggests that DOPrs may play a protective role in chronic alcohol disorders and is being further explored (Margolis et al., 2008; Mitchell et al., 2012; Nielsen et al., 2012; van Rijn et al., 2012). It is tempting to suggest that the influence of DOPrs on the addiction profile of these compounds may not be a direct result of DOPr signalling within the effected cells or pathways but rather an indirect, and concurrent, anxiolytic action of DOPrs (Lutz and Kieffer, 2013; Charbogne et al., 2014).
Learning and memory
Radioligand binding and DOPr–eGFP mice show intense DOPr expression in the hippocampus (Crain et al., 1986; Erbs et al., 2012) where these receptors are found on interneurons and act presynaptically to inhibit GABA release (Rezai et al., 2012; Piskorowski and Chevaleyre, 2013). Further electrophysiological studies demonstrate that DOPrs are required to induce long-term depression of parvalbumin-expressing neurons within CA2 (Piskorowski and Chevaleyre, 2013) and inhibit the excitatory temporoammonic pathway from the entorhinal cortex to CA1 (Rezai et al., 2013). DOPrs are also critical for the induction of long-term potentiation in dentate granule cells (Xie and Lewis, 1995). At the behavioural level, mice lacking DOPrs show impaired hippocampal and striatal-based learning and motor tasks (Le Merrer et al., 2013). Another measure of cognition, the ability to make a decision based on past experience, has recently shown to be mediated by DOPr trafficking and hence function in the cholinergic interneurons of the shell of the NAcc (Laurent et al., 2012; Bertran-Gonzalez et al., 2013).
Hypoxia
The up-regulation of DOPrs during hypoxic preconditioning may induce neuronal, cardiac and retinal protection to subsequent hypoxic events (Gao et al., 2012; Husain et al., 2012; Maslov et al., 2013). The underlying mechanism remains unclear but may be mediated by increased BDNF–TrkB signalling (Tian et al., 2013), modification of micro-RNA expression (He et al., 2013; Yang et al., 2013), and altered mitochondrial and ion channel function (Fischbach et al., 2003). A similar protective effect of DOPr agonists in maintaining cellular integrity has been seen during mammalian hibernation, a state of low-energy stores and oxygen depletion. Indeed circulating opioid peptides are considered a ‘trigger of hibernation’ (Oeltgen et al., 1988) and may play an important role in cell proliferation, scar formation and wound healing in hibernating black bears (Iaizzo et al., 2012).
Immune function
DOPr expression on astroglia and in T cells may explain the reported immunomodulatory roles of DOPr ligands. DOPr forms a heterodimer with CXCR4, a co-receptor for CD4s and an important target receptor for HIV virions. These heterodimers have also been found on astrocytes and neurons where activation by either ligand silences activity of both receptors (Pello et al., 2008). DOPr expression and up-regulation has more recently been found in hepatocellular carcinoma and is associated with enhanced tumour formation (Tang et al., 2013). DOPrs are also expressed on dendritic cells and may trigger chemotaxis in vitro and dendritic cell migration in vivo (Benard et al., 2008).
Other physiological and pathological effects of DOPr signalling
Aside its role in analgesia, the expression of DOPrs in mechanoreceptors in the skin suggests that it also regulates touch. Indeed, DOPr-positive axons have been found surrounding hair follicle endings and the base of Merkel cells in mice (Bardoni et al., 2014). In vitro and in vivo studies also suggest a role for DOPrs in development. The DOPr antagonist ICI 174,864 inhibits embryogenesis (Gallego et al., 2009), and DOPr agonists favour proliferation over neuronal differentiation (Hauser et al., 2000; Persson et al., 2003). Interestingly, studies in rodents and/or non-human primates suggest that DOPr agonists may improve the clinical outlook of Parkinson's disease (Hille et al., 2001; Mabrouk et al., 2009) and of migraine (Pradhan et al., 2014).
Convulsions
In contrast to these many beneficial effects of DOPr activation, some DOPr agonists have a proconvulsant effect that could be a major drawback to any clinical use of DOPr agonists (Comer et al., 1993; Negus et al., 1994; Jutkiewicz et al., 2006). These convulsions are mediated by nitric oxide, tend to be short lived (Khavandgar et al., 2002) and are subject to tolerance (Jutkiewicz et al., 2005a). Importantly, as convulsions may be separable from other functional effects of DOPr agonism (Broom et al., 2002a; b; Jutkiewicz et al., 2005b) and are agonist and dose specific (Hudzik et al., 2011; Saitoh et al., 2011), this drawback could be overcome.
Part II. Novel aspects of DOPr function
The first section of the review discussed the localization of DOPrs, new insights into DOPr interacting and signalling partners, and an update on known functional effects of DOPr activation, suggesting that DOPrs may be a promising target for diverse pathological conditions. This sets the stage for the second part of this review in which two critical aspects of DOPr function will be described in more detail: the role of DOPrs in cells that express MOPrs and the ability of DOPrs to be functionally up-regulated by different stimuli. This next section will thus examine several contentious issues that have recently come to light regarding DOPr function.
DOPr function in MOPr-expressing cells
The activation of MOPr by chronic morphine treatments or other MOPr agonists in vivo was shown to increase the effects of DOPr agonists, that is, DOPr function (Cahill et al., 2001b; Morinville et al., 2003; Hack et al., 2005; Ma et al., 2006; Gendron et al., 2007a). In a similar way, DOPr functions are increased in inflammatory pain models (Hylden et al., 1991; Hurley and Hammond, 2000; Cahill et al., 2003; Patwardhan et al., 2005; Gendron et al., 2006; 2007a; Pettinger et al., 2013; Pradhan et al., 2013), an effect abolished in MOPr knockout mice (Gendron et al., 2007b). Indeed, under various conditions it has been shown that the expression of MOPr is essential for DOPr to be fully functional (Sora et al., 1997a; b; Loh et al., 1998; Matthes et al., 1998; Hosohata et al., 2000; Guo et al., 2003; Morinville et al., 2003; 2004a; Gendron et al., 2007b). Although the exact mechanism by which MOPr can regulate DOPr's functions remains unknown, several lines of evidence point towards direct interactions between MOPr and DOPr and between their signalling cascades.
MOPr–DOPr localization
Despite a significant level of overlap of MOPr and DOPr expression in numerous structures of the CNS and the similar roles they play in pain control, the cellular distribution of these opioid receptors is controversial and highly debated. The controversy was initiated by two different findings: questionable selectivity of the available DOPr antibodies and the cellular and subcellular distribution of the DOPr tagged with a 238 amino acid fluorescent protein, eGFP, in genetically engineered mice (Scherrer et al., 2006). Indeed, it has since been suggested that some DOPr antibodies are non-specific, labelling a protein still expressed in mice lacking DOPrs (Scherrer et al., 2009; Bardoni et al., 2014). These contentious issues have led to further studies, and most antibodies have now been shown to be specific, at least when used under proper conditions (Overland et al., 2009; Riedl et al., 2009; Xie et al., 2009; Billa et al., 2010; Wang et al., 2010; Schuster et al., 2013). More convincingly, Zhang and collaborators used three different commercially available antibodies and showed specific DOPr labelling in wild-type mouse dorsal root ganglia and spinal cords. In the same study, no DOPr labelling was observed with any of these antibodies in DOPr knockout mice (Wang et al., 2010), helping to resolve the first point of contention. With respect to the co-expression of opioid receptors, immunolabelling of MOPrs in DOPr–eGFP mice suggested that DOPr and MOPr were rarely co-expressed in the same neurons. In primary afferents of these mice, DOPr–eGFP was shown to be expressed on Aδ and Aβ fibres, while MOPr-like immunostaining was mainly present on peptidergic nociceptors (Scherrer et al., 2009). In this study, approximately 2% of nociceptive neurons were reported to co-express MOPr and DOPr. In a later study using the same DOPr–eGFP mouse line, the co-expression of MOPr and DOPr was reported at more than 5% of dorsal root ganglia neurons (Bardoni et al., 2014). Using double knockin mice expressing mCherry–MOPr and DOPr–eGFP, Massotte and colleagues recently reported that more than 30% of dorsal root ganglia neurons of all types (i.e. small, medium and large) co-express MOPrs and DOPrs (Erbs et al., 2014). The reasons for these different results from three studies that have used the same DOPr–eGFP knockin mouse line are unclear, but could result from differences in MOPr and GFP immunolabelling technique and the settings or criteria used to define labelled from non-labelled cells.
There is now considerable biochemical evidence supporting that DOPr is expressed in peptidergic primary afferents. In sensory neurons DOPr was shown to interact with the substance P domain of protachykinin in large dense core vesicles (LDCVs) (Guan et al., 2005). Although this phenomenon is not always required (Dubois and Gendron, 2010), the interaction with protachykinin was shown to participate in the sorting of DOPr into the LDCVs. This promotes DOPr insertion into the plasma membrane of peptidergic primary afferents and translates to an increased analgesic potency of DOPr agonists (Guan et al., 2005). Single-cell RT-PCR also revealed the presence of both MOPr and DOPr mRNAs in substance P containing dorsal root ganglion cells (Wang et al., 2010). Functional evidence for the expression of DOPr in these neurons also exists. In small peptidergic neurons, DOPr was indeed shown to be involved in the inhibition of glutamate, substance P and CGRP release (Ueda et al., 1995; Zachariou and Goldstein, 1996; Beaudry et al., 2009; Overland et al., 2009; Kouchek et al., 2013; Normandin et al., 2013). DOPr was also found to synergize with α2A-adrenergic receptors in peptidergic primary afferents via a PKC-dependent mechanism (Overland et al., 2009; Riedl et al., 2009; Schuster et al., 2013). Altogether, these in vivo observations support the conclusions made with DOPr antibodies and therefore endorse the presence of DOPrs on substance P-containing afferent neurons. In a more recent study, Scherrer and collaborators found a higher level of MOPr and DOPr–eGFP co-expression in DOPr–eGFP mice than previously reported and with both receptors being expressed in a population of CGRP-expressing myelinated nociceptors, but not in substance P-containing nociceptors (Bardoni et al., 2014).
Putative MOPr–DOPr heterodimers
The possibility that a MOPr–DOPr heteromer may exist in vivo opens a new era of research and represents an exciting opportunity to develop novel therapeutics with unique pharmacology. For instance, computational studies have described a potential interaction between TM1MOPr and TM4DOPr (Liu et al., 2009). In vitro, overexpression of MOPr and DOPr in the same cells revealed that these receptors can indeed physically interact (George et al., 2000; Gomes et al., 2000; 2004; Hasbi et al., 2007; Decaillot et al., 2008; Gupta et al., 2010; Kabli et al., 2010; Golebiewska et al., 2011). Indeed in heterologous systems, the use of BRET techniques demonstrated that MOPr and DOPr form homo- and hetero-oligomers (Wang et al., 2005; Hasbi et al., 2007). Using this technique, George and collaborators further observed that the heteromer constitutively interact in the ER before being targeted to the plasma membrane as a preassembled signalling complex (Hasbi et al., 2007). This however contrasts with others who suggested that the MOPr–DOPr oligomer associates at the cell surface (Law et al., 2005). In vivo, endogenous MOPr and DOPr were successfully co-immunoprecipitated from mouse spinal cord extracts, suggesting that they can physically associate and interact (Gomes et al., 2004; Xie et al., 2009; He et al., 2011). In the double knockin mice, Massotte and collaborators were also able to co-immunoprecipitate DOPr–eGFP with mCherry–MOPr from the hippocampus (Erbs et al., 2014). However, only few studies thus far revealed direct evidence for the presence of endogenous MOPr–DOPr heteromers in intact tissue. One such example comes from Devi's group who generated an antibody directed against the MOPr–DOPr heterodimer and showed that it is present in various brain areas and in dorsal root ganglion cells (Gupta et al., 2010). In support of such an interaction, the DOPr agonist SNC80 was recently shown to produce antinociception by activating the MOPr–DOPr heteromer (Metcalf et al., 2012). More recently, the high-throughput screening of a small-molecule library gave rise to the identification of the first MOPr–DOPr heteromer-selective biased agonist (Gomes et al., 2013). The activity of the compound CYM51010 was indeed found to be specific to cells expressing both MOPr and DOPr as CYM51010-induced β-arrestin recruitment and 35S-GTPγS binding were only present in cells overexpressing both receptors and were blocked by the MOPr–DOPr heteromer antibody (Gomes et al., 2013).
Measures of MOPr–DOPr function
Because no DOPr splice variants have been identified so far, it has been suggested that the interaction between DOPr and MOPr could be responsible for the two postulated pharmacologically distinct DOPr subtypes, DOPr1 and DOPr2 (van Rijn et al., 2010; 2013). In addition to their ability to physically interact, it has been shown that co-expression of κ opioid receptor (KOPr) or MOPr with DOPr leads to changes in DOPr pharmacology. Indeed, the interaction between KOPr and DOPr results in a new receptor that exhibits distinct ligand binding and functional properties (Jordan and Devi, 1999). In cells expressing both MOPr and DOPr, DPDPE displays a reduced affinity as compared with cells expressing DOPr alone (George et al., 2000). MOPr and DOPr co-expression was also shown to modify the G-protein coupling of the receptors (George et al., 2000; Hasbi et al., 2007). Indeed, although DOPrs and MOPrs recruit the G-protein subunit Gαi when expressed separately, dimerization of MOPr with DOPr is associated with a shift in G-protein coupling from the Gαi to the Gαz subunit. In addition to changes in G-protein coupling, heteromerization of MOPr and DOPr is associated with changes in the kinetics of ERK activation (Rozenfeld and Devi, 2007). In fact, when DOPr is expressed alone it activates ERK in a rapid and transient manner whereas MOPr–DOPr heteromer activation leads to a sustained phosphorylation of ERK. Interestingly, DOPr's trafficking is also modified in cells expressing MOPrs. Indeed, DOPr is co-internalized with MOPr following activation with a MOPr agonist (He et al., 2011; Milan-Lobo and Whistler, 2011). Similarly, MOPr is co-internalized with DOPr and targeted to lysosomal degradation after treatment with a DOPr agonist (He et al., 2011). The latter observations therefore suggest that DOPr can also alter the functions and the trafficking of MOPr. This was further evidenced by the fact that DOPr activation was shown to increase the antinociceptive effects of spinal MOPr agonists (He and Lee, 1998) and that the expression of DOPr contributes to the full expression of MOPr's inhibitory effects on voltage-dependent Ca2+ channels in nociceptive neurons (Walwyn et al., 2009). A direct role of MOPr–DOPr heterodimerization in this effect was supported by the fact that the expression of a dimerization-deficient DOPr mutant in DOPr knockout neurons failed to fully restore the inhibitory coupling of MOPr (Walwyn et al., 2009).
In vivo, the sustained activation of MOPr was shown to increase the level of MOPr–DOPr heteromers in various brain areas and in nociceptive neurons (Gupta et al., 2010). When the formation of the MOPr–DOPr heteromers is prevented, the cell surface expression of DOPr was shown to be reduced and the antinociceptive effects of DOPr agonists decreased (Xie et al., 2009). Disruption of MOPr–DOPr heteromers in the accumbens was also shown to abolish the antidepressant- and anxiolytic-like actions of DOPr agonists (Kabli et al., 2013). Similarly, the heterodimerization of MOPr with DOPr was shown to have important consequences on MOPr functions. Indeed, in acute pain models the absence of DOPr attenuates the development of morphine-induced antinociceptive tolerance (Kest et al., 1996; Zhu et al., 1999; Chefer and Shippenberg, 2009; Walwyn et al., 2009). The disruption of the MOPr–DOPr heteromer was also shown to increase morphine analgesia and decrease tolerance (Xie et al., 2009; He et al., 2011). Taken together, these results provide evidence for MOPr–DOPr heteromers as a distinct functional target for opioid ligands and represent a mechanism to regulate the functions of DOPr.
Functional up-regulation of DOPrs
A brief history
The ability of DOPrs to undergo a functional up-regulation, first described in the 1980s, was attributed to an increase in receptor function (Young et al., 1982; 1983; Barg et al., 1984) that could be influenced by chronic morphine and ethanol (Charness et al., 1986; Danks et al., 1988; Rothman et al., 1989). Simantov and colleagues then found that the DOPr ligand, DPDPE, but not other ligands, increased the levels of Gα subunits in cultured cells (Vogel et al., 1990), and Inturissi and colleagues found that an increase in DOPr sensitivity could not be explained by increased receptor expression (Jenab and Inturrisi, 1997). Studies from the late 1990s and 2000s have shown that even in different systems, cell types and under different pathological conditions such as chronic pain, cell division, hypoxia and scar formation, DOPr function could be enhanced (Chen et al., 1997; Dickenson, 1997; Thorlin et al., 1997; 1999; Cahill et al., 2003; Morinville et al., 2003; Ma et al., 2005; Cheng et al., 2008). The development of mutant mice lacking opioid receptors or ligands demonstrated how opioid receptor function can also be regulated by ligand availability (Brady et al., 1999). The underlying mechanisms of DOPr up-regulation were then suggested to be a result of enhanced DOPr trafficking to the cell membrane (Cahill et al., 2001b), making DOPr a promising analgesic target (Cahill et al., 2007). During the past decade, up-regulation of endogenous DOPr has been shown in different models of pain (Cahill et al., 2003; Morinville et al., 2004b; Pradhan et al., 2013), alcohol (van Rijn et al., 2012), chronic morphine (Chieng and Christie, 2009; Morgan et al., 2009), hypoxia (Peng et al., 2009) and in the progression of cancer (Otis et al., 2011; Tang et al., 2013).
Cell surface receptor levels
Enhanced DOPr function is commonly defined by enhanced efficacy of a bound agonist in either a cellular or a behavioural context (Chieng and Christie, 2009; Pradhan et al., 2013). A number of studies have shown that this increase in signalling results from an increase in the number of receptors on the cell membrane (Cahill et al., 2001b; Scherrer et al., 2006; Walwyn et al., 2009). Conversely, removing receptors through internalization or degradation decreases the response to a subsequent agonist challenge (Scherrer et al., 2006; Pradhan et al., 2009). Together, this suggests that DOPr signalling, and hence functionality, is sensitive to the number of receptors on the cell membrane. This relationship between cell surface receptor levels and functionality could be influenced by the DOPr biosynthetic pathway (Petaja-Repo et al., 2000), which regulates the number of receptors released to the cell membrane (Dong et al., 2007; Achour et al., 2008). Integral to this concept is that DOPrs are found in an intracellular location close to the cell membrane and can be readily and rapidly released to the cell membrane. As previously discussed, there have been a number of reports of endogenous DOPrs found within the cell either in association with the Golgi, with pre-synaptic vesicles or in the sub-plasmalemmal space. Furthermore, few receptors have been shown to be on the cell membrane (Arvidsson et al., 1995; Cheng et al., 1995; 1997; Zhang et al., 1998; Cahill et al., 2001a; b; Bao et al., 2003; Lucido et al., 2005; Fristad et al., 2006; Gendron et al., 2006; Wang et al., 2008b). Many of these reports examined endogenous DOPr localization in paraformaldehyde-fixed tissue using an anti-DOPr antibody and electron microscopy to visualize the gold particles. Conversely, when imaged with an alternative technique, that is, by imaging dorsal root ganglia neurons from DOPr–eGFP knockin mice, eGFP-labelled receptors were primarily found on the cell membrane (Scherrer et al., 2006; Bardoni et al., 2014). This could be a result of the eGFP tag. Indeed Zhang and colleagues observed that both N- and C-terminal eGFP-tagged DOPrs are localized on the cell surface whereas DOPrs with smaller tags (e.g. Myc and haemagglutinin) show a vesicular localization (Wang et al., 2008a). Although DOPrs were overexpressed in this study, the different localization of receptors with smaller versus larger tags suggests that the size of the tag may alter DOPr localization. When compared with wild-type mice, the eGFP tag also increased DOPr mRNA and binding levels, DOPr agonist-induced G-protein activation and Ca2+ channel inhibition (Scherrer et al., 2009; Bardoni et al., 2014). Together these data suggest that DOPr trafficking and function may be altered by a C-terminal eGFP fusion protein. Interestingly, DOPr would not be the first GPCR to show altered trafficking and function when fused to eGFP (McLean and Milligan, 2000; Madziva and Edwardson, 2001; McDonald et al., 2007; Roy et al., 2007).
Despite the controversy described above regarding the specificity of antibodies, photoaffinity labelling of DOPrs in the rat striatum with [125I]-azido-DTLET, performed before the widespread use of antibodies, had revealed that this receptor was principally expressed inside the cells (Pasquini et al., 1992). Predominant membrane expression of DOPr has only been observed in the genetically engineered mice expressing DOPr–eGFP using standard confocal or light microscopy. In addition to the effect of the C-terminal tag on receptor trafficking, our ability to distinguish membrane receptors from those present near the plasma membrane may be limited by the resolution of standard confocal or light microscopy. Such microscopy is limited by the diffraction of light, a concept first defined by the German physicist Ernst Karl Abbe in the 1800s, and known as the Abbe diffraction limit. For the GFPe emission wavelength of 488 nm, this limit would be around ∼175–250 nm when a high numerical (NA) objective lens (NA = 1.4) is used. Thus, confocal or light microscopy does not have the resolving power to differentiate DOPrs localized on the cell membrane from those that are 200 nm beneath the cell membrane. As the antibodies used in electron microscopic studies have now been shown to specifically label DOPrs (Xie et al., 2009; Billa et al., 2010; Wang et al., 2010), and the membrane density of DOPr-like immunostaining and function of DOPr can be enhanced under different conditions (as described above), it is likely that the endogenous receptor has a predominant intracellular localization under normal conditions.
Physiological and pathological evidence of DOPr up-regulation
In light of the considerable doubt in the field whether DOPrs are exported to the cell membrane to enhance DOPr responding under either normal or pathological conditions, studies using a functional readout of DOPr signalling have surfaced. A recent example is from a study by Balleine and colleagues who have shown that pavlovian conditioning and pavlovian instrumental transfer, as measured by food reward, induce a translocation of DOPrs, assessed in DOPr–eGFP mice, to the cell membrane of striatal cholinergic interneurons (Bertran-Gonzalez et al., 2013). This could explain the deficit in pavlovian transfer in mice lacking DOPrs (Laurent et al., 2012). Interestingly in these neurons, DOPr–eGFP is described as having an intracellular location under normal conditions. This is in marked contrast with the description of DOPr–eGFPs in dorsal root ganglia neurons (Scherrer et al., 2009; Bardoni et al., 2014).
Another example is the analgesic effect of DOPrs in animal models of chronic pain. The ability of DOPr agonists to relieve acute mechanical pain is unremarkable (Pradhan et al., 2013). However, chronic pain induced by inflammatory injury or neuropathic insult increases the analgesic efficacy of DOPr agonists (Kabli and Cahill, 2007; Pradhan et al., 2013), suggesting that chronic pain up-regulates DOPrs. Factors associated with this pathological condition such as bradykinin and arachidonic acid (Patwardhan et al., 2005) may ‘prime’ DOPrs and increase receptor function (Rowan et al., 2009). Other pathological conditions such as chronic alcohol exposure (van Rijn et al., 2012) and hypoxia (Gao et al., 2012) have also been shown to enhance DOPr responding.
The role of β-arrestin 1 and the actin cytoskeleton in regulating DOPr trafficking and function
Dynamic remodelling of the cytoskeleton, particularly of the actin filaments, provides the network along which intracellular proteins may be trafficked as needed. This mechanism allows the Golgi apparatus to sort and traffic newly synthesized proteins to the cell membrane (Salvarezza et al., 2009; Lowe, 2011). Both the actin severing protein, cofilin, and the upstream kinase, Lim domain kinase (LIMK), control the release of specific proteins from the Golgi to the cell membrane, demonstrating how dynamic cytoskeletal remodelling controls protein export (Heimann et al., 1999; Egea et al., 2006; Salvarezza et al., 2009). A similar dynamic regulation of actin turnover to alter the leading and trailing edges of lymphocytes and allow directed cell migration outlines a role for β-arrestin 1 or 2 in cytoskeletal remodelling. This is likely a result of these arrestin subunits binding with cofilin, the inactivating phosphatase chronophin, and the activating kinase LIMK, resulting in a spatiotemporal regulation of actin turnover by these scaffolding proteins (DeFea, 2007; Zoudilova et al., 2007; 2010; Xiao et al., 2010). We have recently shown a similar role of β-arrestin 1, but not 2, in regulating LIMK and cofilin to affect actin turnover and regulate DOPr function in dorsal root ganglion neurons (Mittal et al., 2013).
The described cellular and behavioural studies (Mittal et al., 2013) allowed us to propose the following pathway: under normal or wild-type conditions, agonist binding to DOPrs activates the RhoA-ROCK (RhoA-associated coiled-coil containing protein kinase) LIMK pathway resulting in an enhanced but local activation of cofilin. This leads to a controlled export of DOPr-containing cargo vesicles to the cell membrane and allows a limited response to a DOPr agonist such as SNC80. This pathway can be enhanced by removing β-arrestin 1. In this scenario, SNC80 activates LIMK through the RhoA-ROCK pathway but cofilin dephosphorylation and activation does not occur. This leaves stable actin ‘tracks’ in place resulting in enhanced export of DOPrs from the Golgi to the plasma membrane, and enhanced DOPr function. This pathway can be blocked by inhibiting ROCK, the kinase responsible for phosphorylating LIMK and inactivating cofilin. In this scenario, agonist-induced activation of the pathway does not occur and additional receptors are not released to the cell membrane (see the schematic model in Figure 1).
Figure 1.
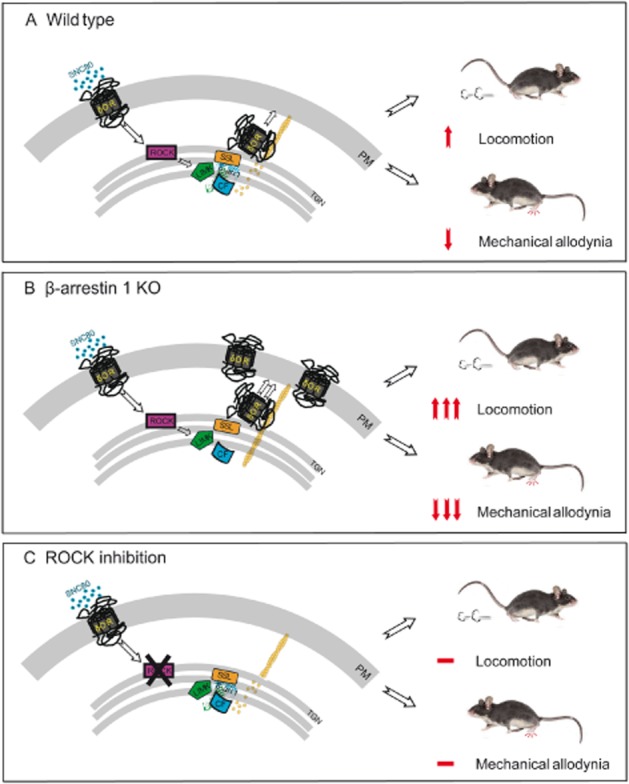
A schematic model of ROCK-LIMK–β-arrestin 1 dependent regulation of DOPr function. (A) The DOPr agonist, SNC80, binds with DOPrs to activate RhoA-ROCK. As β-arrestin 1 is associated with LIMK and one of the phosphatases, possibly slingshot (SSL), within the trans-Golgi network, cofilin is activated to increase actin filament severing and turnover. This allows a regulated release of DOPrs to the cell membrane to influence the functional effect of the DOPr agonist, SNC80. (B) In the absence of β-arrestin 1, LIMK phosphorylates and inactivates cofilin. This leaves stable actin ‘tracks’ in place to enhance DOPr release to the plasma membrane and increases SNC80-induced locomotion and the pain-relieving effects of SNC80 following a mechanical stimulus (C). Preventing ROCK phosphorylation of LIMK prevents DOPr activation of the pathway and agonist-induced DOPr release to the cell membrane blocking the locomotor and analgesic effects of SNC80 (modified from Mittal et al., 2013).
Such regulated release of DOPrs in an agonist-dependent manner may be required to obtain an initial functional response to a DOPr agonist. Thereafter, the properties of DOPr ligands, receptor phosphorylation, β-arrestin 1 or 2 recruitment, the roles of other regulatory proteins such as PKC and bradykinin, and subsequent trafficking, internalization and resensitization may further regulate DOPr function.
Physiological relevance of the ROCK-LIMK–β-arrestin 1 pathway
We found that the behavioural effects of the DOPr agonist, SNC80, can be influenced by genetic deletion or pharmacological inhibition of different proteins within this pathway (Figure 1). In mice lacking β-arrestin 1, the hyperlocomotor and analgesic effects of SNC80 are enhanced; this can be blocked by the δ antagonist, naltrindole. Pharmacological inhibition of ROCK reduced both the hyperlocomotor and analgesic effects of SNC80. Furthermore, the enhanced efficacy of SNC80 in the complete Freund's adjuvant (CFA) model of chronic inflammatory pain (Pradhan et al., 2013) was inhibited by ROCK (Mittal et al., 2013).
In these assays the DOPr agonists, SNC80 and DPDPE, were found to be the principle activators of this pathway. But it is also possible that other receptors or molecules may either initiate activation or are important intermediates. For example, bradykinin, arachidonic acid or perhaps DOPr auto-antibodies, but not endogenous opioids, may activate this pathway in the CFA model of chronic pain (Patwardhan et al., 2005; Gendron et al., 2007b; Ranganathan et al., 2009; Rowan et al., 2009; Pettinger et al., 2013). Other receptors and kinases such as PAR2 or PKC could also be involved in up-regulating DOPrs (Patwardhan et al., 2005; Norcini et al., 2009; Rowan et al., 2009; Hagenacker et al., 2010).
Summary
Undoubtedly, MOPrs and DOPrs can interact to form heteromers in a heterologous system where the receptors are often overexpressed. Although of a particular interest for the regulation of these receptors and their downstream signalling cascades, the MOPr–DOPr dimer is only of clinical interest if demonstrated in vivo. We have recently witnessed the first in vivo evidence of the existence of MOPr–DOPr heteromer. Although much still needs to be carried out to describe the role of this receptor complex, we now have insights that this complex may play distinct physiological roles in the regulation of pain and depression. Concerns of DOPr antibody specificity have also cast some doubt whether DOPr functional up-regulation results from enhanced DOPr trafficking to the cell membrane. In assessing recent findings based on cellular and behavioural measures of DOPr function, it appears that DOPrs are indeed trafficked to the cell membrane in a regulated manner and that this could explain how DOPr signalling is enhanced under different physiological and pathological conditions.
Conclusion
The ability of MOPr and DOPr agonists or various pathological conditions to enhance DOPr function suggests that this receptor may represent a promising clinical target to treat different pathologies. As current findings suggest that DOPr agonists induce fewer side effects and have a reduced potential for abuse than MOPrs, DOPr agonists may indeed provide an alternate target for the treatment of chronic pain and other pathologies. Furthermore, the exciting possibility that DOPrs and MOPrs could form heteromers in vivo with distinct pharmacology and physiological effects represents an opportunity to develop novel classes of therapeutics. The discovery of the pathway by which DOPr function may be influenced by receptor trafficking to the cell membrane provides a new approach to manipulate receptor function. Together these recent advances in our understanding of DOPr function clarify current issues and provide new insight into possible clinical use of these opioid receptors.
Acknowledgments
This work was supported in part by the NIH grants DA05010 and DA30866 and by the Hatos Foundation (W. W. and N. M.) and by the Gates Millennium Scholars program (N. M.). It was also supported in part by the Canadian Institute of Health Research (CIHR) grants MOP84538 and MOP123399 (L. G.). L. G. holds a Junior 2 Salary support from the Fonds de Recherche Québec – Santé.
Glossary
Abbreviations1
- DOPr
δ opioid receptor
- DPDPE
[D-Pen2,5]enkephalin, [D-Pen2,D-Pen5]enkephalin
- KOPr
κ opioid receptor
- LIMK
Lim domain kinase
- MOPr
μ opioid receptor
- ROCK
Rho-associated coiled-coil containing protein kinase
- SNC80
4-[(R)-[(2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl](3-methoxyphenyl)methyl]-N,N-diethylbenzamide
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321:702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Achour L, Labbe-Jullie C, Scott MG, Marullo S. An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci. 2008;29:528–535. doi: 10.1016/j.tips.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology. 2011;115:1363–1381. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G-Protein Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Torrecilla M, Kobayashi K, Okano H, Williams JT. Separation of mu-opioid receptor desensitization and internalization: endogenous receptors in primary neuronal cultures. J Neurosci. 2006;26:4118–4125. doi: 10.1523/JNEUROSCI.0303-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, et al. delta-Opioid receptor immunoreactivity: distribution in brainstem and spinal cord, and relationship to biogenic amines and enkephalin. J Neurosci. 1995;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet N, Charfi I, Mnie-Filali O, Amraei M, Chabot-Dore AJ, Millecamps M, et al. Differential association of receptor-Gβγ complexes with β-arrestin2 determines recycling bias and potential for tolerance of δ opioid receptor agonists. J Neurosci. 2012;32:4827–4840. doi: 10.1523/JNEUROSCI.3734-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, et al. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37:121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Tawfik VL, Wang D, François A, Solorzano C, Shuster SA, et al. Delta opioid receptors presynaptically regulate cutaneous mechanosensory neuron input to the spinal cord dorsal horn. Neuron. 2014;81:1312–1327. doi: 10.1016/j.neuron.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg J, Levy R, Simantov R. Up-regulation of opiate receptors by opiate antagonists in neuroblastoma-glioma cell culture: the possibility of interaction with guanosine triphosphate-binding proteins. Neurosci Lett. 1984;50:133–137. doi: 10.1016/0304-3940(84)90475-0. [DOI] [PubMed] [Google Scholar]
- Beaudry H, Proteau-Gagne A, Li S, Dory Y, Chavkin C, Gendron L. Differential noxious and motor tolerance of chronic delta opioid receptor agonists in rodents. Neuroscience. 2009;161:381–391. doi: 10.1016/j.neuroscience.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard A, Boue J, Chapey E, Jaume M, Gomes B, Dietrich G. Delta opioid receptors mediate chemotaxis in bone marrow-derived dendritic cells. J Neuroimmunol. 2008;197:21–28. doi: 10.1016/j.jneuroim.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Laurent V, Chieng BC, Christie MJ, Balleine BW. Learning-related translocation of delta-opioid receptors on ventral striatal cholinergic interneurons mediates choice between goal-directed actions. J Neurosci. 2013;33:16060–16071. doi: 10.1523/JNEUROSCI.1927-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billa SK, Xia Y, Moron JA. Disruption of morphine-conditioned place preference by a delta2-opioid receptor antagonist: study of mu-opioid and delta-opioid receptor expression at the synapse. Eur J Neurosci. 2010;32:625–631. doi: 10.1111/j.1460-9568.2010.07314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ. Endogenous opiates and behavior: 2012. Peptides. 2013;50C:55–95. doi: 10.1016/j.peptides.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Bosse KE, Jutkiewicz EM, Schultz-Kuszak KN, Mabrouk OS, Kennedy RT, Gnegy ME, et al. Synergistic activity between the delta-opioid agonist SNC80 and amphetamine occurs via a glutamatergic NMDA-receptor dependent mechanism. Neuropharmacology. 2014;77:19–27. doi: 10.1016/j.neuropharm.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady LS, Herkenham M, Rothman RB, Partilla JS, Konig M, Zimmer AM, et al. Region-specific up-regulation of opioid receptor binding in enkephalin knockout mice. Brain Res Mol Brain Res. 1999;68:193–197. doi: 10.1016/s0169-328x(99)00090-x. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague-Dawley rats. Psychopharmacology (Berl) 2002a;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002b;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell D, et al. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001a;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001b;21:7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, et al. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cen B, Xiong Y, Ma L, Pei G. Direct and differential interaction of beta-arrestins with the intracellular domains of different opioid receptors. Mol Pharmacol. 2001a;59:758–764. doi: 10.1124/mol.59.4.758. [DOI] [PubMed] [Google Scholar]
- Cen B, Yu Q, Guo J, Wu Y, Ling K, Cheng Z, et al. Direct binding of beta-arrestins to two distinct intracellular domains of the delta opioid receptor. J Neurochem. 2001b;76:1887–1894. doi: 10.1046/j.1471-4159.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: opioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology. 2014;76:204–217. doi: 10.1016/j.neuropharm.2013.08.028. (Pt B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charness ME, Querimit LA, Diamond I. Ethanol increases the expression of functional delta-opioid receptors in neuroblastoma x glioma NG108-15 hybrid cells. J Biol Chem. 1986;261:3164–3169. [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Dymshitz J, Vasko MR. Regulation of opioid receptors in rat sensory neurons in culture. Mol Pharmacol. 1997;51:666–673. doi: 10.1124/mol.51.4.666. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol. 2006;95:3086–3096. doi: 10.1152/jn.01343.2005. [DOI] [PubMed] [Google Scholar]
- Cheng B, Liu HW, Fu XB, Sheng ZY, Li JF. Coexistence and upregulation of three types of opioid receptors, mu, delta and kappa, in human hypertrophic scars. Br J Dermatol. 2008;158:713–720. doi: 10.1111/j.1365-2133.2008.08449.x. [DOI] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, et al. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- Chieng B, Christie MJ. Chronic morphine treatment induces functional delta-opioid receptors in amygdala neurons that project to periaqueductal grey. Neuropharmacology. 2009;57:430–437. doi: 10.1016/j.neuropharm.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Chu P, Murray S, Lissin D, von Zastrow M. Delta and kappa opioid receptors are differentially regulated by dynamin-dependent endocytosis when activated by the same alkaloid agonist. J Biol Chem. 1997;272:27124–27130. doi: 10.1074/jbc.272.43.27124. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, De Costa BR, et al. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;267:888–895. [PubMed] [Google Scholar]
- Cox BM, Christie MJ, Devi L, Toll L, Traynor JR. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br J Pharmacol. 2015;172:317–323. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain BJ, Chang KJ, McNamara JO. Quantitative autoradiographic analysis of mu and delta opioid binding sites in the rat hippocampal formation. J Comp Neurol. 1986;246:170–180. doi: 10.1002/cne.902460203. [DOI] [PubMed] [Google Scholar]
- Crist RC, Ambrose-Lanci LM, Vaswani M, Clarke TK, Zeng A, Yuan C, et al. Case-control association analysis of polymorphisms in the delta-opioid receptor, OPRD1, with cocaine and opioid addicted populations. Drug Alcohol Depend. 2013;127:122–128. doi: 10.1016/j.drugalcdep.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2012;165:1704–1716. doi: 10.1111/j.1476-5381.2011.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Chieng B, Azriel Y, Christie MJ. Cellular morphine tolerance produced by betaarrestin-2-dependent impairment of mu-opioid receptor resensitization. J Neurosci. 2011;31:7122–7130. doi: 10.1523/JNEUROSCI.5999-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks JA, Tortella FC, Long JB, Bykov V, Jacobson AE, Rice KC, et al. Chronic administration of morphine and naltrexone up-regulate[3H][D-Ala2,D-leu5]enkephalin binding sites by different mechanisms. Neuropharmacology. 1988;27:965–974. doi: 10.1016/0028-3908(88)90125-6. [DOI] [PubMed] [Google Scholar]
- Decaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U S A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- Dickenson AH. Plasticity: implications for opioid and other pharmacological interventions in specific pain states. Behav Brain Sci. 1997;20:392–403. doi: 10.1017/s0140525x97241488. discussion 435–513. [DOI] [PubMed] [Google Scholar]
- Dikshtein Y, Barnea R, Kronfeld N, Lax E, Roth-Deri I, Friedman A, et al. Beta-endorphin via the delta opioid receptor is a major factor in the incubation of cocaine craving. Neuropsychopharmacology. 2013;38:2508–2514. doi: 10.1038/npp.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Poll F, Koch T, Hollt V, Schulz S. Agonist-selective patterns of micro-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol. 2011;164:298–307. doi: 10.1111/j.1476-5381.2011.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta. 2007;1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois D, Gendron L. Delta opioid receptor-mediated analgesia is not altered in preprotachykinin A knockout mice. Eur J Neurosci. 2010;32:1921–1929. doi: 10.1111/j.1460-9568.2010.07466.x. [DOI] [PubMed] [Google Scholar]
- Egea G, Lazaro-Dieguez F, Vilella M. Actin dynamics at the Golgi complex in mammalian cells. Curr Opin Cell Biol. 2006;18:168–178. doi: 10.1016/j.ceb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Elde R, Arvidsson U, Riedl M, Vulchanova L, Lee JH, Dado R, et al. Distribution of neuropeptide receptors. New views of peptidergic neurotransmission made possible by antibodies to opioid receptors. Ann N Y Acad Sci. 1995;757:390–404. doi: 10.1111/j.1749-6632.1995.tb17497.x. [DOI] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Kessler P, Hentsch D, Vonesch JL, et al. Distribution of delta opioid receptor-expressing neurons in the mouse hippocampus. Neuroscience. 2012;221:203–213. doi: 10.1016/j.neuroscience.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbs E, Faget L, Scherrer G, Matifas A, Filliol D, Vonesch JL, et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0717-9. [E-pub ahead of print] doi: 10.1007/500429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach PS, Barrett TD, Reed NJ, Lucchesi BR. SNC-80-induced preconditioning: selective activation of the mitochondrial adenosine triphosphate-gated potassium channel. J Cardiovasc Pharmacol. 2003;41:744–750. doi: 10.1097/00005344-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Fourla DD, Papakonstantinou MP, Vrana SM, Georgoussi Z. Selective interactions of spinophilin with the C-terminal domains of the delta- and mu-opioid receptors and G proteins differentially modulate opioid receptor signaling. Cell Signal. 2012;24:2315–2328. doi: 10.1016/j.cellsig.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Fristad I, Berggreen E, Haug SR. Delta (delta) opioid receptors in small and medium-sized trigeminal neurons supporting the dental pulp of rats. Arch Oral Biol. 2006;51:273–281. doi: 10.1016/j.archoralbio.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Gallego MJ, Porayette P, Kaltcheva MM, Meethal SV, Atwood CS. Opioid and progesterone signaling is obligatory for early human embryogenesis. Stem Cells Dev. 2009;18:737–740. doi: 10.1089/scd.2008.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao CJ, Niu L, Ren PC, Wang W, Zhu C, Li YQ, et al. Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience. 2012;202:352–362. doi: 10.1016/j.neuroscience.2011.11.060. [DOI] [PubMed] [Google Scholar]
- Gaudriault G, Nouel D, Dal Farra C, Beaudet A, Vincent JP. Receptor-induced internalization of selective peptidic mu and delta opioid ligands. J Biol Chem. 1997;272:2880–2888. doi: 10.1074/jbc.272.5.2880. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Delta opioid receptor analgesia: recent contributions from pharmacology and molecular approaches. Behav Pharmacol. 2011;22:405–414. doi: 10.1097/FBP.0b013e32834a1f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Nozaki C, Nadal X, Hever XC, Weibel R, Matifas A, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011;152:1238–1248. doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Ge X, Loh HH, Law PY. mu-Opioid receptor cell surface expression is regulated by its direct interaction with Ribophorin I. Mol Pharmacol. 2009;75:1307–1316. doi: 10.1124/mol.108.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, et al. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O'Donnell D, Vincent JP, et al. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007a;144:263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Pintar JE, Chavkin C. Essential role of mu opioid receptor in the regulation of delta opioid receptor-mediated antihyperalgesia. Neuroscience. 2007b;150:807–817. doi: 10.1016/j.neuroscience.2007.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georganta EM, Agalou A, Georgoussi Z. Multi-component signaling complexes of the delta-opioid receptor with STAT5B and G proteins. Neuropharmacology. 2010;59:139–148. doi: 10.1016/j.neuropharm.2010.04.010. [DOI] [PubMed] [Google Scholar]
- George SR, Fan T, Xie Z, Tse R, Tam V, Varghese G, et al. Oligomerization of mu- and delta-opioid receptors. Generation of novel functional properties. J Biol Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- Georgoussi Z, Georganta EM, Milligan G. The other side of opioid receptor signalling: regulation by protein-protein interaction. Curr Drug Targets. 2012;13:80–102. doi: 10.2174/138945012798868470. [DOI] [PubMed] [Google Scholar]
- Golebiewska U, Johnston JM, Devi L, Filizola M, Scarlata S. Differential response to morphine of the oligomeric state of mu-opioid in the presence of delta-opioid receptors. Biochemistry. 2011;50:2829–2837. doi: 10.1021/bi101701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Fujita W, Gupta A, Saldanha AS, Negri A, Pinello CE, et al. Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci U S A. 2013;110:12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Guo XH, Fairbanks CA, Stone LS, Loh HH. DPDPE-UK14,304 synergy is retained in mu opioid receptor knockout mice. Pain. 2003;104:209–217. doi: 10.1016/s0304-3959(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenacker T, Hillebrand I, Wissmann A, Busselberg D, Schafers M. Anti-allodynic effect of the flavonoid myricetin in a rat model of neuropathic pain: involvement of p38 and protein kinase C mediated modulation of Ca(2) + channels. Eur J Pain. 2010;14:992–998. doi: 10.1016/j.ejpain.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Hamel E, Beaudet A. Electron microscopic autoradiographic localization of opioid receptors in rat neostriatum. Nature. 1984;312:155–157. doi: 10.1038/312155a0. [DOI] [PubMed] [Google Scholar]
- Hasbi A, Allouche S, Sichel F, Stanasila L, Massotte D, Landemore G, et al. Internalization and recycling of delta-opioid receptor are dependent on a phosphorylation-dephosphorylation mechanism. J Pharmacol Exp Ther. 2000;293:237–247. [PubMed] [Google Scholar]
- Hasbi A, Nguyen T, Fan T, Cheng R, Rashid A, Alijaniaram M, et al. Trafficking of preassembled opioid mu-delta heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46:12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W., 3rd Opioids intrinsically inhibit the genesis of mouse cerebellar granule neuron precursors in vitro: differential impact of mu and delta receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000;12:1281–1293. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–1186. [PubMed] [Google Scholar]
- He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- He X, Yang Y, Zhi F, Moore ML, Kang X, Chao D, et al. delta-Opioid receptor activation modified microRNA expression in the rat kidney under prolonged hypoxia. PLoS ONE. 2013;8:e61080. doi: 10.1371/journal.pone.0061080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann K, Percival JM, Weinberger R, Gunning P, Stow JL. Specific isoforms of actin-binding proteins on distinct populations of Golgi-derived vesicles. J Biol Chem. 1999;274:10743–10750. doi: 10.1074/jbc.274.16.10743. [DOI] [PubMed] [Google Scholar]
- Henry AG, White IJ, Marsh M, von Zastrow M, Hislop JN. The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic. 2010;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille CJ, Fox SH, Maneuf YP, Crossman AR, Brotchie JM. Antiparkinsonian action of a delta opioid agonist in rodent and primate models of Parkinson's disease. Exp Neurol. 2001;172:189–198. doi: 10.1006/exnr.2001.7763. [DOI] [PubMed] [Google Scholar]
- Holdridge SV, Cahill CM. Spinal administration of a delta opioid receptor agonist attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Eur J Pain. 2007;11:685–693. doi: 10.1016/j.ejpain.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, et al. delta-Opioid receptor agonists produce antinociception and [35S]GTPgammaS binding in mu receptor knockout mice. Eur J Pharmacol. 2000;388:241–248. doi: 10.1016/s0014-2999(99)00897-3. [DOI] [PubMed] [Google Scholar]
- Hudzik TJ, Maciag C, Smith MA, Caccese R, Pietras MR, Bui KH, et al. Preclinical pharmacology of AZD2327: a highly selective agonist of the delta-opioid receptor. J Pharmacol Exp Ther. 2011;338:195–204. doi: 10.1124/jpet.111.179432. [DOI] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S, Abdul Y, Potter DE. Non-analgesic effects of opioids: neuroprotection in the retina. Curr Pharm Des. 2012;18:6101–6108. doi: 10.2174/138161212803582441. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Thomas DA, Iadarola MJ, Nahin RL, Dubner R. Spinal opioid analgesic effects are enhanced in a model of unilateral inflammation/hyperalgesia: possible involvement of noradrenergic mechanisms. Eur J Pharmacol. 1991;194:135–143. doi: 10.1016/0014-2999(91)90097-a. [DOI] [PubMed] [Google Scholar]
- Iaizzo PA, Laske TG, Harlow HJ, McClay CB, Garshelis DL. Wound healing during hibernation by black bears (Ursus americanus) in the wild: elicitation of reduced scar formation. Integr Zool. 2012;7:48–60. doi: 10.1111/j.1749-4877.2011.00280.x. [DOI] [PubMed] [Google Scholar]
- Jenab S, Inturrisi CE. Activation of protein kinase A prevents the ethanol-induced up-regulation of delta-opioid receptor mRNA in NG108-15 cells. Brain Res Mol Brain Res. 1997;47:44–48. doi: 10.1016/s0169-328x(97)00061-2. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Mu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in rat. Neuroscience. 2010;171:344–350. doi: 10.1016/j.neuroscience.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Woods JH, Winsauer PJ. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav Pharmacol. 2003;14:509–516. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Kaminsky ST, Rice KC, Traynor JR, Woods JH. Differential behavioral tolerance to the delta-opioid agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl ]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005a;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Traynor JR, Woods JH. Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacology (Berl) 2005b;182:588–596. doi: 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Baladi MG, Folk JE, Rice KC, Woods JH. The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther. 2006;317:1337–1348. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kabli N, Martin N, Fan T, Nguyen T, Hasbi A, Balboni G, et al. Agonists at the delta-opioid receptor modify the binding of micro-receptor agonists to the micro-delta receptor hetero-oligomer. Br J Pharmacol. 2010;161:1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabli N, Nguyen T, Balboni G, O'Dowd BF, George SR. Antidepressant-like and anxiolytic-like effects following activation of the μ-δ opioid receptor heteromer in the nucleus accumbens. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.115. [E-pub ahead of print] doi: 10.1038/mp.2013-115. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, et al. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Kest B, Lee CE, McLemore GL, Inturrisi CE. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Res Bull. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- Khavandgar S, Homayoun H, Dehpour AR. The role of nitric oxide in the proconvulsant effect of delta-opioid agonist SNC80 in mice. Neurosci Lett. 2002;329:237–239. doi: 10.1016/s0304-3940(02)00417-2. [DOI] [PubMed] [Google Scholar]
- Ko JL, Arvidsson U, Williams FG, Law PY, Elde R, Loh HH. Visualization of time-dependent redistribution of delta-opioid receptors in neuronal cells during prolonged agonist exposure. Brain Res Mol Brain Res. 1999;69:171–185. doi: 10.1016/s0169-328x(99)00094-7. [DOI] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, et al. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlinska JH, Gibula-Bruzda E, Witkowska E, Izdebski J. Involvement of delta and mu opioid receptors in the acute and sensitized locomotor action of cocaine in mice. Peptides. 2010;48:89–95. doi: 10.1016/j.peptides.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Kouchek M, Takasusuki T, Terashima T, Yaksh TL, Xu Q. Effects of intrathecal SNC80, a delta receptor ligand, on nociceptive threshold and dorsal horn substance p release. J Pharmacol Exp Ther. 2013;347:258–264. doi: 10.1124/jpet.113.206573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent beta-arrestin mutants with constitutive activity in cells. J Biol Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- Kramer HK, Andria ML, Kushner SA, Esposito DH, Hiller JM, Simon EJ. Mutation of tyrosine 318 (Y318F) in the delta-opioid receptor attenuates tyrosine phosphorylation, agonist-dependent receptor internalization, and mitogen-activated protein kinase activation. Brain Res Mol Brain Res. 2000;79:55–66. doi: 10.1016/s0169-328x(00)00097-8. [DOI] [PubMed] [Google Scholar]
- Lamberts JT, Smith CE, Li MH, Ingram SL, Neubig RR, Traynor JR. Differential control of opioid antinociception to thermal stimuli in a knock-in mouse expressing regulator of G-protein signaling-insensitive Galphao protein. J Neurosci. 2013;33:4369–4377. doi: 10.1523/JNEUROSCI.5470-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Leung B, Maidment N, Balleine BW. mu- and delta-opioid-related processes in the accumbens core and shell differentially mediate the influence of reward-guided and stimulus-guided decisions on choice. J Neurosci. 2012;32:1875–1883. doi: 10.1523/JNEUROSCI.4688-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Wong YH, Loh HH. Mutational analysis of the structure and function of opioid receptors. Biopolymers. 1999;51:440–455. doi: 10.1002/(SICI)1097-0282(1999)51:6<440::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Law PY, Kouhen OM, Solberg J, Wang W, Erickson LJ, Loh HH. Deltorphin II-induced rapid desensitization of delta-opioid receptor requires both phosphorylation and internalization of the receptor. J Biol Chem. 2000;275:32057–32065. doi: 10.1074/jbc.M002395200. [DOI] [PubMed] [Google Scholar]
- Law PY, Erickson-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, et al. Heterodimerization of mu- and delta-opioid receptors occurs at the cell surface only and requires receptor-G protein interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Plaza-Zabala A, Del Boca C, Matifas A, Maldonado R, Kieffer BL. Deletion of the delta opioid receptor gene impairs place conditioning but preserves morphine reinforcement. Biol Psychiatry. 2011;69:700–703. doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Faget L, Matifas A, Kieffer BL. Cues predicting drug or food reward restore morphine-induced place conditioning in mice lacking delta opioid receptors. Psychopharmacology (Berl) 2012;223:99–106. doi: 10.1007/s00213-012-2693-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL. Impaired hippocampus-dependent and facilitated striatum-dependent behaviors in mice lacking the delta opioid receptor. Neuropsychopharmacology. 2013;38:1050–1059. doi: 10.1038/npp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontiadis LJ, Papakonstantinou MP, Georgoussi Z. Regulator of G protein signaling 4 confers selectivity to specific G proteins to modulate mu- and delta-opioid receptor signaling. Cell Signal. 2009;21:1218–1228. doi: 10.1016/j.cellsig.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Leskela TT, Markkanen PM, Pietila EM, Tuusa JT, Petaja-Repo UE. Opioid receptor pharmacological chaperones act by binding and stabilizing newly synthesized receptors in the endoplasmic reticulum. J Biol Chem. 2007;282:23171–23183. doi: 10.1074/jbc.M610896200. [DOI] [PubMed] [Google Scholar]
- Liu X, Kai M, Jin L, Wang R. Molecular modeling studies to predict the possible binding modes of endomorphin analogs in mu opioid receptor. Bioorg Med Chem Lett. 2009;19:5387–5391. doi: 10.1016/j.bmcl.2009.07.121. [DOI] [PubMed] [Google Scholar]
- Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. mu Opioid receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Brain Res Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- Lowe JD, Celver JP, Gurevich VV, Chavkin C. mu-Opioid receptors desensitize less rapidly than delta-opioid receptors due to less efficient activation of arrestin. J Biol Chem. 2002;277:15729–15735. doi: 10.1074/jbc.M200612200. [DOI] [PubMed] [Google Scholar]
- Lowe M. Structural organization of the Golgi apparatus. Curr Opin Cell Biol. 2011;23:85–93. doi: 10.1016/j.ceb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Lucido AL, Morinville A, Gendron L, Stroh T, Beaudet A. Prolonged morphine treatment selectively increases membrane recruitment of delta-opioid receptors in mouse basal ganglia. J Mol Neurosci. 2005;25:207–214. doi: 10.1385/JMN:25:3:207. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang Y, Kalyuzhny AE, Pan ZZ. Emergence of functional delta-opioid receptors induced by long-term treatment with morphine. Mol Pharmacol. 2006;69:1137–1145. doi: 10.1124/mol.105.019109. [DOI] [PubMed] [Google Scholar]
- Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y. Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem. 2005;280:16208–16218. doi: 10.1074/jbc.M408055200. [DOI] [PubMed] [Google Scholar]
- Mabrouk OS, Marti M, Salvadori S, Morari M. The novel delta opioid receptor agonist UFP-512 dually modulates motor activity in hemiparkinsonian rats via control of the nigro-thalamic pathway. Neuroscience. 2009;164:360–369. doi: 10.1016/j.neuroscience.2009.08.058. [DOI] [PubMed] [Google Scholar]
- Madziva MT, Edwardson JM. Trafficking of green fluorescent protein-tagged muscarinic M4 receptors in NG108-15 cells. Eur J Pharmacol. 2001;428:9–18. doi: 10.1016/s0014-2999(01)01266-3. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Isobolographic and dose–response analyses of the interaction between intrathecal mu and delta agonists: effects of naltrindole and its benzofuran analog (NTB) J Pharmacol Exp Ther. 1992;263:264–275. [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994a;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 1994b;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov LN, Naryzhnaia NV, Tsibulnikov SY, Kolar F, Zhang Y, Wang H, et al. Role of endogenous opioid peptides in the infarct size-limiting effect of adaptation to chronic continuous hypoxia. Life Sci. 2013;93:373–379. doi: 10.1016/j.lfs.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald NA, Henstridge CM, Connolly CN, Irving AJ. Generation and functional characterization of fluorescent, N-terminally tagged CB1 receptor chimeras for live-cell imaging. Mol Cell Neurosci. 2007;35:237–248. doi: 10.1016/j.mcn.2007.02.016. [DOI] [PubMed] [Google Scholar]
- McLean AJ, Milligan G. Ligand regulation of green fluorescent protein-tagged forms of the human beta(1)- and beta(2)-adrenoceptors; comparisons with the unmodified receptors. Br J Pharmacol. 2000;130:1825–1832. doi: 10.1038/sj.bjp.0703506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennicken F, Zhang J, Hoffert C, Ahmad S, Beaudet A, O'Donnell D. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- Metcalf MD, Yekkirala AS, Powers MD, Kitto KF, Fairbanks CA, Wilcox GL, et al. The delta opioid receptor agonist SNC80 selectively activates heteromeric mu-delta opioid receptors. ACS Chem Neurosci. 2012;3:505–509. doi: 10.1021/cn3000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Taiwo YO, Levine JD. Kappa- and delta-opioid agonists synergize to produce potent analgesia. Brain Res. 1990;509:165–168. doi: 10.1016/0006-8993(90)90327-8. [DOI] [PubMed] [Google Scholar]
- Miaskowski C, Sutters KA, Taiwo YO, Levine JD. Comparison of the antinociceptive and motor effects of intrathecal opioid agonists in the rat. Brain Res. 1991;553:105–109. doi: 10.1016/0006-8993(91)90236-o. [DOI] [PubMed] [Google Scholar]
- Milan-Lobo L, Whistler JL. Heteromerization of the mu- and delta-opioid receptors produces ligand-biased antagonism and alters mu-receptor trafficking. J Pharmacol Exp Ther. 2011;337:868–875. doi: 10.1124/jpet.111.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Margolis EB, Coker AR, Fields HL. Alcohol self-administration, anxiety, and cortisol levels predict changes in delta opioid receptor function in the ventral tegmental area. Behav Neurosci. 2012;126:515–522. doi: 10.1037/a0029027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N, Roberts K, Pal K, Bentolila L, Fultz E, Minasyan A, et al. Select G-protein-coupled receptors modulate agonist-induced signaling via a ROCK, LIMK and β-arrestin 1 pathway. Cell Rep. 2013;5:1010–1021. doi: 10.1016/j.celrep.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MM, Ashley MD, Ingram SL, Christie MJ. Behavioral consequences of delta-opioid receptor activation in the periaqueductal gray of morphine tolerant rats. Neural Plast. 2009;2009:516328. doi: 10.1155/2009/516328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, et al. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23:4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, et al. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24:5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109:266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Nagasaka H, Yaksh TL. Effects of intrathecal mu, delta, and kappa agonists on thermally evoked cardiovascular and nociceptive reflexes in halothane-anesthetized rats. Anesth Analg. 1995;80:437–443. doi: 10.1097/00000539-199503000-00001. [DOI] [PubMed] [Google Scholar]
- Navratilova E, Eaton MC, Stropova D, Varga EV, Vanderah TW, Roeske WR, et al. Morphine promotes phosphorylation of the human delta-opioid receptor at serine 363. Eur J Pharmacol. 2005;519:212–214. doi: 10.1016/j.ejphar.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, DeCosta B, Winger G, Woods JH. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Nichols ML, Bian D, Ossipov MH, Lai J, Porreca F. Regulation of morphine antiallodynic efficacy by cholecystokinin in a model of neuropathic pain in rats. J Pharmacol Exp Ther. 1995;275:1339–1345. [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Li R, Mill D, Yi H, Feduccia AA, et al. δ-opioid receptor function in the dorsal striatum plays a role in high levels of ethanol consumption in rats. J Neurosci. 2012;32:4540–4552. doi: 10.1523/JNEUROSCI.5345-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MM, Guen SL, Kieffer BL, Roques BP, Noble F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Norcini M, Vivoli E, Galeotti N, Bianchi E, Bartolini A, Ghelardini C. Supraspinal role of protein kinase C in oxaliplatin-induced neuropathy in rat. Pain. 2009;146:141–147. doi: 10.1016/j.pain.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Normandin A, Luccarini P, Molat JL, Gendron L, Dallel R. Spinal μ and δ opioids inhibit both thermal and mechanical pain in rats. J Neurosci. 2013;33:11703–11714. doi: 10.1523/JNEUROSCI.1631-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeltgen PR, Nilekani SP, Nuchols PA, Spurrier WA, Su TP. Further studies on opioids and hibernation: delta opioid receptor ligand selectively induced hibernation in summer-active ground squirrels. Life Sci. 1988;43:1565–1574. doi: 10.1016/0024-3205(88)90406-7. [DOI] [PubMed] [Google Scholar]
- Otis V, Sarret P, Gendron L. Spinal activation of delta opioid receptors alleviates cancer-related bone pain. Neuroscience. 2011;183:221–229. doi: 10.1016/j.neuroscience.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overland AC, Kitto KF, Chabot-Dore AJ, Rothwell PE, Fairbanks CA, Stone LS, et al. Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord. J Neurosci. 2009;29:13264–13273. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini F, Bochet P, Garbay-Jaureguiberry C, Roques BP, Rossier J, Beaudet A. Electron microscopic localization of photoaffinity-labelled delta opioid receptors in the neostriatum of the rat. J Comp Neurol. 1992;326:229–244. doi: 10.1002/cne.903260206. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, et al. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25:8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Agonist-dependent phosphorylation of the mouse delta-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase C. Mol Pharmacol. 1995;48:173–177. [PubMed] [Google Scholar]
- Pello OM, Martinez-Munoz L, Parrillas V, Serrano A, Rodriguez-Frade JM, Toro MJ, et al. Ligand stabilization of CXCR4/delta-opioid receptor heterodimers reveals a mechanism for immune response regulation. Eur J Immunol. 2008;38:537–549. doi: 10.1002/eji.200737630. [DOI] [PubMed] [Google Scholar]
- Peng PH, Huang HS, Lee YJ, Chen YS, Ma MC. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem. 2009;108:741–754. doi: 10.1111/j.1471-4159.2008.05807.x. [DOI] [PubMed] [Google Scholar]
- Perl ER. Ideas about pain, a historical view. Nat Rev Neurosci. 2007;8:71–80. doi: 10.1038/nrn2042. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, et al. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275:13727–13736. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Bhalla S, Walker P, Bouvier M. Newly synthesized human delta opioid receptors retained in the endoplasmic reticulum are retrotranslocated to the cytosol, deglycosylated, ubiquitinated, and degraded by the proteasome. J Biol Chem. 2001;276:4416–4423. doi: 10.1074/jbc.M007151200. [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Bhalla S, Laperriere A, Morello JP, Bouvier M. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinger L, Gigout S, Linley JE, Gamper N. Bradykinin controls pool size of sensory neurons expressing functional delta-opioid receptors. J Neurosci. 2013;33:10762–10771. doi: 10.1523/JNEUROSCI.0123-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Daniel K, Puzicha M, Caron MG, Lefkowitz RJ, et al. Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J Biol Chem. 1993;268:3201–3208. [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- Piskorowski RA, Chevaleyre V. Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the hippocampus. J Neurosci. 2013;33:14567–14578. doi: 10.1523/JNEUROSCI.0649-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther. 1984;230:341–348. [PubMed] [Google Scholar]
- Pradhan A, Smith M, McGuire B, Evans C, Walwyn W. Chronic inflammatory injury results in increased coupling of delta opioid receptors to voltage-gated Ca2+ channels. Mol Pain. 2013;9:8. doi: 10.1186/1744-8069-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Becker JA, Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581–590. doi: 10.1016/j.tips.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155:269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psifogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, Gold SJ, et al. A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia. J Neurosci. 2011;31:5617–5624. doi: 10.1523/JNEUROSCI.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Sora I, Ren K, Uhl G, Dubner R. Enhanced delta-opioid receptor-mediated antinociception in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2000;387:163–169. doi: 10.1016/s0014-2999(99)00813-4. [DOI] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci. 2011;31:4434–4443. doi: 10.1523/JNEUROSCI.4874-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall-Thompson JF, Pescatore KA, Unterwald EM. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan P, Chen H, Adelman MK, Schluter SF. Autoantibodies to the delta-opioid receptor function as opioid agonists and display immunomodulatory activity. J Neuroimmunol. 2009;217:65–73. doi: 10.1016/j.jneuroim.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai X, Faget L, Bednarek E, Schwab Y, Kieffer BL, Massotte D. Mouse delta opioid receptors are located on presynaptic afferents to hippocampal pyramidal cells. Cell Mol Neurobiol. 2012;32:509–516. doi: 10.1007/s10571-011-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai X, Kieffer BL, Roux MJ, Massotte D. Delta opioid receptors regulate temporoammonic-activated feedforward inhibition to the mouse CA1 hippocampus. PLoS ONE. 2013;8:e79081. doi: 10.1371/journal.pone.0079081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl MS, Schnell SA, Overland AC, Chabot-Dore AJ, Taylor AM, Ribeiro-da-Silva A, et al. Coexpression of alpha 2A-adrenergic and delta-opioid receptors in substance P-containing terminals in rat dorsal horn. J Comp Neurol. 2009;513:385–398. doi: 10.1002/cne.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Whistler JL, Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr Opin Pharmacol. 2010;10:73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Brissett DI, Whistler JL. Emergence of functional spinal delta opioid receptors after chronic ethanol exposure. Biol Psychiatry. 2012;71:232–238. doi: 10.1016/j.biopsych.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijn RM, Defriel JN, Whistler JL. Pharmacological traits of delta opioid receptors: pitfalls or opportunities? Psychopharmacology (Berl) 2013;228:1–18. doi: 10.1007/s00213-013-3129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero G, Gabilondo AM, Garcia-Fuster MJ, La Harpe R, Garcia-Sevilla JA, Meana JJ. Differential regulation of RGS proteins in the prefrontal cortex of short- and long-term human opiate abusers. Neuropharmacology. 2012;62:1044–1051. doi: 10.1016/j.neuropharm.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Roman-Vendrell C, Yu YJ, Yudowski GA. Fast modulation of mu-opioid receptor (MOR) recycling is mediated by receptor agonists. J Biol Chem. 2012;287:14782–14791. doi: 10.1074/jbc.M111.319616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Bykov V, Long JB, Brady LS, Jacobson AE, Rice KC, et al. Chronic administration of morphine and naltrexone up-regulate mu-opioid binding sites labeled by [3H][D-Ala2,MePhe4,Gly-ol5]enkephalin: further evidence for two mu-binding sites. Eur J Pharmacol. 1989;160:71–82. doi: 10.1016/0014-2999(89)90655-9. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Ruparel NB, Patwardhan AM, Berg KA, Clarke WP, Hargreaves KM. Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol. 2009;602:283–287. doi: 10.1016/j.ejphar.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Rached M, Gallo-Payet N. Differential regulation of the human adrenocorticotropin receptor [melanocortin-2 receptor (MC2R)] by human MC2R accessory protein isoforms alpha and beta in isogenic human embryonic kidney 293 cells. Mol Endocrinol. 2007;21:1656–1669. doi: 10.1210/me.2007-0041. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J. 2007;21:2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Sugiyama A, Nemoto T, Fujii H, Wada K, Oka J, et al. The novel delta opioid receptor agonist KNT-127 produces antidepressant-like and antinociceptive effects in mice without producing convulsions. Behav Brain Res. 2011;223:271–279. doi: 10.1016/j.bbr.2011.04.041. [DOI] [PubMed] [Google Scholar]
- Salvarezza SB, Deborde S, Schreiner R, Campagne F, Kessels MM, Qualmann B, et al. LIM kinase 1 and cofilin regulate actin filament population required for dynamin-dependent apical carrier fission from the trans-Golgi network. Mol Biol Cell. 2009;20:438–451. doi: 10.1091/mbc.E08-08-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster DJ, Kitto KF, Overland AC, Messing RO, Stone LS, Fairbanks CA, et al. Protein kinase C{epsilon} is required for spinal analgesic synergy between delta opioid and alpha-2A adrenergic receptor agonist pairs. J Neurosci. 2013;33:13538–13546. doi: 10.1523/JNEUROSCI.4013-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered mu- and delta-, but not kappa-, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302:1146–1150. doi: 10.1124/jpet.102.033167. [DOI] [PubMed] [Google Scholar]
- Sora I, Funada M, Uhl GR. The mu-opioid receptor is necessary for [D-Pen2,D-Pen5]enkephalin-induced analgesia. Eur J Pharmacol. 1997a;324:R1–R2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, et al. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997b;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Hammond DL. Activation of spinal delta-1 or delta-2 opioid receptors reduces carrageenan-induced hyperalgesia in the rat. J Pharmacol Exp Ther. 1994;268:701–708. [PubMed] [Google Scholar]
- Stratinaki M, Varidaki A, Mitsi V, Ghose S, Magida J, Dias C, et al. Regulator of G protein signaling 4 [corrected] is a crucial modulator of antidepressant drug action in depression and neuropathic pain models. Proc Natl Acad Sci U S A. 2013;110:8254–8259. doi: 10.1073/pnas.1214696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutters KA, Miaskowski C, Taiwo YO, Levine JD. Analgesic synergy and improved motor function produced by combinations of mu-delta- and mu-kappa-opioids. Brain Res. 1990;530:290–294. doi: 10.1016/0006-8993(90)91297-t. [DOI] [PubMed] [Google Scholar]
- Tang B, Li Y, Yuan S, Tomlinson S, He S. Upregulation of the delta opioid receptor in liver cancer promotes liver cancer progression both in vitro and in vivo. Int J Oncol. 2013;43:1281–1290. doi: 10.3892/ijo.2013.2046. [DOI] [PubMed] [Google Scholar]
- Thorlin T, Eriksson PS, Hansson E, Ronnback L. [D-Pen2,5]enkephalin and glutamate regulate the expression of delta-opioid receptors in rat cortical astrocytes. Neurosci Lett. 1997;232:67–70. doi: 10.1016/s0304-3940(97)00583-1. [DOI] [PubMed] [Google Scholar]
- Thorlin T, Persson PA, Eriksson PS, Hansson E, Ronnback L. Delta-opioid receptor immunoreactivity on astrocytes is upregulated during mitosis. Glia. 1999;25:370–378. doi: 10.1002/(sici)1098-1136(19990215)25:4<370::aid-glia6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tian X, Hua F, Sandhu HK, Chao D, Balboni G, Salvadori S, et al. Effect of delta-opioid receptor activation on BDNF-TrkB vs. TNF-alpha in the mouse cortex exposed to prolonged hypoxia. Int J Mol Sci. 2013;14:15959–15976. doi: 10.3390/ijms140815959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng LF, Narita M, Mizoguchi H, Kawai K, Mizusuna A, Kamei J, et al. Delta-1 opioid receptor-mediated antinociceptive properties of a nonpeptidic delta opioid receptor agonist, (-)TAN-67, in the mouse spinal cord. J Pharmacol Exp Ther. 1997;280:600–605. [PubMed] [Google Scholar]
- Tuusa JT, Leskela TT, Petaja-Repo UE. Human delta opioid receptor biogenesis is regulated via interactions with SERCA2b and calnexin. FEBS J. 2010;277:2815–2829. doi: 10.1111/j.1742-4658.2010.07699.x. [DOI] [PubMed] [Google Scholar]
- Ueda M, Sugimoto K, Oyama T, Kuraishi Y, Satoh M. Opioidergic inhibition of capsaicin-evoked release of glutamate from rat spinal dorsal horn slices. Neuropharmacology. 1995;34:303–308. doi: 10.1016/0028-3908(94)00160-t. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Cheng P, Moriwaki A, Uhl GR, Pickel VM. Ultrastructural evidence for prominent distribution of the mu-opioid receptor at extrasynaptic sites on noradrenergic dendrites in the rat nucleus locus coeruleus. J Neurosci. 1996;16:5037–5048. doi: 10.1523/JNEUROSCI.16-16-05037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z, Barg J, Attali B, Simantov R. Differential effect of mu, delta, and kappa ligands on G protein alpha subunits in cultured brain cells. J Neurosci Res. 1990;27:106–111. doi: 10.1002/jnr.490270116. [DOI] [PubMed] [Google Scholar]
- Walwyn W, John S, Maga M, Evans CJ, Hales TG. Delta receptors are required for full inhibitory coupling of mu-receptors to voltage-dependent Ca(2+) channels in dorsal root ganglion neurons. Mol Pharmacol. 2009;76:134–143. doi: 10.1124/mol.109.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sun X, Bohn LM, Sadee W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- Wang HB, Guan JS, Bao L, Zhang X. Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells. Neurochem Res. 2008a;33:2028–2034. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Van Bockstaele EJ, Liu-Chen LY. In vivo trafficking of endogenous opioid receptors. Life Sci. 2008b;83:693–699. doi: 10.1016/j.lfs.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel R, Reiss D, Karchewski L, Gardon O, Matifas A, Filliol D, et al. Mu opioid receptors on primary afferent nav1.8 neurons contribute to opiate-induced analgesia: insight from conditional knockout mice. PLoS ONE. 2013;8:e74706. doi: 10.1371/journal.pone.0074706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Tsao P, von Zastrow M. A phosphorylation-regulated brake mechanism controls the initial endocytosis of opioid receptors but is not required for post-endocytic sorting to lysosomes. J Biol Chem. 2001;276:34331–34338. doi: 10.1074/jbc.M104627200. [DOI] [PubMed] [Google Scholar]
- Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, et al. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of mu-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65:223–254. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, et al. Global phosphorylation analysis of beta-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc Natl Acad Sci U S A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CW, Lewis DV. Endogenous opioids regulate long-term potentiation of synaptic inhibition in the dentate gyrus of rat hippocampus. J Neurosci. 1995;15((5 Pt 2)):3788–3795. doi: 10.1523/JNEUROSCI.15-05-03788.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WY, He Y, Yang YR, Li YF, Kang K, Xing BM, et al. Disruption of Cdk5-associated phosphorylation of residue threonine-161 of the delta-opioid receptor: impaired receptor function and attenuated morphine antinociceptive tolerance. J Neurosci. 2009;29:3551–3564. doi: 10.1523/JNEUROSCI.0415-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhi F, He X, Moore ML, Kang X, Chao D, et al. delta-opioid receptor activation and microRNA expression of the rat cortex in hypoxia. PLoS ONE. 2013;7:e51524. doi: 10.1371/journal.pone.0051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E, Olney J, Akil H. Increase in delta, but not mu, receptors in MSG-treated rats. Life Sci. 1982;31:1343–1346. doi: 10.1016/0024-3205(82)90377-0. [DOI] [PubMed] [Google Scholar]
- Young E, Olney J, Akil H. Selective alterations of opiate receptor subtypes in monosodium glutamate-treated rats. J Neurochem. 1983;40:1558–1564. doi: 10.1111/j.1471-4159.1983.tb08126.x. [DOI] [PubMed] [Google Scholar]
- Yu YJ, Dhavan R, Chevalier MW, Yudowski GA, von Zastrow M. Rapid delivery of internalized signaling receptors to the somatodendritic surface by sequence-specific local insertion. J Neurosci. 2010;30:11703–11714. doi: 10.1523/JNEUROSCI.6282-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Goldstein BD. Delta-Opioid receptor modulation of the release of substance P-like immunoreactivity in the dorsal horn of the rat following mechanical or thermal noxious stimulation. Brain Res. 1996;736:305–314. doi: 10.1016/0006-8993(96)00718-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82:1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang F, Chen X, Li J, Xiang B, Zhang YQ, et al. Beta-arrestin1 and beta-arrestin2 are differentially required for phosphorylation-dependent and -independent internalization of delta-opioid receptors. J Neurochem. 2005;95:169–178. doi: 10.1111/j.1471-4159.2005.03352.x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Ma GQ. Sorting of neuropeptides and neuropeptide receptors into secretory pathways. Prog Neurobiol. 2010;90:276–283. doi: 10.1016/j.pneurobio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Zhao B, Wang HB, Lu YJ, Hu JW, Bao L, Zhang X. Transport of receptors, receptor signaling complexes and ion channels via neuropeptide-secretory vesicles. Cell Res. 2011;21:741–753. doi: 10.1038/cr.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- Zoudilova M, Kumar P, Ge L, Wang P, Bokoch GM, DeFea KA. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- Zoudilova M, Min J, Richards HL, Carter D, Huang T, DeFea KA. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem. 2010;285:14318–14329. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]


